-
无根藤(Cassytha filiformis)系樟科无根藤属(Cassytha)寄生缠绕草本植物,又名无娘藤、无爷藤、罗网藤等,主要分布于热带和亚热带地区,在我国主产于海南、广西、台湾、贵州以及福建等地区[1]。无根藤为我国民间常用药用植物,常用于糖尿病、风湿关节炎、肾炎水肿以及尿路结石等多种疾病的治疗[2-3]。无根藤的药用成分丰富,包括阿朴菲类生物碱、黄酮及挥发油类等[4]。目前的研究表明,阿朴菲类生物碱是无根藤的特征成分,从无根藤中分离得到30余个阿朴菲类生物碱,主要包括4种结构类型:阿朴菲类生物碱、氧化阿朴菲类生物碱、原阿朴菲类生物碱和吗啡烷类生物碱[5]。近年来,随着药理学和分子生物学的发展,研究人员陆续发现,阿朴菲类生物碱在抗糖尿病方面展示了较好的活性[6-7]。目前,阿朴菲类生物碱在抗糖尿病方面取得一定的研究成果,展示良好的应用前景。但是无根藤来源阿朴菲类生物碱的研究主要集中在抗肿瘤及抗寄生虫方面[8],而在抗糖尿病的研究较少。
为了进一步探究无根藤中阿朴菲类生物碱成分,笔者所在的课题组通过酸溶碱沉和乙酸乙酯萃取法得到无根藤总生物碱浸膏,进一步运用硅胶柱色谱、葡聚糖凝胶、制备薄层色谱法和半制备高效液相色谱等多种色谱方法进行分离纯化,利用核磁共振波谱和高分辨质谱等技术进行结构鉴定。同时,采用葡萄糖氧化酶法评价了所得化合物对HL-7702细胞葡萄糖消耗活性,挖掘无根藤中具有降糖活性的阿朴菲类生物碱,旨在为该植物资源的开发利用提供科学依据。
-
SB-1100型旋转蒸发仪(上海爱朗仪器有限公司),BRUKERAV Ⅲ600型核磁共振仪(德国布鲁克公司),SHZ-D(Ⅲ)型循环水式多用真空泵(河南省予华仪器有限公司),超声清洗仪(昆山市超声仪器有限公司),万特C20002型电子分析天平(杭州万特衡器有限公司),ATY124型电子天平(岛津菲律宾工厂),101-1AB型电热恒温干燥箱(天津市泰斯特仪器有限公司),ZF-20D暗箱式紫外分析仪(上海宝山顾村电光仪器厂),DLSB-5L/25型低温冷却液循环泵(巩义市予华仪器有限责任公司),DK-98-П型电热恒温水浴锅(天津市泰斯特仪器有限公司),DB-XAB型电热板(邦西仪器科技(上海)有限公司),赛默飞LTQ液质离子阱质谱仪液质联用仪(赛默飞公司),高效液相色谱用岛津液相色谱LC-16系统、配SPD-16检测器和YMC柱(ODS,2.5 mm ×250 mm, 5 μm)(日本岛津公司),薄层色谱硅胶板GF254(青岛海洋化工厂),Sephadex LH-20凝胶柱填料(Healthcare公司),柱色谱硅胶粉200目~300目(青岛海洋化工厂),酶标仪(Model 680型)(美国Bio-Rad公司),所用试剂为分析纯(西陇科学股份有限公司)或色谱纯(天津康科德科技有限公司),DMEM 培养基、RPMI Medium 1640培养基、胎牛血清、胰酶溶液、PBS均购自美国Gibco公司,盐酸二甲双胍(上海源叶生物有限公司),葡萄糖氧化反应检测试剂盒(北京九强生物技术有限公司),HL-7702细胞购自中国科学院细胞生物学研究所细胞库(中国上海)。
-
供试的无根藤于2020年11月,采自海南省文昌市铜鼓岭,由海南医学院曾念开教授鉴定为樟科无根藤,全株植物标本保存于海南医学院天然药物化学实验室,样本编号为No.CF202011。
-
无根藤(50 kg),剪断研碎,95%乙醇加热回流,重复提取3次,每次2 h,过滤收集,减压浓缩,收集样品,无醇味,得到乙醇提取浸膏。将乙醇浸膏加水分散,用2%盐酸调节pH2 ~ 3,然后用乙酸乙酯进行萃取3遍除去脂溶性成分和非生物碱相成分,水相用氢氧化钠调节pH9 ~ 10,用乙酸乙酯萃取3遍,减压浓缩后得到乙酸乙酯相即总生物碱相(352 g)。然后将总生物碱相进行硅胶柱色谱分离,以二氯甲烷−丙酮体系[(100∶0) ~ (1∶1)]为流动相梯度洗脱,经TLC检识后合并为6个部分(Fr. A ~ F)。
Fr. C部分经Sephadex LH-20(MeOH)柱色谱进一步分离,每50 mL接为1个组分,共得到29个组分后,根据薄层板检识和高效液相色谱初步摸索,然后,经半制备型HPLC以甲醇−水混合液(V甲醇∶V水=65∶35,流速2.0 mL·min−1)洗脱,得到1(12.2 mg)、2(2.8 mg)和3(2.1 mg)。
Fr. D部分根据薄层板检识,第17至25部分进行合并后,经Sephadex LH-20(MeOH)柱色谱进一步分离,每50 mL接为1个组分,共得到14个组分,然后经半制备型HPLC以甲醇−水混合液(V甲醇∶V水=60∶40,流速2.0 mL·min−1)洗脱,得到化合物4(3.3 mg)、5(2.1 mg);第48~82部分进行合并后,经硅胶柱色谱分离,以三氯甲烷−甲醇体系[(100∶0 )~(1∶1)]为流动相梯度洗脱纯化,收集到8个组分即Fr. D. a~Fr. D. h。进一步通过Sephadex LH-20(MeOH)柱色谱分离后,然后,通过制备薄层板层析(V三氯甲烷∶V丙酮=2∶1)刮板富集后,经半制备型HPLC以甲醇−水混合液(V甲醇∶V水=80∶20,流速2.0 mL·min−1)洗脱,得到化合物6(3.2 mg)。
Fr. E部分根据薄层板检识,第7至25部分进行合并后,经硅胶柱色谱层析,以二氯甲烷−甲醇体系[(100∶0 )~( 1∶1)]为流动相进行梯度洗脱,得10个组分即Fr. E. a~Fr. E. j,进一步通过制备薄层色谱(二氯甲烷:甲醇=12∶1)分离,然后经半制备型HPLC以甲醇−甲酸混合液(V甲醇∶V0.2%甲酸=55∶45,流速2.0 mL·min−1)进一步纯化,得到化合物7(2.8 mg),8(3.5 mg);第26至32部分进行合并后,进一步通过制备薄层色谱(V二氯甲烷∶V甲醇=8∶1)分离,经半制备型HPLC以甲醇−甲酸混合液(V甲醇∶V0.2%甲酸=50∶50,流速2.0 mL·min−1)进一步纯化,得到化合物9(2.3 mg);第38至43部分进行合并后,经Sephadex LH-20(MeOH)柱色谱分离,每50 mL接为1个组分,共得到17个组分,进一步通过制备薄层色谱(V二氯甲烷∶V甲醇=9∶1)重复2次刮板分离,然后分别经半制备型HPLC以甲醇−0.2%甲酸混合液(V甲醇∶V0.2%甲酸=50∶50,流速2.0 mL·min−1)进一步纯化,得到化合物10(13.4 mg),11(20.5 mg);经甲醇−甲酸混合液(V甲醇∶V0.2%甲酸=65∶35,流速2.0 mL·min−1)洗脱,得到化合物12(2.7 mg);第44至47部分进行合并后,进一步通过制备薄层色谱(二氯甲烷:甲醇=8:1)分离,然后经半制备型HPLC以甲醇−甲酸混合液(V甲醇∶V0.2%甲酸=50∶50,流速2.0 mL·min−1)进一步纯化,得到化合物13(18.3 mg)。
-
采用葡萄糖氧化酶法[9]评价了化合物1~8、10~12对HL-7702细胞葡萄糖消耗活性,以盐酸二甲双胍(Met,2 mmol·L−1)为阳性对照。具体步骤如下:HL-7702细胞用RPMI Medium 1640培养基(含10% FBS,1% P/S)培养,放置相对湿度为95%,温度为37 ℃,5%CO2 培养箱中,待细胞生长至一定汇合度时开始实验。细胞铺96板1×104个·孔−1,待细胞长到80%~90%的密度时,进行待测化合物给药处理,分组设置为正常对照组(CK组)、待测化合物不同浓度处理组和阳性对照组(Met组)。设置5个复孔,干预24 h后,从各孔中吸出2 μL细胞上清液到新的孔板里,做好对应分组标记,并设置标准液孔,每孔加入200 μL的葡萄糖消耗检测工作液,96孔板振荡器混匀,37 ℃培养箱孵育10 min,505 nm下检测吸光度。独立重复实验操作3次,以下式计算消耗的葡萄糖。
葡萄糖消耗浓度=标准液葡萄糖浓度−(标准液葡萄糖浓度×待测药物吸光度÷标准液吸光度)。
-
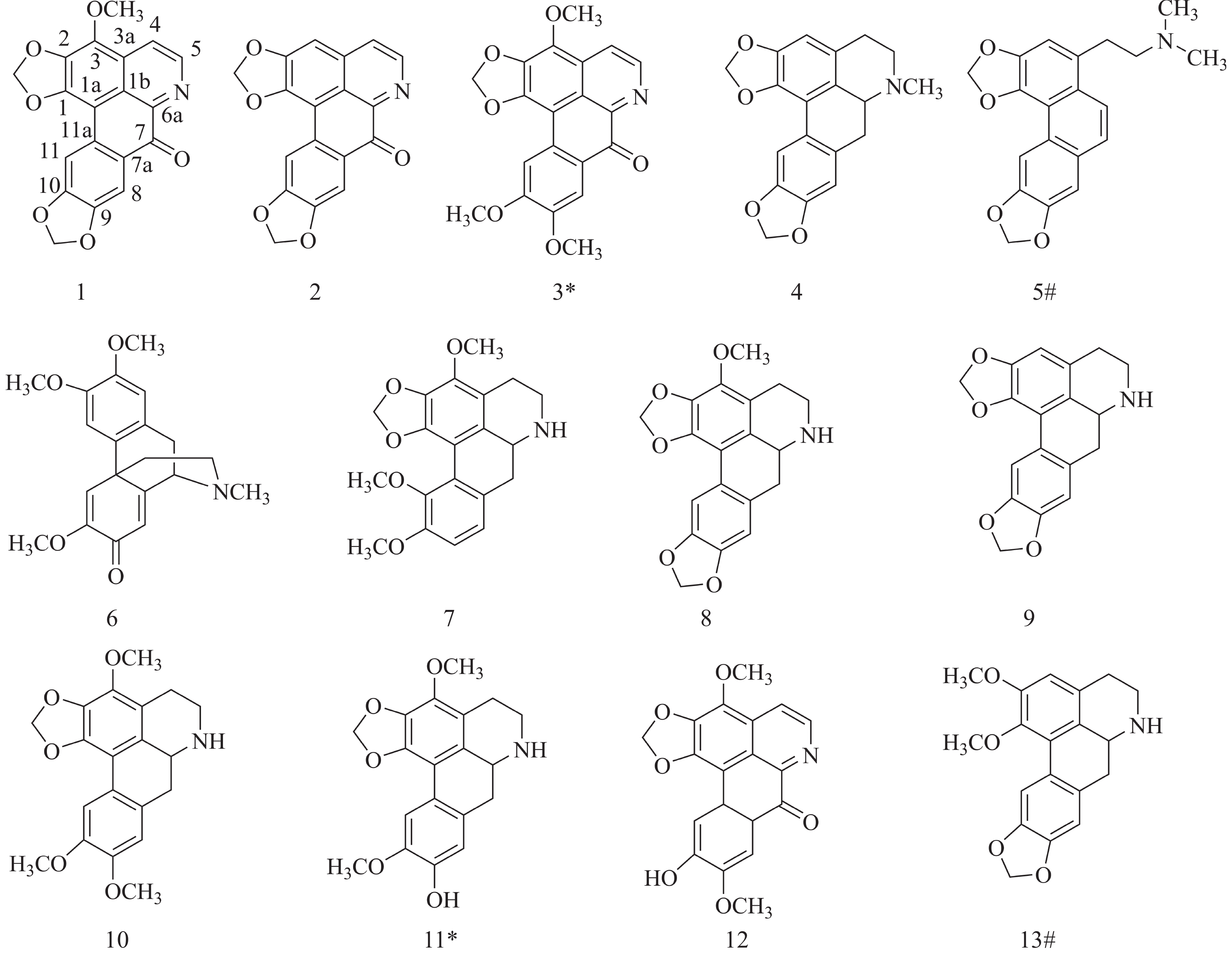
从无根藤中一共分离得到13个化合物,根据1H-NMR、13C-NMR 和HRESIMS数据鉴定了化合物1~13的结构(图1)。
化合物1:橘红色粉末;C19H11NO6。1H-NMR(600 MHz,CDCl3)δH:4.30(3H,s,3-OCH3),6.14(2H,s,9-OCH2O-10),6.32(2H,s,1-OCH2O-2),7.97(1H,s,H-8),8.01(1H,s,H-11),8.15(1H,d,J = 5.3 Hz,H-5),8.91(1H,d,J = 5.3Hz,H-4);13C-NMR(150 MHz,CDCl3)δC:60.3(3-OCH3),102.2(9-OCH2O-10),102.3(1-OCH2O-2),103.1(C-1a),106.0(C-11),107.5(C-8), 119.1(C-5),122.2(C-1b),127.1(C-11a),130.2(C-7a),130.7(C-3a),136.3(C-3),136.6(C-2),144.6(C-6a),145.1(C-4),147.9(C-10),149.2(C-9),153.0(C-1),181.1(C-7)。以上数据与文献[10]报道基本一致,故鉴定化合物1为无根藤米丁(cassamedine)。
化合物2:黄色粉末;ESI-MS m/z 320.0554 [M + H]+,C18H9NO5。1H-NMR(600 MHz,CDCl3)δH:8.89(1H,d,J = 7.9 Hz),8.14(1H,s),8.00(1H,s),7.77(1H,d,J = 7.7 Hz),7.19(1H,s),6.36(2H,s,-O-CH2-O-),6.15(2H,s,-O-CH2-O-)。以上数据与文献[11]报道基本一致,故鉴定化合物2为无根藤米里丁(cassameridine)。
化合物3:橘红色粉末;ESI-MS m/z 366.0969 [M + H]+,C20H15NO6。1H-NMR(600 MHz,CDCl3)δH:8.92(1H,d,J = 8.0 Hz),8.16(1H,d,J = 7.9 Hz),8.00(1H,s),7.98(1H,s),6.32(2H,s),4.28(3H,s,-OCH3),4.06(3H,s,-OCH3),4.05(3H,s,-OCH3)。以上数据与文献[12]报道基本一致,故鉴定化合物3为小唐松草宁碱(thalicminine)。
化合物4:红色粉末;C19H17NO4。1H-NMR(600 MHz,CD3OD)δH:2.51(1H,m),2.54(3H,s),2.66(1H,s),3.05-3.16(5H,m),5.95(2H,t,J = 1.6 Hz),5.92(1H,d,J = 1.3 Hz),6.06(1H,d,J = 1.2 Hz),6.54(1H,s),6.81(1H,s),7.57(1H,s);13C-NMR(150 MHz,CD3OD)δC:29.6(C-4),35.1(C-7),43.9(N-CH3),54.5(C-5),63.7(C-6a),102.1(-OCH2-O-),102.4(-OCH2-O-),107.7(C-3),108.3(C-11),109.5(C-8),117.8(C-1a),125.7(C-11a),127.0(C-1b),127.6(C-3a),130.6(C-7a),143.4(C-1),148.1(C-10),148.3(C-9),148.5(C-2)。以上数据与文献[12]报道基本一致,故鉴定化合物4 为新木姜子素(neolitsine)。
化合物5:白色粉末;C20H19NO4。1H-NMR(600 MHz,DMSO-d6)δH:3.17(6H,d,J = 4.4 Hz,N-(CH3)2),2.78(2H,m,CH2β),3.21(2H,m,CH2α),6.18(2H,s,6-OCH2O-7),6.26(2H,s,3-OCH2O-4),7.29(1H,s,H-2),7.43(1H,s,H-8),7.60(1H,d,J = 9.2 Hz,H-9),7.81(1H,d,J = 9.2 Hz,H-10),8.41(1H,s,H-5);13C-NMR(150 MHz,DMSO-d6)δC:31.0(CH2β) ,48.6(N-(CH3)2),63.0(CH2α),101.0(OCH2O,C-3,4),101.5(OCH2O,C-6,7),104.4(C-5),105.3(C-8),110.6(C-2),116.3(C-4a),121.3(C-10),123.5(C-5a),124.4(C-9),125.0(C-10a),128.2(C-8a),131.5(C-1),140.6(C-4),144.1(C-3),147.1(C-6),147.1(C-7)。以上数据与文献[13]报道基本一致,故鉴定化合物5为N-metilseconeolitsine。
化合物6:淡黄色粉末;C20H25NO4。1H-NMR(600 MHz,DMSO-d6)δH:7.11 (1H,s,H-5),6.88(1H,s,H-4),6.73(1H,s,H-1),6.20(1H,s,H-8),3.68(1H,d,J = 5.9 Hz,H-9),3.77,3.72(6H,s,s,2-OCH3 and 3-OCH3)and 3.69(3H,s,6-OCH3),3.28(1H,d,J = 17.6 Hz,H-10β),2.90(1H,dd,J = 6.6,17.8 Hz,H-10α),2.35-2.48(2H,m,H-16),2.32(3H,s,N-CH3),1.72-1.89(2H,m,H-15);13C-NMR(150 MHz,DMSO-d6)δC:180.0(C-7),162.9(C-14),150.5(C-6),147.7(C-3),147.6(C-2),130.1(C-12),128.5(C-11),121.1(C-8),120.8(C-5),110.5(C-1),109.9(C-4),59.6(C-9),55.9(3-OCH3),55.4(2-OCH3),55.0(6-OCH3),48.6(C-16),42.1(C-13),41.2(N-CH3),40.4(C-15),31.8(C-10)。以上数据与文献[14]报道基本一致,故鉴定化合物6为N-methyl-2, 3, 6-trimethoxymorphin-andien-7-one。
化合物7:褐色粉末;C20H21NO5。1H-NMR(600 MHz,DMSO-d6)δH:6.95(1H,d,J = 8.2Hz,H-8),6.91(1H,d,J = 8.2Hz,H-9),6.03/5.88(2H,d,J = 1.1Hz,1-OCH2O-2),3.98(1H,t,J = 10.7Hz,H-6a),3.95(3H,s,3-OCH3),3.79(3H,s,10-OCH3),3.70(3H,s,11-OCH3),3.44-3.46(2H,m,H-5),2.67-2.73(2H,m,H-4),2.53/3.17(2H,m,H-7);13C-NMR(150 MHz,DMSO-d6)δC:151.6(C-10),146.0(C-11),144.1(C-1),139.9(C-3),134.5(C-2),131.3(C-1b),129.4(C-7a),123.5(C-11a),121.9(C-8),118.6(C-3a),111.6(C-9),108.0(C-1a),100.1(1-OCH2O-2),60.6(11-OCH3),59.1(3-OCH3),55.8(10-OCH3),53.8(C-6a),41.9(C-5),37.0(C-7),23.7(C-4)。以上数据与文献[15]报道基本一致,故鉴定化合物7为10-O-甲基汝兰碱(1, 2-methylenedioxy-3, 10, 11-trimethoxyaporphine)。
化合物8:灰白色粉末;C19H17NO5。1H-NMR(600 MHz,DMSO-d6)δH:7.34(1H,s),6.89(1H,s),6.09/6.00/5.99/5.96(4H,d,J = 1.1Hz,-OCH2O-),3.92(3H,s,-OCH3),3.64(1H,dd,J = 4.8,14.2Hz),3.20(1H,m),2.68-2.78(3H,m),2.54-2.57(2H,m);13C-NMR(150 MHz,DMSO-d6)δC:146.0,145.8,142.8,139.5,134.8,129.1,129.0,124.1,119.3,110.2,108.6,106.3,100.9,100.7,59.7,53.3,42.4,36.3,23.6。以上数据与文献[16]报道基本一致,故鉴定化合物8为无根藤定碱(cassythidine)。
化合物9:褐色粉末;C18H15NO4。1H-NMR(600 MHz,DMSO-d6)δH:7.49(1H, s),6.91(1H, s),6.60(1H, s),6.09/6.02/6.01/5.95(4H,d,J = 1.2 Hz,-OCH2O-),3.69(1H,dd,J = 4.9,14.3Hz),3.12-3.17(1H,m),2.75-2.79(3H,m),2.52-2.55(2H,m);13C-NMR(150 MHz,DMSO-d6)δC:146.3(C-2),146.0(C-9),146.0(C-10),141.1(C-1),130.0(C-7a),129.6(C-3a),127.1(C-1b),124.0(C-11a),115.5(C-1a),108.6(C-8),107.2(C-11),106.6(C-3),101.0(-OCH2O-),100.4(-OCH2O-),53.2(C-6a),42.7(C-5),36.3(C-7),28.9(C-4)。以上数据与文献[17]报道基本一致,故鉴定化合物9为norneolitsine。
化合物10:白色粉末;C20H21NO5。1H-NMR(600 MHz,CD3OD)δH:7.63(1H,s,H-11),6.86(1H,s,H-8),6.08/5.95(2H,d,J = 1.3Hz,1-OCH2O-2),4.09(1H,m,H-6a),4.01(3H,s,3-OCH3),3.83(3H,s,10-OCH3),3.81(3H,s,9-OCH3),3.08/3.54(2H,m,H-5),2.77/2.95(2H,m,H-7),2.81/2.88(2H,m,H-4);13C-NMR(150 MHz,CD3OD)δC:148.3(C-10),148.1(C-9),144.0(C-1),139.5(C-3),135.6(C-2),125.6(C-1b),123.8(C-7a),123.3(C-11a),117.3(C-3a),111.5(C-8),110.6(C-1a),110.4(C-11),101.0(1-OCH2O-2),58.5(3-OCH3),55.2(10-OCH3),55.1(9-OCH3),53.2(C-6a),41.6(C-5),33.7(C-7),21.3(C-4)。以上数据与文献[16]报道基本一致,故鉴定化合物10为O-甲基无根藤碱(O-methylcassythine)。
化合物11:黑色粉末;C19H19NO5。1H-NMR(600 MHz,CD3OD)δH:7.57(1H,s,H-11),6.70(1H,s,H-8),6.11/5.97(2H,d,J = 1.1Hz,1-OCH2O-2),4.21(1H,d,J = 9.7Hz,H-6a),4.03(3H,s,3-OCH3),3.83(3H,s,10-OCH3),3.67/3.19(2H,m,H-5),2.78-2.99(4H,m,H-7/4);13C-NMR(150 MHz,CD3OD)δC:148.3(C-10),147.3(C-9),145.5(C-1),140.6(C-3),137.4(C-2),126.1(C-1b),123.2(C-7a),122.9(C-11a),117.8(C-3a),116.1(C-8),112.2(C-1a),111.7(C-11),102.6(1-OCH2O-2),60.1(3-OCH3),56.5(10-OCH3),54.4(C-6a),42.5(C-5),33.9(C-7),21.8(C-4)。以上数据与文献[18]报道基本一致,故鉴定化合物11为无根藤碱(cassythine)。
化合物12:红色粉末;ESI-MS m/z 352.0821 [M + H]+,C18H9NO5。1H-NMR(600 MHz,DMSO-d6)δH:8.84(1H,d,J = 5.2 Hz,H-5),8.16(1H,d,J = 5.2 Hz,H-4),8.06(1H,s,H-11),7.72(1H,s,H-8),6.48(2H,s,1-O-CH2-O-2),4.22(3H,s,3-OCH3),3.97(3H,s,9-OCH3)。以上数据与文献[19]报道基本一致,故鉴定化合物12为filiformine。
化合物13:褐色粉末;C19H19NO4。1H-NMR(600 MHz,DMSO-d6)δH:7.57(1H,s),7.08(1H,s),6.79(1H,s),6.08/5.93(2H,d,J = 1.6Hz,1-OCH2O-2),4.08(1H,m),4.01(3H,s),3.92(3H,s),3.65(2H,m),3.60(1H,t,J = 4.7Hz),3.53(1H,m),3.00(1H,m),2.92(1H,m),2.81(1H,m),13C-NMR(150 MHz,DMSO-d6)δC:145.7,144.8,143.6,139.8,130.0,130.0,127.5,123.2,118.6,114.4,111.2,109.7,100.9,59.7,56.3,53.6,42.5,35.4,29.9。以上数据与文献[20]报道基本一致,故鉴定化合物13为降南天竹碱(1,2-Dimethoxy-5,6, 6a,7-tetrahydro-4H-benzo[de][1,3]benzodioxolo[5,6-g]quinoline)。
本研究从无根藤中分离鉴定得到13个阿朴菲类生物碱,其中,4个(化合物1~3和12)为氧化阿朴菲类生物碱结构类型,7个(化合物4、7~11和13)为阿朴菲类生物碱结构类型,2个(化合物5和6)为其他阿朴菲类生物碱结构类型。
-
降糖是治疗糖尿病的主要手段之一,检测细胞葡萄糖消耗活性可以反应药物调控细胞糖代谢作用,是研究药物体外降糖活性的重要方法[21]。对从无根藤中分离鉴定所得阿朴菲类生物碱进行细胞葡萄糖消耗活性检测,结果(图2)表明;化合物1~2、4~8、10、12均无显著促进HL-7702细胞葡萄糖消耗活性;化合物3与CK 相比,在0.39~100 μmol·L−1时,显著促进HL-7702细胞葡萄糖消耗(P<0.05,**P<0.01,***P<0.001),有统计学意义;化合物11与CK 相比,在0.156~1.25 μmol·L−1时,显著促进HL-7702细胞葡萄糖消耗(*P<0.05,**P<0.01),有统计学意义。结果表明,化合物3和11能够调节细胞葡萄糖代谢作用。相比两者的活性效果,化合物3相对更优。
-
本研究从无根藤中分离鉴定得到13个阿朴菲类生物碱,其中化合物5和13是首次从该植物中分离得到,且化合物5是一类罕见的阿朴菲类生物碱结构,其余分离获得化合物主要为阿朴菲类及氧化阿朴菲类母核结构,取代基主要为甲氧基和或亚甲二氧基取代,与之前报道的阿朴菲类生物碱母核主要取代基团一致[5],本研究所得的化合物进一步增加了无根藤的阿朴菲类生物碱组成。
近年来,阿朴菲类生物碱在治疗糖尿病方面研究展现较好的活性,降糖研究是治疗糖尿病的重要方法之一[22]。所以,笔者评价了待测化合物的细胞葡萄糖消耗活性,化合物3和11显示具有显著促进HL-7702细胞葡萄糖消耗活性,又以化合物3降糖活性最强,具有调控细胞糖代谢作用。明确化合物构效关系可以更高效地研究开发治疗药物的先导化合物,因此,比较本研究分离所得的4个氧化阿朴菲类生物碱构效关系,结果发现,化合物3与其他3个的结构差异在于9、10位的取代基不同,9、10甲氧基的取代比亚甲二氧基或羟基取代更能显示出降糖活性,所以,9、10位2个甲氧基的取代可能是影响这类阿朴菲类生物碱降糖活性的主要因素;比较无根藤中发现的罕见阿朴菲类生物碱结构类型(化合物5)与文献[23]报道类似结构化合物(N-allylsecoboldine、N-vanillylsecoboldine和N-isopropylsecoboldine)的构效关系,发现化合物5与这3个化合物的结构差异主要在于1、2位和9、10位的取代基不同,这3个化合物在1、2位和9、10位为甲氧基或羟基取代,而化合物5为亚甲二氧基取代,所以,亚甲二氧基取代可能是降低这类化合物降糖活性的主要因素;比较本研究发现阿朴菲类生物碱母核化合物(4、7~8、10~11)及文献[24]报道的降糖活性阿朴菲类生物碱(波尔定碱、新木姜子碱)之间构效关系,发现当1、2位为亚甲二氧基取代时,9、10位为羟基和甲氧基取代的化合物显示出降糖活性,且当1、2位和9、10位均为羟基或甲氧基取代,不含有亚甲二氧基取代的化合物,也能表现出降糖活性。综上,在阿朴菲类生物碱基团的主要取代位点(1、2、9、10位),亚甲二氧基可能是降低阿朴菲类生物碱降糖活性的取代基团,而羟基和甲氧基可能是增强此类化合物降糖活性的取代基团。据报道,9、10位甲氧基和或羟基取代的多种阿朴菲类生物碱进行改善糖尿病机理研究:如新木姜子碱在细胞和动物实验上表明,其可以通过调控AMPK信号通路发挥降糖及改善糖尿病作用[25];波尔定碱和唐松草坡芬在糖尿病模型大鼠中,可以调控胰岛素信号通路发挥降血糖作用[26];波尔定碱、六驳碱和黄肉楠碱在大鼠小肠匀浆模型中,可以抑制α−葡萄糖苷酶活性发挥降糖作用[6]。因此,后续可以围绕化合物3和11调控AMPK信号通路、胰岛素信号通路和α-葡萄糖苷酶活性等方面进行研究,探索阿朴菲类生物碱的构效关系,对化合物结构进行针对性的修饰,为开发治疗糖尿病的药物提供有价值的先导化合物。
本研究成果进一步丰富了无根藤属植物无根藤阿朴菲类生物碱化学成分,发现2个阿朴菲类生物碱具有显著降糖作用,为阿朴菲类生物碱成为降糖先导化合物的研究提供数据支持,为先导化合物的衍生化改造提供了参考,促进了无根藤的抗糖尿病的开发利用研究。
Analysis of aporphine alkaloids in Cassytha filiformis
-
摘要: 为了寻找无根藤(Cassytha filiformis)中具有降糖活性的阿朴菲类生物碱,以无根藤为研究对象,通过酸溶碱沉法提取,乙酸乙酯萃取获取总生物碱,并通过多种色谱方法进行分离和纯化,再通过波谱学技术鉴定化合物的结构,最后采用葡萄糖氧化酶法评价所得阿朴菲类生物碱对HL-7702细胞葡萄糖消耗活性。鉴定结果发现13个阿朴菲类生物碱,分别鉴定为:无根藤米丁( 1 )、无根藤米里丁( 2 )、小唐松草宁碱( 3 )、新木姜子素( 4 )、N-metilseconeolitsine( 5 )、N-methyl-2,3,6-trimethoxymorphin-andien-7-one( 6 )、10-O-甲基汝兰碱( 7 )、无根藤定碱( 8 )、norneolitsine( 9 )、O-甲基无根藤碱( 10 )、无根藤碱( 11 )、filiformine( 12 )、降南天竹碱( 13 ) , 其中,化合物 5 和 13 首次从该植物中分离得到。细胞葡萄糖消耗活性结果显示,化合物 3 和 11 显著促进HL-7702细胞葡萄糖消耗,具有研究开发成降糖先导化合物的潜在价值。Abstract: In order to discover aporphine alkaloids with hypoglycemic activity in Cassytha filiformis, the total alkaloids were extracted from C. filiformis by acid solubilization and alkaline precipitation, obtained by ethyl acetate extraction, separated and purified by various chromatographic methods. The structures of the compounds purified were identified by using spectrometry, and the glucose oxidase method was used to evaluate the glucose consumption activity of the resulting aporphine alkaloids on HL-7702 cells. Thirteen aporphine alkaloids were identified from the extract of C. filiformis: cassamedine ( 1 ), cassameridine ( 2 ), thalicminine ( 3 ), neolitsine ( 4 ), N-metilseconeolitsine ( 5 ), N-methyl-2, 3, 6-trimethoxymorphin-andien-7-one ( 6 ), 1, 2-methylenedioxy-3, 10, 11-trimethoxyaporphine ( 7 ), cassythidine ( 8 ), norneolitsine ( 9 ), O-methylcassythine ( 10 ), cassythine ( 11 ), filiformine ( 12 ), and 1, 2-Dimethoxy-5, 6, 6a, 7-tetrahydro-4H-benzo[de][1, 3]benzodioxolo[56-g]quinoline ( 13 ). Among them, compounds 5 and 13 were first isolated from C. filiformis. Cellular glucose consumption activity assay showed that compounds 3 and 11 significantly promoted glucose consumption in HL-7702 cells and displayed a potential value for research and development into hypoglycemic lead compounds.
-
[1] ZHANG H X, FLORENTINE S, TENNAKOON K U. The angiosperm stem hemiparasitic genus Cassytha(Lauraceae) and its host interactions: a review[J]. Frontiers in Plant Science, 2022, 13: 864110. [2] ZHANG C, MA H, SANCHEZ-PUERTA M V, et al. Horizontal gene transfer has impacted cox1 gene evolution in Cassytha filiformis[J]. Journal of Molecular Evolution, 2020, 88(4): 361 − 371. doi: 10.1007/s00239-020-09937-1 [3] CHEUNG W L, LAW C Y, LEE H C H, et al. Gelsemium poisoning mediated by the non-toxic plant Cassytha filiformis parasitizing gelsemium elegans[J]. Toxicon, 2018, 154: 42 − 49. doi: 10.1016/j.toxicon.2018.09.009 [4] OUATTARA Z A, SANGARÉ N, MAMYRBEKOVA-BEKRO A J, et al. Composition and chemical variability of essential oils isolated from aerial parts of Cassytha filiformis from côte d′ivoire[J]. Natural Product Communications, 2018, 13(2): 217 − 218. [5] 黄兹宝, 张璐, 董琳, 等. 无根藤的研究进展[J]. 海南医学院学报, 2022, 28(12): 954 − 960. doi: 10.13210/j.cnki.jhmu.20210303.003 [6] 刘欣, 孙洋, 窦利民, 等. 阿朴菲生物碱抗糖尿病生物活性研究进展[J]. 药学进展, 2017, 41(9): 704 − 709. [7] WANG F X, ZHU N, ZHOU F, et al. Natural aporphine alkaloids with potential to impact metabolic syndrome[J]. Molecules, 2021, 26(20): 1 − 22. [8] OLI A N, OBAJI M, ENWEANI I B. Combinations of Alchornea cordifolia, Cassytha filiformis and Pterocarpus santalinoides in diarrhoegenic bacterial infections[J]. BMC Research Notes, 2019, 12(1): 649. doi: 10.1186/s13104-019-4687-0 [9] HUANG Z, CAO M, WANG R, et al. Two new aporphine alkaloids with glucose consumption increasing activity from Cassytha filiformis[J]. Phytochemistry Letters, 2022, 51: 23 − 27. doi: 10.1016/j.phytol.2022.06.013 [10] SHAMMA M, CASTENSON R L. Chapter 6 the oxoaporphine alkaloids[J]. The Alkaloids: Chemistry and Physiology. Amsterdam: Elsevier, 1973: 225 − 264. [11] ZHENG B, QU H Y, MENG T Z, et al. Novel total syntheses of oxoaporphine alkaloids enabled by mild Cu-catalyzed tandem oxidation/aromatization of 1-Bn-DHIQs[J]. RSC Advances, 2018, 8(51): 28997 − 29007. doi: 10.1039/C8RA05338C [12] GARCEZ F R, FRANCISCA DA SILVA A G, GARCEZ W S, et al. Cytotoxic aporphine alkaloids from Ocotea acutifolia[J]. Planta Medica, 2011, 77(4): 383 − 387. doi: 10.1055/s-0030-1250401 [13] GARCÍA M T, BLÁZQUEZ M A, FERRÁNDIZ M J, et al. New alkaloid antibiotics that target the DNA topoisomerase I of Streptococcus pneumoniae[J]. Journal of Biological Chemistry, 2011, 286(8): 6402 − 6413. [14] WU J B, CHENG Y D, CHIU N Y, et al. A Novel morphinandienone alkaloid from Fissistigma oldhamii[J]. Planta Medica, 1993, 59(2): 179 − 180. doi: 10.1055/s-2006-959639 [15] TSAI T H, WANG G J, LIN L C. Vasorelaxing alkaloids and flavonoids from Cassytha filiformis[J]. Journal of Natural Products., 2008, 71(2): 289 − 291. doi: 10.1021/np070564h [16] JONES S R, LAMBERTON J A. Cassytha alkaloids. I. new aporphine alkaloids from Cassytha filiformis L[J]. Australian Journal of Chemistry, 1966, 19(2): 297 − 302. doi: 10.1071/CH9660297 [17] STÉVIGNY C, WAUTIER M C, HABIB JIWAN J L, et al. Development and validation of a HPLC method for quantitative determination of aporphine alkaloids from different samples of Cassytha filiformis[J]. Planta Medica, 2004, 70(8): 764 − 770. doi: 10.1055/s-2004-827209 [18] STÉVIGNY C, BLOCK S, DE PAUW-GILLET M C, et al. Cytotoxic aporphine alkaloids from Cassytha filiformis[J]. Planta Medica, 2002, 68(11): 1042 − 1044. doi: 10.1055/s-2002-35651 [19] CHANG F R, CHAO Y C, TENG C M, et al. Chemical constituents from Cassytha filiformis II[J]. Journal of Natural Products, 1998, 61(7): 863 − 866. doi: 10.1021/np970348g [20] PECIC S, MAKKAR P, CHAUDHARY S, et al. Affinity of aporphines for the human 5-HT2A receptor: insights from homology modeling and molecular docking studies[J]. Bioorganic & Medicinal Chemistry, 2010, 18(15): 5562 − 5575. [21] 陈晶, 李璟. 糖尿病细胞模型及研究进展[J]. 临床与病理杂志, 2016, 36(4): 507 − 514. [22] 杨燕, 卓见, 申红霞, 等. 新型降糖药物治疗2型糖尿病的研究进展[J]. 实用医学杂志, 2023, 39(2): 153 − 157. [23] LIN C J, CHEN C H, LIU F W, et al. Inhibition of intestinal glucose uptake by aporphines and secoaporphines[J]. Life Sciences, 2006, 79(2): 144 − 153. doi: 10.1016/j.lfs.2005.12.031 [24] 金燕. 潺槁树生物碱类成分的发现及其降糖活性研究[D]. 海口: 海南医学院, 2019. [25] ZHANG Y, WANG R Q, YANG Y N, et al. Laurolitsine ameliorates type 2 diabetes by regulating the hepatic LKB1-AMPK pathway and gut microbiota[J]. Phytomedicine, 2022, 106: 1 − 14. [26] CHI T C, LEE S S, SU M J. Antihyperglycemic effect of aporphines and their derivatives in normal and diabetic rats[J]. Planta Medica, 2006, 72(13): 1175 − 1180. doi: 10.1055/s-2006-947199 -





 下载:
下载: