-
番茄(Solanum lycopersicum)起源于南美洲的安第斯地区,被认为是最主要的蔬菜作物,为人类的膳食营养作出了巨大贡献[1-2]。食用番茄可以预防缓解贫血,治疗消化性溃疡以及高血压[3-4]。此外,番茄作为一种重要的双子叶模式植物,代表了包括马铃薯、胡椒和茄子在内所有茄科作物的生物研究和遗传改良的模型。番茄由醋栗番茄(Solanum pimpinellifolium, PIM)驯化为樱桃番茄(Slycopersicum var. cerasiforme, CER),进一步经过人工选择改良为现代大果番茄(Solanum lycopersicum, BIG)[5-6]。番茄及其野生近缘植物起源于南美洲的安第斯地区,16世纪后在全世界各地进行传播,随后的迁移和持续的育种选择减少了番茄的遗传多样性。
黄酮类化合物作为一类重要的植物代谢物,是以2-苯基色原酮为母核具有清除自由基、抗氧化、抗衰老、促进免疫调节、抗肿瘤及预防慢性疾病的作用。黄酮类化合物广泛分布于芹菜、甜椒、绿叶蔬菜、柑橘和苹果等水果蔬菜中[7-8]。木犀草素作为一种重要的黄酮类化合物,具有抗炎、抗肥胖和抗非酒精性脂肪肝等多种生物学功能[9-10]。研究发现,木犀草素中的酚羟基可以提供氢原子,与细胞产生的过量自由基发生反应形成醌类游离基中间体,并在酚环共轭作用下趋于稳定,阻断氧化链反应的传递,防止过氧化反应,避免ROS对细胞的损害[11-13]。Choi等[14]研究了木犀草素对2,2-二苯基-1-吡啶并肼基(DPPH)、一氧化氮(NO)、过氧亚硝酸盐(ONOO−)及活性氧(ROS)的清除能力,结果表明,木犀草素对ROS的清除能力优于异荭草苷、荭草苷等类似物。
糖基转移酶(glycosyltransferase,GT)在植物中广泛存在,可催化多种代谢物的糖基化反应生成寡糖、多糖和糖复合物。这些结构多样性的糖分子参与和介导从结构、存储到信号传递等生命活动。糖基转移酶依据氨基酸序列的相似程度、反应机制、保守序列以及糖苷键的立体化学性等总共被划分为 114 个家族[15]。人们已在许多植物中发现大量的糖基转移酶,如胡萝卜(Daucus carota var. sativa)的花青素3-O-半乳糖基转移酶1(Daucus carotacyanidin 3-O-galactosyltransferase 1,DcUCGalT1)、芹菜(Apium graveolens)的花青素3-O-半乳糖基转移酶1(Apium graveolens cyanidin 3-O-galactosyltransferase 1,AgUCGalT1)和中国金莲花(Trollius chinensis)的黄酮C-糖基转移酶(Trollius chinensis 8-C-glucosyltransferase,TcCGT1)等,这些物质经过糖基化修饰后可能表现出与骨架不同的生理活性[16-18]。然而到目前为止,番茄中修饰木犀草素的糖基转移酶鲜有报道,因此探究木犀草素糖苷在番茄中的自然变异与代谢机制,对后续解析木犀草素途径和品种选育具有重要意义。
-
426份番茄材料来自海南大学热带作物学院,低温液氮取果实样品并冻于−80 ℃低温冰箱。果实样品经冷冻干燥后,采用 BM500 球磨仪研磨成粉末(频率30 Hz,持续时间 1.5 min)。称取 100 mg 研磨好的干粉样品,加入1.2 mL 含有 0.1 mg·L−1 利多卡因内标的甲醇提取液,涡旋30 s,低温静置10 min,重复上述操作3次后,放入4 ℃冰箱继续萃取代谢产物12 h,10 300 r·min−1 4 ℃ 离心10 min,取上清液并用 0.22 μm的滤膜过滤收集于进样瓶中。
使用Thermo Scientific™ QExactive Plus-Orbitrap高分辨率质谱分析样品,通过比较保留时间(RT)、前体离子(Q1)和产物碎片信息(MS/MS)来鉴定代谢物。采用UPLC-ESI-MS/MS-QTRAP(AB Sciex QTRAP 6500+)分时段多反应监测模式(sMRM)对代谢物进行相对定量[19]。
-
共使用426份材料的1,476,413个SNPs(single nucleotide polymorphism,单核苷酸多态性)进行全基因组关联分析(MAF>5%,缺失率<10%)。通过EMMAX软件计算基因型与表型之间的关系[20],采用Admixture软件将群体结构矩阵作为随机效应,emmax-kin计算亲属关系(K)矩阵,命令为emmax-kin -v -h -s -d 10。进一步利用 GEC 软件计算独立 SNP 的有效数目,使用Bonferrini方法对显著性阈值进行校正,计算出的全基因组显著性阈值为P=4.19×10−7(0.05 / N, N为独立SNP的有效数目)。
-
利用笔者所在实验室测序的番茄二代DNA数据[6],通过番茄的遗传地理背景将番茄分类为野生种(PIM)、地方种(CER)以及栽培种(BIG)3个群体,并利用VCFtools软件分别对3个群体的全基因组核苷酸多态性进行计算。
-
DNA利用CTAB法提取不同番茄亚群的基因组DNA,提取方法如为:1~2 cm长剪碎的番茄苗叶心幼嫩组织打磨成粉末;加入CTAB抽提液裂解;加入氯仿去除蛋白质,离心后吸上清在无水乙醇中沉淀,75%乙醇洗涤沉淀;自然风干, ddH2O溶解DNA。
RNA提取使用TRIZOL试剂(购自Invitrogen公司),从已经测序的番茄品种Micro Tom叶片中提取总RNA(提取方法根据上述TRIZOL试剂说明书),利用反转录试剂盒Supermix(购自北京全式金公司)将RNA反转录合成cDNA,反应条件:42 ℃ 30 min,80 ℃ 5 s,保存于−40 ℃。
-
以cDNA为模板,设计带有Gateway接头的PCR特异引物,F:5′-aaaaagcaggcttaATGAAGCATCATCATTT-3′;R: 5′-agaaagctgggtaTCACAAACTCTCTAA AA-3′使用超保真KOD酶(大连宝日医生物工程有限公司)扩增基因的全长CDS片段。PCR的反应条件:94 ℃预变性5 min;98 ℃ 10 s,60 ℃ 30 s,68 ℃ 2 min,32个循环;68 ℃延伸5 min。将扩增得到的PCR产物通过Gateway克隆技术(Thermo fisher)的BP反应连入pDonr207入门载体,转化大肠杆菌DH5α感受态细胞。菌落PCR验证以获得阳性克隆,测序得到含有候选基因的无突变的正确克隆(海口擎科生物公司),分别获得pDonr207-SlUGT75C1-like的入门载体,再通过Gateway克隆技术的LR反应连入蛋白表达载体pGEX-6P-1载体。
-
对SlUGT75C1-like进行原核蛋白表达,首先将pGEX-6P-1-SlUGT75C1-like质粒分别转化至大肠杆菌感受态细胞BL21(上海唯地生物)中,通过IPTG诱导使大肠杆菌分别表达出候选基因所编码的蛋白。进一步收集菌体,加入Lysis buffer缓冲液悬浮菌体,置于冰上。使用高压细胞破碎仪(苏州安拓思纳米技术有限公司)对悬浮菌体进行细胞破碎。以SDS-PAGE电泳检测总蛋白和上清中是否有目标蛋白。剩余蛋白存于−80 ℃备用。
蛋白纯化:用Lysis buffer缓冲液充分洗涤含有谷胱甘肽琼脂糖4B琼脂糖(美国GE Healthcare公司)的纯化柱子,加入含有靶蛋白的上清液,收集流出液,再次过柱子。用Lysis buffer缓冲液(5倍柱体积)充分洗涤柱子。待洗涤充分后向纯化柱中加入还原型谷胱甘肽洗脱液(上海生工股份有限公司),每次加1 mL,同时收集1 mL穿流液重复5~6次即可。通过SDS-PAGE确认所收集的穿流液中是否含有目标蛋白。依次获得纯化良好的SlUGT75C1-like蛋白融合GST标签蛋白的重组蛋白,将上述蛋白分装保存于−80 ℃冰箱待用。
-
对SlUGT75C1-like进行糖基转移酶体外酶活反应,体系为10 μL,含有1 mmol·L−1的木犀草素底物1 μL、1.5 mmol·L−1 UGP-glucose 1 μL、500 ng纯化蛋白、1 mol·L−1 Tris-HCl缓冲液(pH7.4)1μL和50 mmol·L−1的MgCl2 1 μL,ddH2O补齐至10 μL,在37 ℃孵育30 min后,加入50 μL预冷的甲醇终止反应。将反应混合物通过4 ℃、12 000 r·min−1离心10 min,取上清50 μL用于LC-MS检测分析。
-
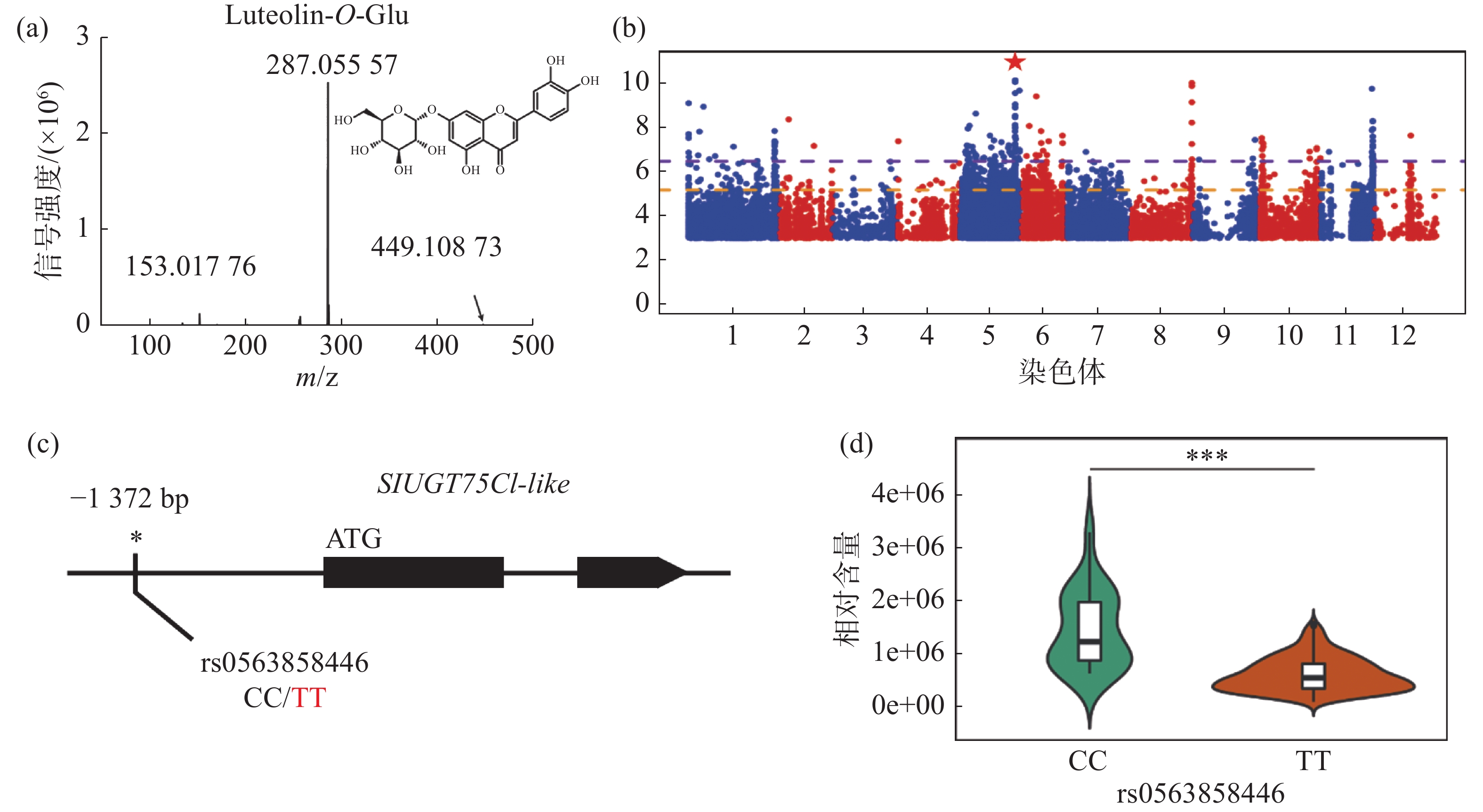
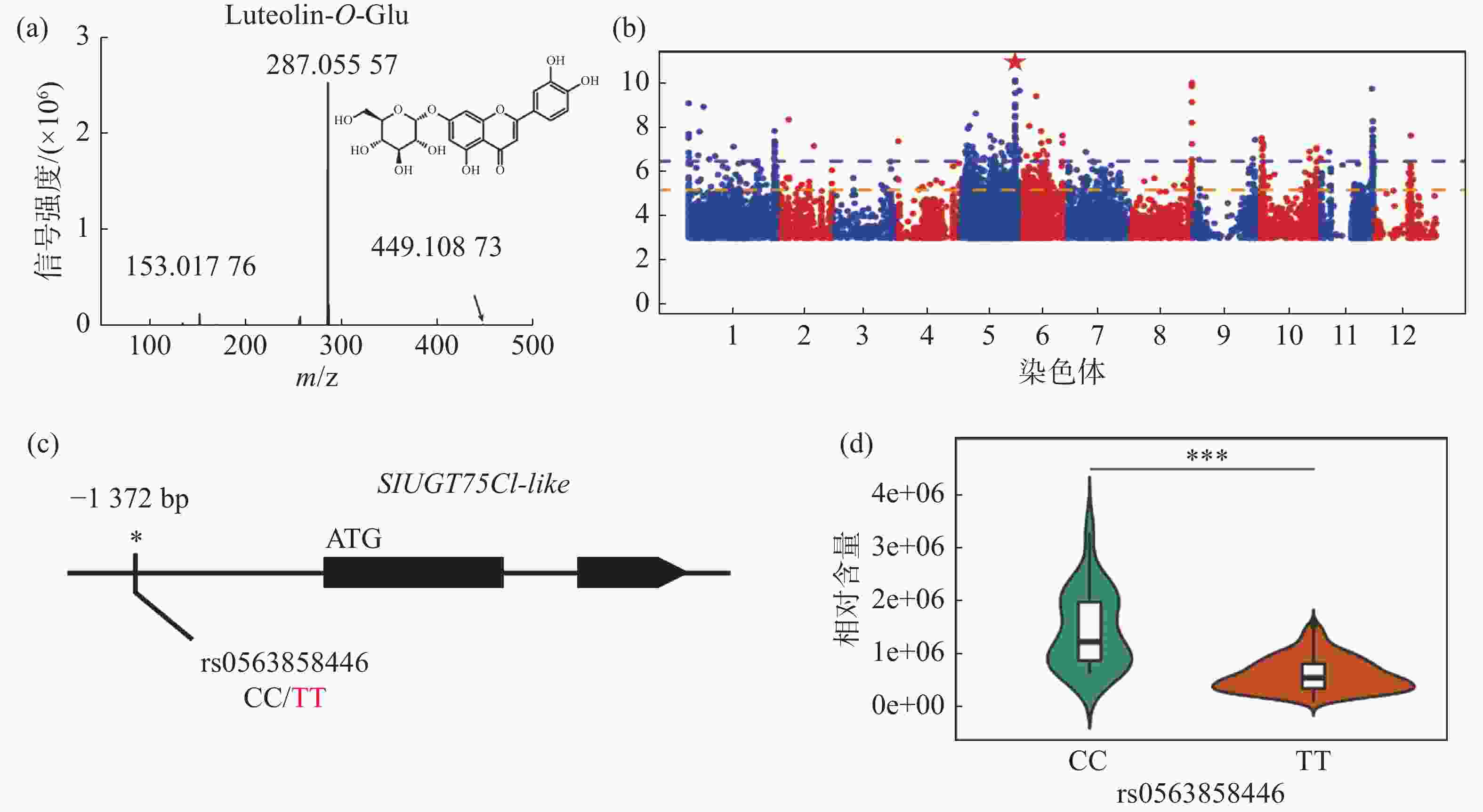
对生长条件时期相同的426份番茄果实进行代谢组检测,整合群体重测序数据进行全基因组关联分析,结果发现,番茄中木犀草素-7-O-葡萄糖苷的相对含量与5号染色体的1个基因型存在显著关联,结果表明番茄群体中木犀草素-7-O-葡萄糖苷的自然变异主要受5号染色体的位点调控(图1-a,图1-b)。对不同品种中的基因型进行比较分析,发现位于SlUGT75C1-like(Solyc05g053820)的上游存在一个SNP位点拥有最显著的关联性(图1-c),进一步分析结果表明SlUGT75C1-like中的突变(rs0563858446)与番茄中木犀草素-7-O-葡萄糖苷含量密切关联(图1-d),该基因型为CC型时,番茄中该代谢物含量极显著(P < 0.001)高于该基因型为TT型时的相对含量,因此这个SNP可以代表这个基因型的功能多态性,是SlUGT75C1-like的自然遗传变异造成番茄群体中木犀草素-7-O-葡萄糖苷含量差异的原因。
-
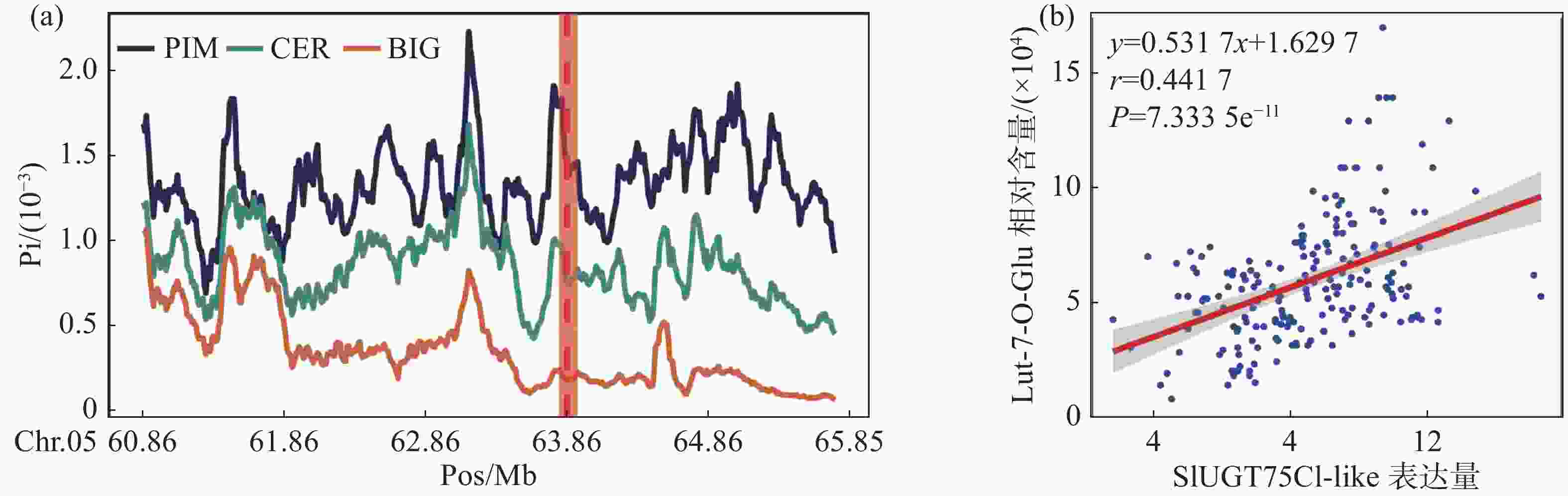
利用笔者所在实验室测序的番茄二代DNA数据[6],通过番茄的遗传地理背景将番茄分类为野生种(PIM)、地方种(CER)以及栽培种(BIG)3个群体,分别对3个群体的全基因组核苷酸多态性进行计算。分析结果表明,SlUGT75C1-like所处的区域在PIM、CER和BIG中存在明显的差异(图2-a)。这意味着该基因在基因组所在位置中PIM亚群番茄变异更为复杂而地方种和栽培种可能受到人类选择降低了SNP的多样性,因此我们认为该基因可能为人类育种选择的关键位点。
为了进一步确认该基因的确具有修饰木犀草素的功能,利用426份番茄代谢组和转录组数据进行相关性分析。结果表明,SlUGT75C1-like的转录水平与木犀草素-7-O-葡萄糖苷的相对含量存在极显著的正相关(图2-b),表明SlUGT75C1-like基因的高转录水平正向推动木犀草素糖苷的合成。
-
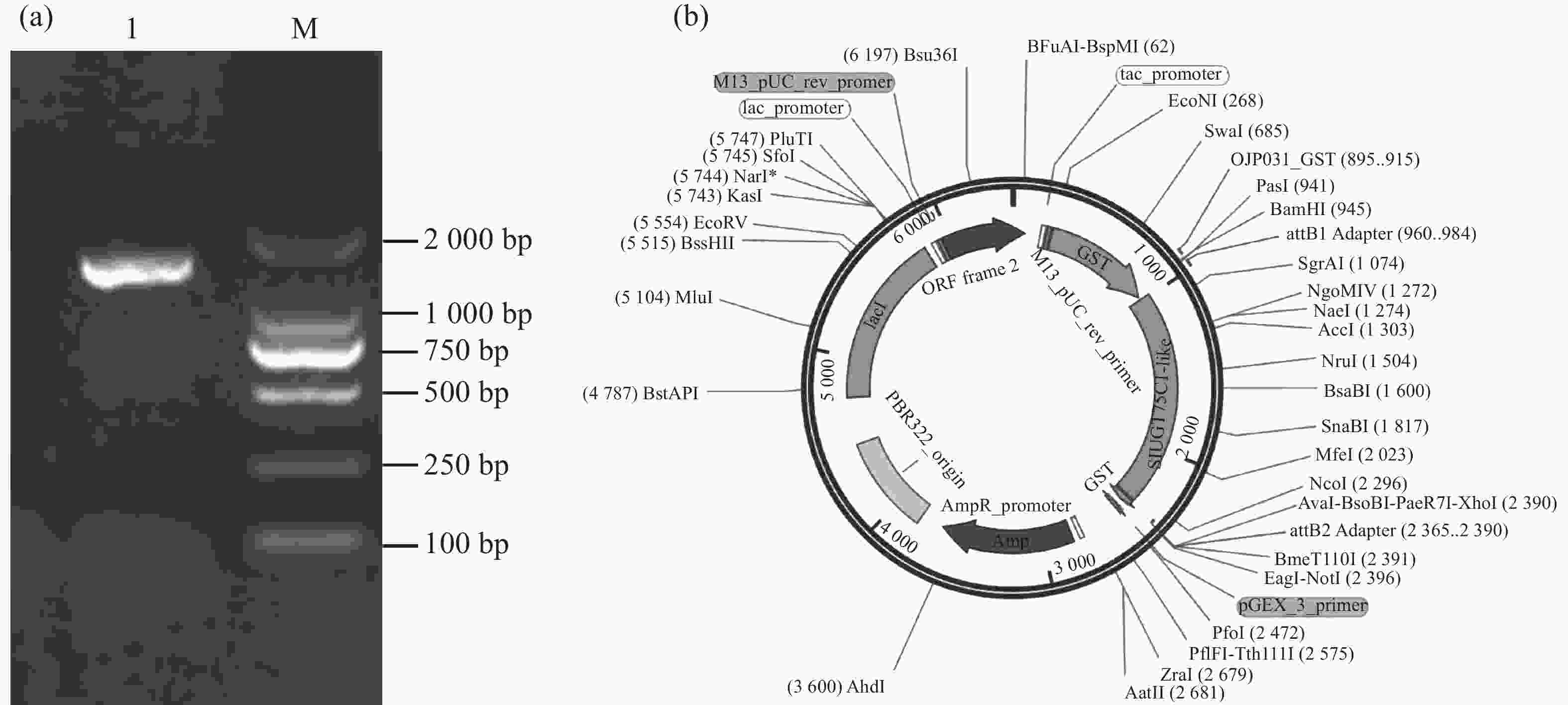
以番茄cDNA为模板,以特异性引物扩增SlUGT75C1-like的编码序列,琼脂糖凝胶电泳结果显示该片段约为1 700 bp(图3-a)。通过Gateway反应将目的片段构建至pGEX6P-1载体(图3-b),送至擎科生物公司进行测序,比对正确即pGEX6P-1-SlUGT75C1-like载体构建成功。
-
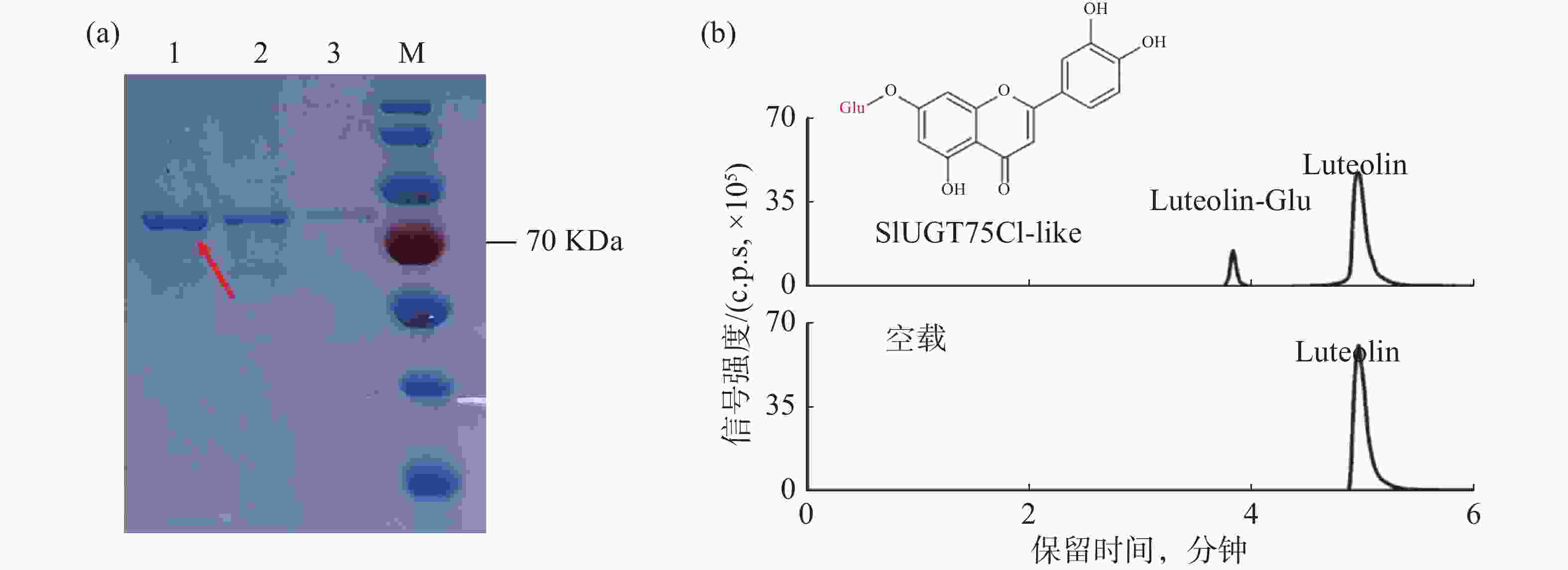
为了验证候选基因SlUGT75C1-like的功能,将pGEX6P-1-SlUGT75C1-like转入BL21大肠感受态中表达蛋白。经过诱导表达和GST亲和层析获得可用于体外酶活测定实验的SlUGT75C1-like-GST 融合蛋白(图4-a)。在蛋白体外酶活反应体系中,木犀草素和葡萄糖基供体用作反应底物,纯化后的蛋白或空载蛋白进行孵育反应,产物上清用于 LC-MS分析。从图4-b可知,加入蛋白的反应产物出现两个明显的色谱峰,并且其保留时间和碎片模式与推测的糖基化产物一致;加入空载的反应产物并出现新的色谱峰,说明SlUGT75C1-like蛋白能够催化木犀草素发生糖基化反应。
-
植物代谢组的多样性源于基因组进化、遗传多样性、发育阶段和环境刺激, mGWAS是鉴定代谢多样性基础上常见遗传变异的有力工具[21]。Klein等[22]成功地进行了第1次GWAS,并确定了补体因子H基因的1个变体与年龄相关性黄斑变性密切相关。在过去10年中,将基因组、转录组及代谢组学联用,从原因和结果两个层次分析生物体的内在变化,锁定与代谢物变化相关的重要代谢通路,构建核心调控网络,从而全面探究生物体的生长发育,从整体水平解释生物学问题,是当今多组学的研究热点。例如,Shang等人利用 115 个黄瓜品种的苦味性状进行遗传图谱分析,定位到了控制黄瓜苦味素生物合成的第一步的催化酶 Bi 基因,之后利用多种研究手段共揭示了负责黄瓜中苦味素生物合成的9 个基因,其中包含 Bi 在内的 6 个基因在 6 号染色上聚集排列,形成了苦味素生物合成的代谢基因簇[23];Chen 等[24]对500多份水稻品种中的八百多种代谢物进行了 mGWAS分析,获得 600多个能够控制代谢物自然变异的显著遗传位点,确定了36个候选基因并进行了验证,它们能够调节代谢产物的水平并在生理和营养方面也具有潜在的重要性。在本研究中,对426份番茄果实进行代谢组检测,整合群体重测序数据进行全基因组关联分析,结果发现,番茄中木犀草素-7-O-葡萄糖苷的相对含量与5号染色体的一个基因型存在显著关联,表明番茄群体中木犀草素-7-O-葡萄糖苷的自然变异主要受5号染色体的位点调控。对不同品种中的基因型进行比较分析,发现位于候选基因SlUGT75C1-like的下游存在一个SNP单核苷酸多态性位点拥有最显著的关联性。
在自然界中类黄酮一般由糖基转移酶催化成糖苷的形式存在,黄酮类化合物的糖基化赋予其结构的复杂性、分子的溶解性和稳定性及亚细胞的可运输性[25]。例如,花色素苷的糖基化已被证明在一系列花卉中对这些色素的溶解性和稳定性起着关键作用[26];Zhao等[27-28]的研究表明,多药物及毒素外排转运蛋白(MATE)对表儿茶素3′-糖苷和花青素糖苷具有较高的转运能力。此外,柑橘类物种的苦味是由区域特异性黄烷酮糖基化引起的[29],而黄酮醇3-O-糖苷已被证明在红茶中赋予涩味[30]。Liu等[31]发现,木犀草素在体外实验中能够表现很好的抗炎作用,而木犀草素葡萄糖苷未能发挥很好的体外抗炎功能,因此,含有较高木犀草素含量的植物能够更好地治疗炎症相关疾病。在本研究验证了SlUGT75C1-like在体外能够催化木犀草素糖基化生成木犀草素-O-葡萄糖苷,但在番茄体内的2种物质的含量变化及其导致的生理表型仍有待研究。
番茄糖基转移酶基因SlUGT75C1-like的功能研究及自然变异分析
DOI: 10.15886/j.cnki.rdswxb.20230062
 CSTR: 32425.14.j.cnki.rdswxb.20230062
CSTR: 32425.14.j.cnki.rdswxb.20230062
Functional study and natural variation analysis of glycosyltransferase gene SlUGT75C1-like in tomato
-
摘要: 为了解析番茄(Solanum lycopersicum)群体中与木犀草素葡萄糖苷含量相关的自然变异,以426份番茄为材料,进行了基于代谢组学的全基因组关联分析(metabolome Genome-Wide Association Study,mGWAS),发现番茄SlUGT75C1-like基因下游的存在一个与番茄木犀草素-7-O-葡萄糖苷含量紧密关联的突变位点。进一步在3个亚群的番茄群体中,进行全基因组核苷酸多态性计算,发现SlUGT75C1-like所处的区域在不同亚群中存在明显的差异;进一步分析代谢组和转录组数据相关性发现SlUGT75C1-like的转录水平与木犀草素-7-O-葡萄糖苷的相对含量存在极显著的正相关关系,最后,通过蛋白原核表达获得SlUGT75C1-like融合蛋白并利用体外酶活试验证明该基因催化木犀草素的糖基化反应。研究结果说明,番茄SlUGT75C1-like基因的自然变异造成了木犀草素-7-O-葡萄糖苷在番茄群体中的含量差异,mGWAS在识别新的植物代谢基因簇方面具有较大的优势和巨大潜力,在为植物精细育种提供分子基础方面具有重要的应用价值。Abstract: In order to analyze the natural variation related to luteolin glucoside content in tomato population, 426 tomato samples were used as materials to performed metabolome genome-wide association study (mGWAS) analysis, and found that there was a mutation site downstream of tomato SlUGT75C1-like gene which was closely related to the content of luteolin-7-O-glucoside. Furthermore, the nucleotide polymorphism of the whole genome was calculated in three subgroups of tomato population, and it was found that the region where SlUGT75C1-like was located was obviously different among different subgroups. Further analysis of the correlation between metabolomics and transcriptome data showed that the transcription level of SlUGT75C1-like was positively correlated with the relative content of luteolin-7-O-glucoside. Finally, the fusion protein of SlUGT75C1-like was obtained by prokaryotic expression of the protein, and the enzyme activity test in vitro showed that SlUGT75C1-like catalyzed the glycosylation of luteolin. The results indicated that the natural variation of SlUGT75C1-like gene causes the content difference of luteolin-7-O-glucoside in tomato population. mGWAS has a large advantage and great potential in identifying new plant metabolic gene clusters, and is important in providing the molecular basis for fine plant breeding.
-
Key words:
- tomato /
- genome-wide association study /
- luteolin /
- transcriptome /
- metabolomics
-
-
[1] 王守创. 番茄育种过程中代谢组的变化及遗传基础研究[D]. 武汉: 华中农业大学. 2018. [2] ZHU Y, SIMS C A, KLEE H J, et al. Sensory and flavor characteristics of tomato juice from garden gem and roma tomatoes with comparison to commercial tomato juice[J]. Journal of Food Science, 2018, 83(1): 153 − 161. doi: 10.1111/1750-3841.13980 [3] TOOR R K, SAVAGE G P. Changes in major antioxidant components of tomatoes during post-harvest storage[J]. Food Chemistry, 2006, 99(4): 724 − 727. doi: 10.1016/j.foodchem.2005.08.049 [4] SORREQUIETA A, FERRARO G. , BOGGIO S B, et al. Free amino acid production during tomato fruit ripening: a focus on L-glutamate[J]. Amino Acids, 2010, 38(5): 1523 − 1532. doi: 10.1007/s00726-009-0373-1 [5] LI Y, CHEN Y, ZHOU L, et al. MicroTom metabolic network: rewiring tomato metabolic regulatory network throughout the growth cycle[J]. Molecular Plant, 2020, 13(8): 1203 − 1218. doi: 10.1016/j.molp.2020.06.005 [6] ZHU G, WANG S, HUANG Z, et al. Rewiring of the fruit metabolome in tomato breeding[J]. Cell, 2018, 172(1/2): 249 − 261. doi: 10.1016/j.cell.2017.12.019 [7] CROZIER A, DEL RIO D, CLIFFORD M N. Bioavailability of dietary flavonoids and phenolic compounds[J]. Molecular Aspects of Medicine, 2010, 31(6): 446 − 467. doi: 10.1016/j.mam.2010.09.007 [8] 陈璇. 黄酮类化合物(槲皮素、木犀草素)与类胡萝卜素(番茄红素、叶黄素)协同抗氧化作用及分子机制[D].南昌: 南昌大学,2022. [9] 詹鑫, 徐帆, 祝钧, 等. 木犀草素的生理作用及制剂研究进展[J]. 日用化学工业(中英文), 2023, 53(4): 437 − 444. [10] CALABRESE E J, AGATHOKLEOUS E, KAPOOR R, et al. Luteolin and hormesis[J]. Mechanisms of Ageing and Development, 2021, 199: 111559. doi: 10.1016/j.mad.2021.111559 [11] 王璐. 花生果实荚壳中木犀草素的提取纯化、纳米粒制备及其稳定Pickering乳液的设计与研究[D]. 哈尔滨: 东北林业大学, 2020. [12] XU T, WANG C, JIANG S, et al. Glycosylation of luteolin in hydrophilic organic solvents and structure–antioxidant relationships of luteolin glycosides[J]. RSC Advances, 2022, 12(28): 18232 − 18237. doi: 10.1039/D2RA03300C [13] APAK R, ÖZYÜREK M, GÜÇLÜ K, et al. Antioxidant activity/capacity measurement. 2. hydrogen atom transfer (HAT)-based, mixed-mode (electron transfer (ET)/HAT), and lipid peroxidation assays[J]. Journal of Agricultural and Food Chemistry, 2016, 64(5): 1028 − 1045. doi: 10.1021/acs.jafc.5b04743 [14] CHOI M Y, SONG H S, HUR H S, et al. Whitening activity of luteolin related to the inhibition of cAMP pathway in α-MSH-stimulated B16 melanoma cells[J]. Archives of Pharmacal Research, 2008, 31(9): 1166 − 1171. doi: 10.1007/s12272-001-1284-4 [15] RAHIMI S, KIM J, MIJAKOVIC I, et al. Triterpenoid-biosynthetic UDP-glycosyltransferases from plants[J]. Biotechnology Advances, 2019, 37(7): 107394. doi: 10.1016/j.biotechadv.2019.04.016 [16] FENG K, XU Z S, LIU J X, et al. Isolation, purification, and characterization of AgUCGalT1, a galactosyltransferase involved in anthocyanin galactosylation in purple celery (Apium graveolens L.)[J]. Planta, 2018, 247(6): 1363 − 1375. doi: 10.1007/s00425-018-2870-5 [17] XU Z, MA J, WANG F, et al. Identification and characterization of DcUCGalT1, a galactosyltransferase responsible for anthocyanin galactosylation in purple carrot (Daucus carota L.) taproots[J]. Scientific Reports, 2016, 6(1): 27356. doi: 10.1038/srep27356 [18] HE J B, ZHAO P, HU Z, et al. Molecular and structural characterization of a promiscuous C-glycosyltransferase from trollius chinensis[J]. Angewandte Chemie International Edition, 2019, 58(33): 11513 − 11520. doi: 10.1002/anie.201905505 [19] KANG H M, SUL J H, SERVICE S K, et al. Variance component model to account for sample structure in genome-wide association studies[J]. Nature Genetics, 2010, 42(4): 348 − 354. doi: 10.1038/ng.548 [20] 毛梦迪. 番茄转录因子SlERF. D6在调控茄碱代谢和果实成熟中的功能研究[D].海口: 海南大学. 2022. [21] FANG C, LUO J. Metabolic GWAS-based dissection of genetic bases underlying the diversity of plant metabolism[J]. The Plant Journal, 2019, 97(1): 91 − 100. doi: 10.1111/tpj.14097 [22] KLEIN R J, ZEISS C, CHEW E Y, et al. Complement factor H polymorphism in age-related macular degeneration[J]. Science, 2005, 308(5720): 385 − 389. doi: 10.1126/science.1109557 [23] SHANG Y, MA Y, ZHOU Y, et al. Biosynthesis, regulation, and domestication of bitterness in cucumber[J]. Science, 2014, 346(6213): 1084 − 1088. doi: 10.1126/science.1259215 [24] CHEN W, GAO Y, XIE W, et al. Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism[J]. Nature Genetics, 2014, 46(7): 714 − 721. doi: 10.1038/ng.3007 [25] PENG M, SHAHZAD R, GUL A, et al. Differentially evolved glucosyltransferases determine natural variation of rice flavone accumulation and UV-tolerance[J]. Nature Communications, 2017, 8(1): 1975. doi: 10.1038/s41467-017-02168-x [26] TANAKA Y, SASAKI N , OHMIYA A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids[J]. The Plant Journal, 2008, 54(4): 733 − 749. doi: 10.1111/j.1365-313X.2008.03447.x [27] PANG Y, PEEL G J, SHARMA S B, et al. A transcript profiling approach reveals an epicatechin-specific glucosyltransferase expressed in the seed coat of Medicago truncatula[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(37): 14210 − 14215. doi: 10.1073/pnas.0805954105 [28] ZHAO J AND DIXON R A. transporters facilitate vacuolar uptake of epicatechin 3'-O-glucoside for proanthocyanidin biosynthesis inMedicago truncatula and Arabidopsis[J]. The Plant Cell, 2009, 21(8): 2323 − 2340. doi: 10.1105/tpc.109.067819 [29] FRYDMAN A, WEISSHAUS O, BAR-PELED M, et al. Citrus fruit bitter flavors: isolation and functional characterization of the gene Cm1, 2RhaT encoding a 1, 2 rhamnosyltransferase, a key enzyme in the biosynthesis of the bitter flavonoids of citrus[J]. The Plant Journal, 2004, 40(1): 88 − 100. doi: 10.1111/j.1365-313X.2004.02193.x [30] SCHARBERT S , HOFMANN T. Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments[J]. Journal of Agricultural and Food Chemistry, 2005, 53(13): 5377 − 5384. doi: 10.1021/jf050294d [31] LIU C W, LIN H W, YANG D J, et al. Luteolin inhibits viral-induced inflammatory response in RAW264.7 cells via suppression of STAT1/3 dependent NF-κB and activation of HO-1[J]. Free Radical Biology and Medicine, 2016, 95: 180 − 189. doi: 10.1016/j.freeradbiomed.2016.03.019 -




 下载:
下载: