-
近年来,由于人类活动频繁,二氧化碳排放量居高不下导致全球气候变暖和环境污染,这两大诱因导致海洋中低氧现象频发[1]。低氧通常是指水中溶解氧浓度低于2 mg·L−1[2]。凡纳滨对虾具有抗逆性强、生长快、繁殖期长、对养殖条件要求低、便于运输等优点,成为世界对虾养殖的三大品种之一[3]。水体中的溶解氧是影响凡纳滨对虾生长发育的重要因素。目前对甲壳类动物应激的研究主要集中在其对行为、代谢、免疫等方面。甲壳动物对低氧胁迫的分子调控机制主要是通过在HIF-1信号通路、AMPK信号通路及细胞凋亡信号通路上发挥作用[4]。HIF-1作为参与低氧反应的重要转录调节因子,是由HIF-1α和HIF-1β两个亚基组成的,但 HIF-1α亚基在常氧状态下表达受到抑制,只有在生物体处于低氧环境中,HIF-1α才被激活;该亚基与β亚基共同调控低氧应答部分基因的转录[5]。目前的研究成果主要是围绕HIF-1α参与调节低氧条件下凡纳滨对虾葡萄糖代谢途径中一些基因的表达及其相应的生理响应和抗病毒能力[6-13]。
无脊椎动物缺乏特异性免疫系统,只能依靠血淋巴这一非特异性免疫实现[14]。血淋巴中的血细胞和各种免疫因子在体内共同发挥免疫作用;其中血细胞在吞噬、包囊和结节形成等方面均发挥重要作用[15];血蓝蛋白(Hemocyanin,HC)是节肢动物中重要的呼吸蛋白,还在酚氧化酶和抗菌抗病毒等方面发挥免疫作用[16]。在血细胞中,有许多基因调控血细胞的增殖稳态和免疫,造血激素(Astakine,AST)通过降低细胞外TGase活性从而刺激了血细胞的释放,在甲壳动物中造血的重要性已被证实[17];血细胞稳态相关蛋白( Hemocyte homeostasis-associated protein ,HHAP)在维持血细胞稳态中发挥重要作用,该基因表达降低会导致血细胞数减少[18];铁蛋白(Ferritin,FT)作为一种铁清除蛋白,可以通过抑制铁含量从而抑制细菌生长,最终阻碍病原体的存活[17]。在虾类血清中,酸性磷酸酶(Acid phosphatase,ACP )和碱性磷酸酶(Alkaline phosphatase,AKP)是甲壳动物体内吞噬溶酶体的重要组成部分,能促进血细胞的吞噬和包裹;酚氧化酶(Phenoloxidase,PO)活化后可快速吸附在体外异物上,从而进行识别和调节 ,对于血细胞的吞噬和包裹及识别消灭体外异物发挥积极作用[19]。然而,在低氧条件下HIF-1α对凡纳滨对虾的免疫性能的影响尚不清楚,且血细胞作为无脊椎动物重要的免疫系统,特别是在低氧胁迫条件下,凡纳滨对虾血淋巴中HIF-1α对各项免疫指标的影响有待深入研究[20]。因此,本研究着重探讨了HIF-1α在低氧胁迫下对凡纳滨对虾血淋巴在免疫反应中的影响,旨在为凡纳滨对虾的免疫性能研究及其健康养殖提供一定的理论依据。
-
凡纳滨对虾( L. vannamei) 于2022年3月份取自海南省东方市海南中正水产科技有限公司,选择质量为(13.0 ± 2.0)g的健康对虾作为实验对虾。实验前,在100 L的水族箱中驯养7 d,水体盐度、温度和pH分别为(35.0 ± 1.0)‰、(25.0 ± 0.5)℃和 7.9 ± 0.2,水体溶氧浓度为(6.5 ± 0.5) mg·L−1。驯养结束后,在水族箱中随机挑选72尾凡纳滨对虾进行后续实验。
-
试剂:TRIzol™ 试剂购自赛默飞世尔科技(中国)有限公司;PrimeSTAR® HS DNA Polymerase、TB Green® Premix Ex Taq™ II(Tli RNaseH Plus)、PrimeScript™ 1st Strand cDNA Synthesis Kit、PrimeScript™ RT reagent Kit with gDNA Eraser(Perfect Real Time)、in vitro Transcription T7 Kit(for siRNA Synthesis)均购自宝生物工程(大连)有限公司。SanPrep 柱式 DNA 胶回收试剂盒、PBS购自生工生物工程(上海)股份有限公司;酶活试剂盒购自南京建成生物工程研究所有限公司。仪器:高通量组织研磨器(Scientz-48),低温离心机(Eppendorf),微量分光光度计(Thermo nanodrop 2000),PCR仪(Bio-rad T100),电泳仪(Bio-rad),凝胶成像分析系统(Bio-rad Gel Doc XR+),荧光定量PCR仪(Analytik-qTOEWER3),恒温水浴锅,溶氧仪( HANNA,HI 9146),pH 计( HANNA,HI 8424),盐度计,温度计,氮气罐。
-
本实验的qRT-PCR引物(表1)通过primer5.0设计,并全部由合成生工生物工程(上海)股份有限公司合成。
表 1 实验用引物
引物名称 引物序列(5′-3′) 用途 GenBank HIF-1α-F GGAGAGCGAGATCTTCACG HIF-1α cDNA扩增 FJ807918 HIF-1α-R CTCGATGTTGGACGGGTG HIF-1α cDNA扩增 T7HIF-1α-F GATCACTAATACGACTCACTATAGG
GGGAGAGCGAGATCTTCACG含有T7 Promoter序列的HIF-1α cDNA扩增 FJ807918 T7HIF-1α-R GATCACTAATACGACTCACTATA
GGGCTCGATGTTGGACGGGTG含有T7 Promoter序列的HIF-1α cDNA扩增 rtHIF-1α-F GACTTGACCCACTTGGCTCC qRT-PCR目的基因扩增 FJ807918 rtHIF-1α-R CCTGCTGCTAAGACGCTTC qRT-PCR目的基因扩增 AST-F GCCGTGTCCAGTGCAGTCAGAA qRT-PCR目的基因扩增 HM594944 AST-R TTCATGATGCGACACCAGTCCC qRT-PCR目的基因扩增 HHAP-F CGTACCAAGGCAACAGAAGAACCTG qRT-PCR目的基因扩增 KP676891 HHAP-R CGGATCTCCTTCTTTGGCTCCTC qRT-PCR目的基因扩增 AY955373 FT-F CCACAGAATTTGGATGGAAG qRT-PCR目的基因扩增 FT-R ATGATTACCAAGCTGAAGCG qRT-PCR目的基因扩增 L8-F GTCTACTGCGGCAAGAAGGC qRT-PCR内参基因扩增 DQ316258 L8-R CCTGAAGGGAGCTTTACACG qRT-PCR内参基因扩增 -
利用T7 RNA Polymerase进行体外转录,以含有T7 Promoter序列的HIF-1α双链DNA为模板,可以对Promoter下游的DNA序列进行转录,高效合成单链RNA。并按照说明书通过 DNase I 降解 DNA 模板和纯化转录RNA。使用 NanoDrop 微量分光光度计和 1% 琼脂糖凝胶电泳确认dsRNA 的纯度和浓度,并将一部分送去生工生物工程(上海)股份有限公司测序验证片段的正确性,剩余纯化 dsRNA储存在 −20 ℃备用。用于体外转录的PCR 片段来源于文献[8],1个 580 bp 片段对应于编码序列的位置 243~822,GenBank 登录号 FJ807918。
-
将合成的dsRNA溶于1×PBS,每100 μL PBS中含有13 μg dsRNA。将制备好的dsRNA溶液注射至凡纳滨对虾腹部肌肉,保证1 g虾注射1 μg dsRNA,为干扰组;将注射100 μL 1×PBS的凡纳滨对虾作为对照组。注射后,为了使dsRNA充分发挥作用,且尽可能让对虾消除注射应激效应,将2组对虾放回常氧水体中观察24 h,再进行常氧组的血淋巴采集。实验设置常氧组[(6.5 ± 0.5) mg·L−1]和低氧组[(2.0 ± 0.5 )mg·L−1],每组设置3个重复。低氧胁迫实验开始时,先向缸中通入大量氮气来实现急性低氧,待溶氧浓度到达2.0 mg·L−1时,减少氮气的输入量,再通过调节水体中充入氮气和空气的比例维持溶氧浓度,并且每隔30 min使用HANNA溶氧仪监测溶解氧浓度。低氧胁迫实验持续12 h,分别在3、6、12 h进行低氧组血淋巴的采集,用提前加入抗凝剂(30 mmol·L−1柠檬酸三钠,0.34 mol·L−1氯化钠,10 mmol·L−1 EDTA,0.115 mol·L−1葡萄糖)的注射器从虾围心腔中采集血淋巴样品,置于冰上备用,每组采集3尾实验虾。
-
血细胞计数使用血球计数板,在血球计数板的计数室中加10 μL的血淋巴,然后用光学显微镜进行观察计数。将血淋巴在4℃,352 r·min−1条件下离心10 min,取100 μL上清液并添加2 900 μL缓冲液(50 mmol·L−1 Tris)。用紫外分光光度计(1 cm光通)在335 nm处测定其OD值,根据公式E335 nm( g·L−1)= 2. 3×OD 值(式中, E335 nm为血蓝蛋白浓度,2. 3 为消光系数)计算血蓝蛋白浓度。
-
向1.6实验中血淋巴离心后的沉淀中加入Trizol试剂提取凡纳滨对虾血细胞的总RNA,并按说明书用反转录试剂盒制备 cDNA。实时荧光定量 PCR 扩增体系:TB Green Premix Ex Taq II(Tli RNaseH Plus)(2×) 10.0 μL, 上、下游引物(10 μmol·L−1 )各0.8 μL,cDNA模板1.0 μL,灭菌水7.4 μL,总体系共20.0 μL。PCR扩增程序:95 ℃,30 s; 40个(95 ℃, 5 s; 60 ℃,30 s)循环;绘制融解曲线。选择L8作为内参基因,基因表达水平采用 2 −ΔΔCt 法进行计算[21]。
-
ACP、AKP、PO酶活的测定均采用南京建成生物工程研究所生产的试剂盒进行测定,具体操作按其说明书进行。
-
用 SPSS Statistics 23 软件进行方差分析,并利用 GraphPad Prism 9.0 软件作图。
-
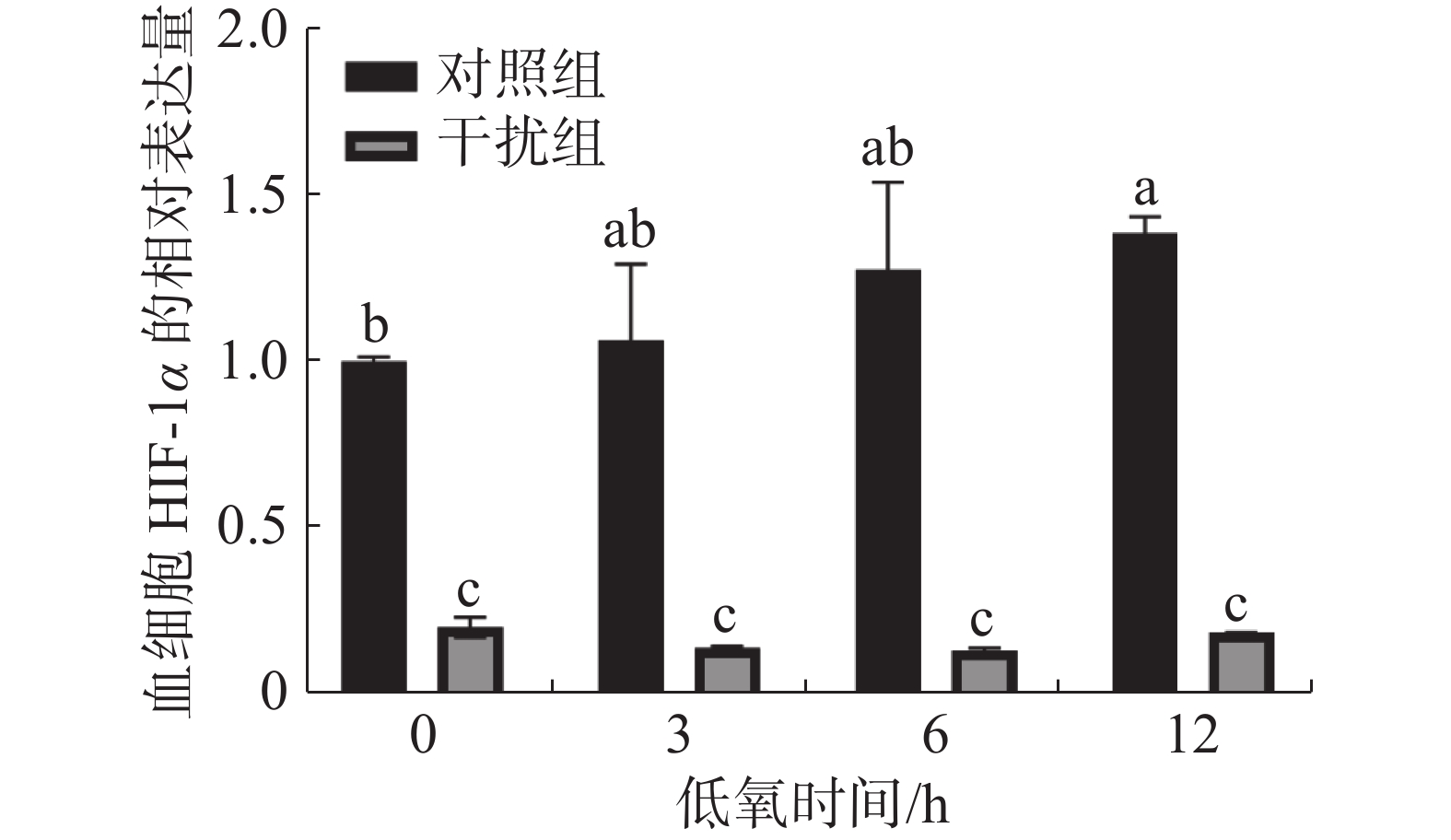
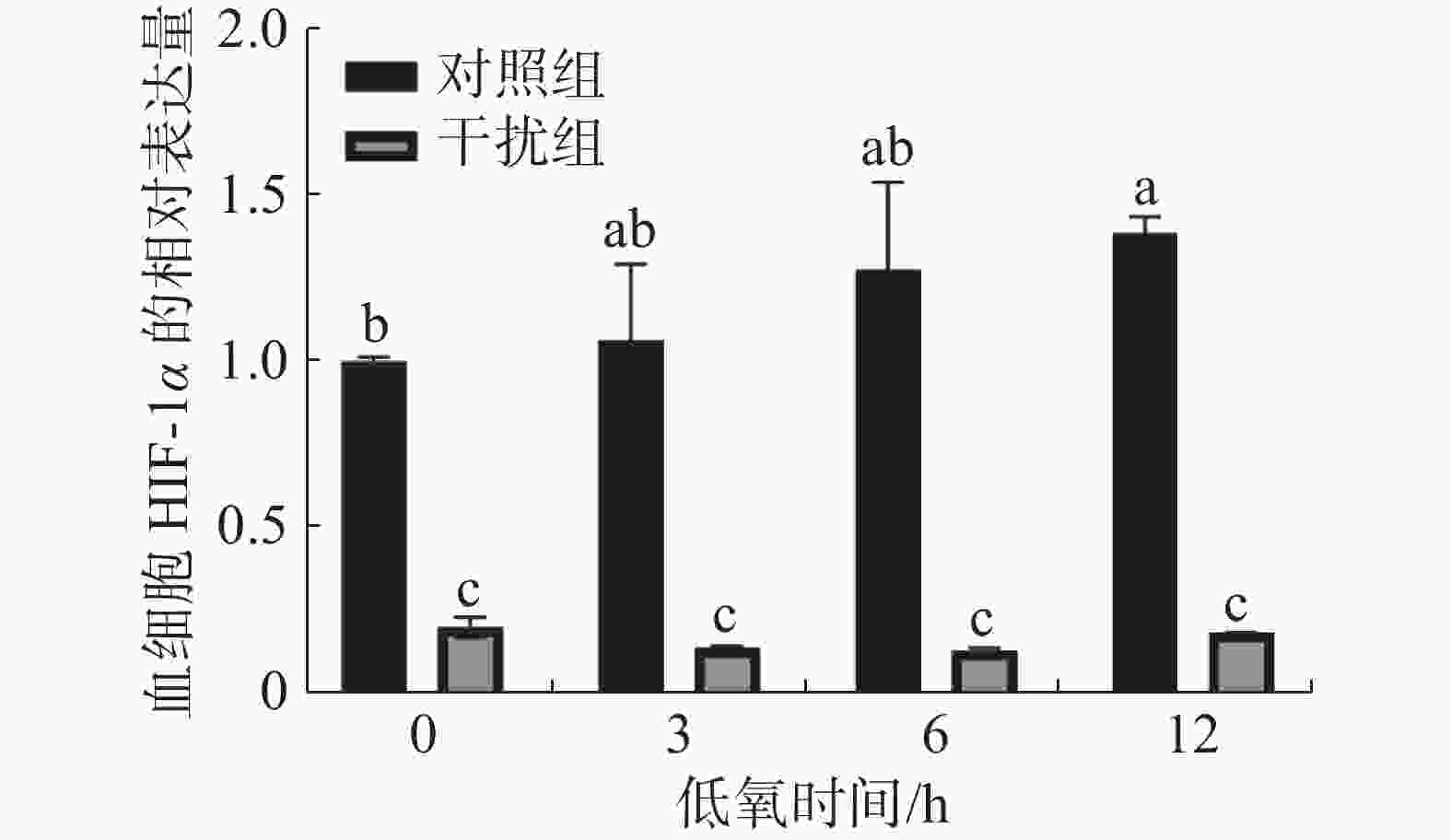
在低氧胁迫第0、3、6、12 h,通过 RT-qPCR 检测凡纳滨对虾血细胞中HIF-1α 的表达水平(图1)。在低氧0 h(常氧状态)时,干扰组较对照组HIF-1α的表达水平下降了80.1%,这说明实验中HIF-1α的敲低对凡纳滨对虾有显著的影响(P < 0.05)。随着低氧持续时间的增加,对照组HIF-1α的表达水平呈升高的趋势。低氧0 h(常氧状态)和低氧持续12 h组之间有显著性差异(P < 0.05);干扰组HIF-1α的表达水平在低氧过程中无显著性差异(P > 0.05)。
-
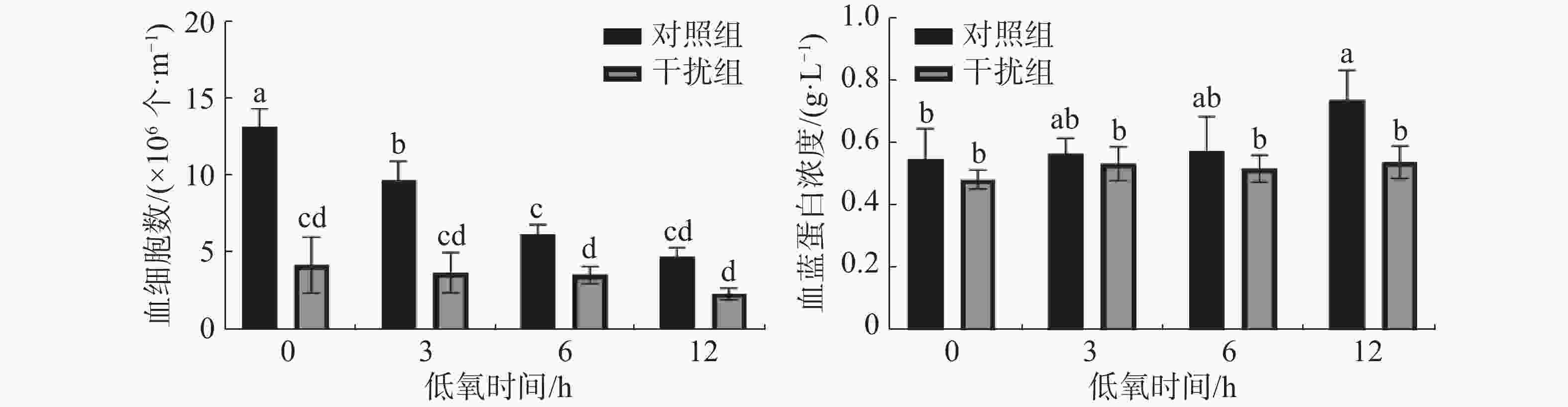
图2表明,凡纳滨对虾的血细胞数在低氧0 h(常氧状态)时干扰组较对照组显著降低(P < 0.05),说明HIF-1α的敲低对血细胞数有显著的影响;对照组随着低氧时间的增加血细胞数显著下降(P < 0.05),而干扰组无显著差异(P > 0.05)。对照组血蓝蛋白含量在低氧胁迫下较干扰组有着显著升高(P < 0.05);对照组血蓝蛋白的浓度随着低氧持续时间的增加而上升(P < 0.05),干扰组无显著差异(P > 0.05)。
-
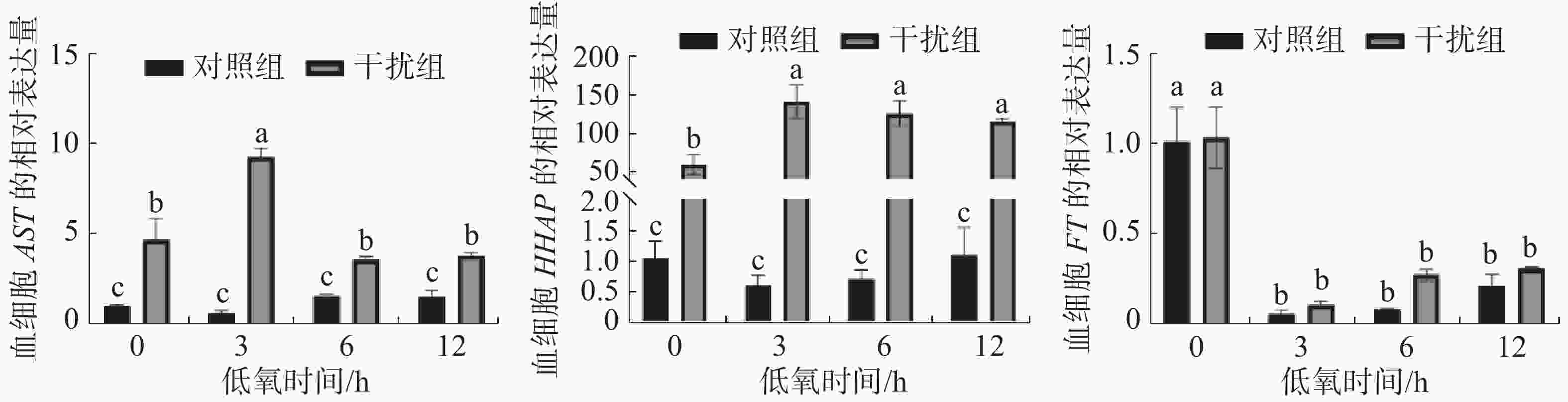
图3表明了低氧条件下敲低HIF-1α基因后,其免疫相关基因AST、HHAP和FT的表达情况。对照组AST基因的表达在低氧胁迫下无显著性变化,干扰组基本呈先升后降的趋势(P < 0.05),在低氧持续3 h时到达顶点。在低氧胁迫下HHAP基因的表达对照组差异不显著,而干扰组基本呈上升后小幅度下降的趋势(P < 0.05),且在低氧持续3h时表达量达到最高; FT基因的表达在低氧胁迫下对照组和干扰组无显著差异(P > 0.05),两组在低氧胁迫初期就显著降低(P<0.05),后期有小幅度恢复的趋势(P > 0.05)。
-
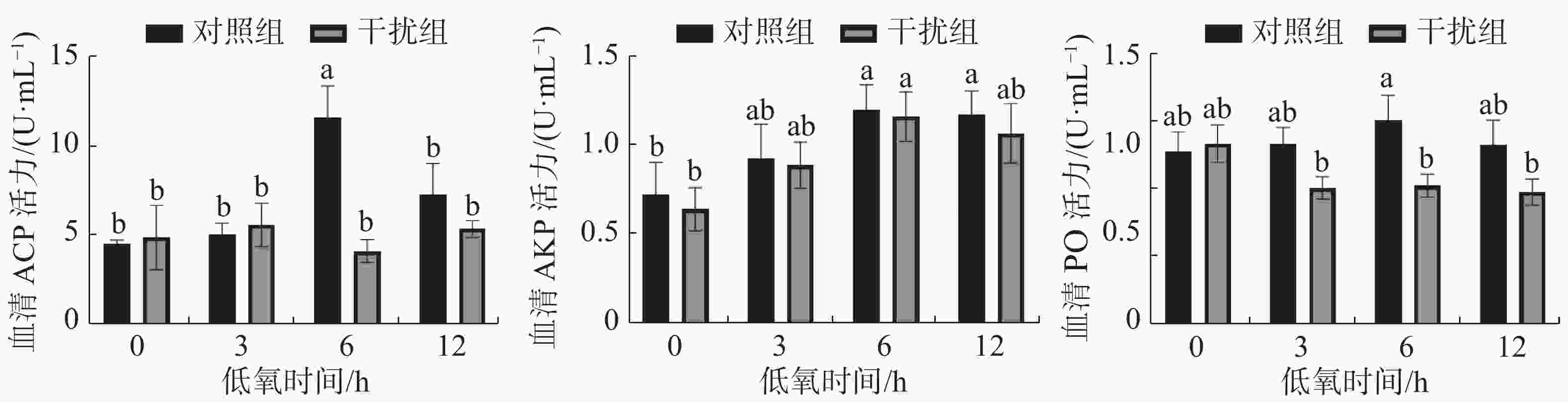
图4表明了低氧胁迫下凡纳滨对虾血清的ACP、AKP、PO酶活力变化情况。对照组在血清中ACP活力变化的趋势呈先升高后降低的趋势(P < 0.05),在低氧持续6 h到达顶点,而干扰组无显著性差异(P > 0.05);对照组和干扰组在血清中AKP活力先升高后降低,在低氧持续6 h到达顶点,两组间无显著性差异(P > 0.05);对照组在血清中PO活力呈现先升高后降低的趋势(P > 0.05),而干扰组的PO活力在低氧胁迫也有降低的趋势(P > 0.05),在低氧持续6 h时,对照组的PO活力较干扰组有显著提高(P < 0.05)。
-
凡纳滨对虾作为无脊椎动物不具备完善的免疫系统,只能依靠非特异性免疫防御,包括细胞防御和体液防御2种类型,且此2种防御体系紧密联系[22]。细胞防御包括血细胞介导的吞噬、结节形成和包裹,而体液防御涉及酚氧化酶原激活系统、凝血级联反应和免疫相关蛋白(如抗菌肽、抗病毒肽、蛋白酶和蛋白酶抑制剂)的活性[23]。
根据HIF-1α的表达水平,结合凡纳滨对虾的血细胞和血淋巴的生理指标可以综合评判HIF-1α在低氧胁迫中发挥的免疫调控作用。在低氧和注射ds HIF-1α的双重作用处理下12 h后,双链 RNA 成功抑制了凡纳滨对虾中 HIF-1α的表达水平。对虾血细胞可以通过诱导细胞和体液反应(包括凝血和溶解病原体以及伤口愈合)来抵抗病原体[22]。有研究表明低氧胁迫会减少凡纳滨对虾的血细胞数[24],而实验中干扰组的血细胞数较对照组显著降低,证明敲低HIF-1α基因可以通过调控凡纳滨对虾的血细胞数量,且比低氧胁迫的影响更为明显。血蓝蛋白是重要的呼吸蛋白,在结合和运输氧的过程中发挥着重要作用[25],且血蓝蛋白作为无脊椎动物血淋巴的主要蛋白质,在非特异性免疫尤其是抗病毒例如对虾白斑综合征病毒(WSSV)发挥着重要作用[26]。实验结果表明低氧胁迫会促进凡纳滨对虾血蓝蛋白浓度的升高[27],而敲低HIF-1α基因使低氧胁迫下血蓝蛋白浓度无显著变化(P>0.05)。结果表明,低氧胁迫下HIF-1α的敲低对于血细胞和血蓝蛋白免疫功能具有抑制作用。
此外, AST和HHAP作为免疫相关基因在实验中均表现出显著的差异表达,而对照组和干扰组的FT基因表达无显著差异。当生物体被外界病原体感染后会导致造血细胞数量急剧下降, AST对于维持甲壳类动物的免疫功能至关重要[28],本研究发现,与对照组相比,干扰组中AST的表达水平显著升高,这与在拟穴青蟹中的结果一致[29]。因AST基因具有促进血细胞增殖分化的作用,干扰组AST基因的显著升高可能与血细胞数降低有直接关系。除了AST基因,在对虾中还发现有HHAP基因参与造血调控,该蛋白在造血组织中高表达[30],通过注射特异性双链RNA敲低黑虎虾的HHAP基因30 h导致100%的死亡率,血细胞的数量显著减少且发生了严重损伤,表明HHAP基因在血细胞稳态中发挥重要作用[18,31]。本实验中凡纳滨对虾HIF-1α基因的敲低导致血细胞数的大幅降低或是提高HHAP表达的诱因之一,结果表明凡纳滨对虾HIF-1α的表达影响其造血功能。铁蛋白是一种细胞溶质铁储存蛋白,它可以在其内核中隔离多达 4500 个铁离子,以保护细胞免受铁的毒性作用[32]。通过注射铁蛋白可以提高凡纳滨对虾的血细胞数、PO活力和呼吸爆发[33],而且敲低FT也会导致WSSV的感染率增加[34],表明 FT参与了其免疫功能。对照组和干扰组的FT表达虽无显著差异,但低氧胁迫会导致FT表达的显著下降从而影响凡纳滨对虾的免疫功能。
ACP作为溶酶体中的标志酶,在酸性环境下ACP能够通过水解表面带有磷酸酯的异物从而预防感染,还可以通过修饰外来异物的表面识别系统从而增强血细胞对体内外来病原体的识别[35],从而提高吞噬细胞消灭体内异物的速度[36]。AKP参与磷酸基团的代谢,在钙吸收和磷酸钙沉积过程中发挥重要作用,可以加速物质的摄取和转运[37]。本研究结果表明,敲低凡纳滨HIF-1α基因在低氧持续6 h时发现血清中的ACP的活力有明显的下降,而AKP的活力则无显著变化。PO 以酶原的形式存在于大颗粒细胞和小颗粒细胞的颗粒中,在无脊椎动物中有愈合伤口、参与非特异性免疫的功能[38]。本实验中敲低HIF-1α基因在低氧条件下会使PO活力降低,在低氧持续6 h时有显著差异。3种免疫相关酶在低氧条件下对照组整体上有升高的趋势,这与文献[39]研究结果一致。由此可见,低氧胁迫下敲低HIF-1α基因降低了部分免疫相关酶的活力。
低氧胁迫条件下HIF-1α对凡纳滨对虾血淋巴免疫性能的影响
DOI: 10.15886/j.cnki.rdswxb.20220084
 CSTR: 32425.14.j.cnki.rdswxb.20220084
CSTR: 32425.14.j.cnki.rdswxb.20220084
Effects of HIF-1α on the immune response of the hemolymph of Litopenaeus vannamei under hypoxic stress
-
摘要: 为了探究低氧水平下低氧诱导因子-1α(HIF-1α)对凡纳滨对虾(Litopenaeus vannamei)血淋巴免疫性能的影响,本研究通过对凡纳滨对虾体内注射dsRNA,干扰HIF-1α基因的表达,同时对凡纳滨对虾进行低氧胁迫(2.0 mg·L−1),并测定其血细胞数、血蓝蛋白含量、基因表达和酶活等免疫指标。结果表明,RNA干扰组HIF-1α的表达水平较对照组下降了80.1%。干扰组在低氧条件下血细胞数较对照组有显著降低,此外干扰组血蓝蛋白的浓度较对照组有显著升高。低氧条件下干扰组的造血激素基因(AST)、血细胞稳态相关蛋白基因(HHAP)在血细胞中的表达水平较对照组有显著的升高,而铁蛋白基因(FT)在血细胞中的表达水平无显著差异。低氧条件下RNA干扰组血清中ACP、AKP和PO三种酶活力均有不同程度的降低。以上结果表明,凡纳滨对虾的HIF-1α直接或间接地参与了免疫功能的调控。Abstract: In order to investigate the effects of hypoxic-induced-factor-1α (HIF-1α) on the immune response of the hemolymph of Litopenaeus vannamei under hypoxia stress, dsRNA was injected into L. vannamei to interfere with the expression of HIF-1α gene under the hypoxic stress (2.0 mg·L−1) to analyze the regulation effect of hypoxic stress on HIF-1α gene expression level in the hemocyte of L. vannamei. Immune indicators such as hemocyte counts, hemocyanin concentration, gene expression level, enzymatic activity, etc were determined. The results showed that the expression level of HIF-1α in the RNA interference group decreased by 80.1% compared with the control group. The hemocyte counts were significantly lower under hypoxic conditions in the interference group compared with the control group (P < 0.05). Additionally, the concentration of hemocyanin was significantly higher in the interference group than in the control group (P < 0.05). The expression levels of Astakine (AST) and hemocyte homeostasis-associated protein (HHAP) in the hemocytes were significantly higher in the RNA interference group under hypoxic conditions than in the control group, while the expression levels of Ferritin (FT) in the hemocytes were not significantly different between both the interference and control groups. The enzymatic activities of ACP, AKP and PO in the serum in the RNA interference group under hypoxic conditions were reduced to different degrees. By interfering with the expression of HIF-1α gene, it was found that HIF-1α gene played an important role in hemocyte counts, hemocyanin concentration, expression of immune-related genes and the activity of immune-related enzymes in L. vannamen.
-
Key words:
- Litopenaeus vannamei /
- HIF-1α /
- gene /
- enzyme activity /
- hemolymph
-
表 1 实验用引物
引物名称 引物序列(5′-3′) 用途 GenBank HIF-1α-F GGAGAGCGAGATCTTCACG HIF-1α cDNA扩增 FJ807918 HIF-1α-R CTCGATGTTGGACGGGTG HIF-1α cDNA扩增 T7HIF-1α-F GATCACTAATACGACTCACTATAGG
GGGAGAGCGAGATCTTCACG含有T7 Promoter序列的HIF-1α cDNA扩增 FJ807918 T7HIF-1α-R GATCACTAATACGACTCACTATA
GGGCTCGATGTTGGACGGGTG含有T7 Promoter序列的HIF-1α cDNA扩增 rtHIF-1α-F GACTTGACCCACTTGGCTCC qRT-PCR目的基因扩增 FJ807918 rtHIF-1α-R CCTGCTGCTAAGACGCTTC qRT-PCR目的基因扩增 AST-F GCCGTGTCCAGTGCAGTCAGAA qRT-PCR目的基因扩增 HM594944 AST-R TTCATGATGCGACACCAGTCCC qRT-PCR目的基因扩增 HHAP-F CGTACCAAGGCAACAGAAGAACCTG qRT-PCR目的基因扩增 KP676891 HHAP-R CGGATCTCCTTCTTTGGCTCCTC qRT-PCR目的基因扩增 AY955373 FT-F CCACAGAATTTGGATGGAAG qRT-PCR目的基因扩增 FT-R ATGATTACCAAGCTGAAGCG qRT-PCR目的基因扩增 L8-F GTCTACTGCGGCAAGAAGGC qRT-PCR内参基因扩增 DQ316258 L8-R CCTGAAGGGAGCTTTACACG qRT-PCR内参基因扩增 -
[1] VAQUER-SUNYER R, DUARTE C M. Thresholds of hypoxia for marine biodiversity [J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(40): 15452 − 15457. doi: 10.1073/pnas.0803833105 [2] WU R S S. Hypoxia: from molecular responses to ecosystem responses [J]. Marine Pollution Bulletin, 2002, 45(1/2/3/4/5/6/7/8/9/10/11/12): 35 − 45. doi: 10.1016/S0025-326X(02)00061-9 [3] 王兴强, 马甡, 董双林. 凡纳滨对虾生物学及养殖生态学研究进展[J]. 海洋湖沼通报, 2004(4): 94 − 100. doi: 10.3969/j.issn.1003-6482.2004.04.016 [4] 孙盛明, 祝孟茹, 潘方艳,等. 低氧对甲壳动物的影响及其分子调控研究进展[J]. 水产学报, 2020, 44(4): 690 − 704. [5] WENGER R H, STIEHL D P, CAMENISCH G. Integration of oxygen signaling at the consensus HRE [J]. Science,s STKE:signal transduction knowledge environment, 2005, 2005(306): e3062005re12. [6] CAMACHO-JIMÉNEZ L, LEYVA-CARRILLO L, PEREGRINO-URIARTE A B, et al. Regulation of glyceraldehyde-3-phosphate dehydrogenase by hypoxia inducible factor 1 in the white shrimp Litopenaeus vannamei during hypoxia and reoxygenation [J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 2019, 235: 56 − 65. [7] COTA-RUIZ K, LEYVA-CARRILLO L, PEREGRINO-URIARTE A B, et al. Role of HIF-1 on phosphofructokinase and fructose 1, 6-bisphosphatase expression during hypoxia in the white shrimp Litopenaeus vannamei [J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 2016, 198: 1 − 7. [8] DUARTE-GUTIÉRREZ J, PEREGRINO-URIARTE A B, GÓMEZ-JIMÉNEZ S, et al. HIF-1 is involved in the regulation of expression of metallothionein and apoptosis incidence in different oxygen conditions in the white shrimp Litopenaeus vannamei [J]. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 2021, 262: 111072. [9] GODOY-LUGO J A, MIRANDA-CRUZ M M, ALFREDO ROSAS-RODRÍGUEZ J A, et al. Hypoxia inducible factor-1 regulates WSSV-induced glycolytic genes in the white shrimp Litopenaeus vannamei [J]. Fish & Shellfish Immunology, 2019, 92: 165 − 171. [10] MIRANDA-CRUZ M M, POOM-LLAMAS J J, GODOY-LUGO J A, et al. Silencing of HIF-1 in WSSV-infected white shrimp: Effect on viral load and antioxidant enzymes [J]. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 2018, 213: 19 − 26. [11] SONANEZ-ORGANIS J G, PEREGRINO-URIARTE A B, GÓMEZ-JIMÉNEZ S, et al. Molecular characterization of hypoxia inducible factor-1 (HIF-1) from the white shrimp Litopenaeus vannamei and tissue-specific expression under hypoxia [J]. Comparative Biochemistry and Physiology. Part C:Toxicology & Pharmacology, 2009, 150(3): 395 − 405. [12] SOÑANEZ-ORGANIS J G, PEREGRINO-URIARTE A B, SOTELO-MUNDO R R, et al. Hexokinase from the white shrimp Litopenaeus vannamei: cDNA sequence, structural protein model and regulation via HIF-1 in response to hypoxia [J]. Comparative Biochemistry and Physiology. Part B:Biochemistry & Molecular Biology, 2011, 158(3): 242 − 249. [13] SOÑANEZ-ORGANIS J G, RACOTTA I S, YEPIZ-PLASCENCIA G. Silencing of the hypoxia inducible factor 1-HIF-1-obliterates the effects of hypoxia on glucose and lactate concentrations in a tissue-specific manner in the shrimp Litopenaeus vannamei [J]. Journal of Experimental Marine Biology and Ecology, 2010, 393(1/2): 51 − 58. doi: 10.1016/j.jembe.2010.06.031 [14] CERENIUS L, JIRAVANICHPAISAL P, LIU H-P, et al. Crustacean Immunity[M]. // Soderhaill K. Invertebrate Immunity. Springer US. 2010: 239-259. [15] 陈钦胜. 拟穴青蟹在盐度胁迫下血细胞免疫调控机制的研究[D]. 海口: 海南大学, 2020. [16] 杨留冰. 凡纳滨对虾(Litopenaeus vannamei)血蓝蛋白免疫作用的初步研究[D]. 青岛: 中国海洋大学, 2013. [17] SÖDERHÄLL I. Crustacean hematopoiesis [J]. Developmental and Comparative Immunology, 2016, 58: 129 − 141. doi: 10.1016/j.dci.2015.12.009 [18] CHAROENSAPSRI W, SANGSURIYA P, LERTWIMOL T, et al. Laminin receptor protein is implicated in hemocyte homeostasis for the whiteleg shrimp Penaeus (Litopenaeus) vannamei [J]. Developmental and Comparative Immunology, 2015, 51(1): 39 − 47. doi: 10.1016/j.dci.2015.02.012 [19] 王冰心, 叶均安. 虾类血清中免疫相关酶的研究进展[J]. 中国饲料, 2009(3): 27 − 28. doi: 10.3969/j.issn.1004-3314.2009.03.010 [20] 桂建芳, 朱作言. 水产动物重要经济性状的分子基础及其遗传改良[J]. 科学通报, 2012, 57(19): 1719 − 1729. [21] LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT Method [J]. Methods (San Diego, Calif. ), 2001, 25(4): 402 − 408. doi: 10.1006/meth.2001.1262 [22] KULKARNI A, KRISHNAN S, ANAND D, et al. Immune responses and immunoprotection in crustaceans with special reference to shrimp [J]. Reviews in Aquaculture, 2021, 13(1): 431 − 459. doi: 10.1111/raq.12482 [23] 陈琪, 康翠洁. 虾类血细胞的分类与功能研究进展[J]. 生物工程学报, 2021, 37(1): 53 − 66. doi: 10.13345/j.cjb.200269 [24] WEI L, LI Y, QIU L, et al. Comparative studies of hemolymph physiology response and HIF-1 expression in different strains of Litopenaeus vannamei under acute hypoxia [J]. Chemosphere, 2016, 153: 198 − 204. doi: 10.1016/j.chemosphere.2016.03.064 [25] 黄天鸽, 金彩霞, 潘鲁青. 低氧胁迫对凡纳滨对虾血蓝蛋白、自由氨基酸含量和体内Cu2+转运的影响[J]. 海洋湖沼通报, 2015(2): 16 − 22. doi: 10.13984/j.cnki.cn37-1141.2015.02.003 [26] COATES C J, NAIRN J. Diverse immune functions of hemocyanins [J]. Developmental and Comparative Immunology, 2014, 45(1): 43 − 55. doi: 10.1016/j.dci.2014.01.021 [27] 李玉虎. 凡纳滨对虾生长发育规律及对低氧胁迫响应的研究[D]. 海口: 海南大学, 2015. [28] 梁高峰. 凡纳滨对虾造血激素(astakine)的表达及其功能研究[D]. 青岛: 中国海洋大学, 2011. [29] WANG J, XU Z, HE J. The role of HIF-1 α in the energy metabolism and immune responses of hypoxic Scylla paramamosain[J]. Aquaculture Reports, 2021, 20: 100740. [30] 杨丰, 李钫. 甲壳动物造血机制研究进展[J]. 应用海洋学学报, 2019, 38(4): 484 − 489. doi: 10.3969/J.ISSN.2095-4972.2019.04.004 [31] PRAPAVORARAT A, VATANAVICHARN T, SÖDERHÄLL K, et al. A novel viral responsive protein is involved in Hemocyte homeostasis in the Black Tiger Shrimp, Penaeus monodon [J]. The Journal of Biological Chemistry, 2010, 285(28): 21467 − 21477. doi: 10.1074/jbc.M110.130526 [32] HSIEH S L, CHIU Y C, KUO C M. Molecular cloning and tissue distribution of ferritin in Pacific white shrimp (Litopenaeus vannamei) [J]. Fish & Shellfish Immunology, 2006, 21(3): 279 − 283. [33] RUAN Y H, KUO C M, LO C F, et al. Ferritin administration effectively enhances immunity, physiological responses, and survival of Pacific white shrimp (Litopenaeus vannamei) challenged with white spot syndrome virus [J]. Fish & Shellfish Immunology, 2010, 28(4): 542 − 548. [34] YE T, WU X, WU W, et al. Ferritin protects shrimp Litopenaeus vannamei from WSSV infection by inhibiting virus replication [J]. Fish & Shellfish Immunology, 2015, 42(1): 138 − 143. [35] 刘志鸿, 牟海津, 王清印. 软体动物免疫相关酶研究进展[J]. 海洋水产研究, 2003,24(3): 86 − 90. [36] 田耕晨, 曾文涛. 脂多糖对三角帆蚌酸性磷酸酶和碱性磷酸酶活力的影响[J]. 浙江农业科学, 2018, 59(2): 269 − 270. doi: 10.16178/j.issn.0528-9017.20180232 [37] 管晓娟. 甲壳动物体液免疫相关酶及免疫因子研究概况[J]. 生命科学仪器, 2009, 7(6): 3 − 7. doi: 10.3969/j.issn.1671-7929.2009.06.001 [38] 樊廷俊, 荆昭, 樊现远. 海洋无脊椎动物酚氧化酶的研究进展[J]. 中国海洋大学学报(自然科学版), 2012, 42(Z1): 93 − 98. doi: 10.16441/j.cnki.hdxb.2012.z1.015 [39] 宋芹芹, 李玉虎, 周海龙. 凡纳滨对虾应答低氧-复氧胁迫免疫相关酶活力的时空变化[J]. 热带生物学报, 2015, 6(4): 353 − 358. doi: 10.15886/j.cnki.rdswxb.2015.04.001 -




 下载:
下载: