-
肝簇虫是顶复门(Apicomplexa)真球目(Encoudida)肝簇虫属(Hepatozoon spp.)的原虫,属于血液寄生虫的8个属之一。目前所发现的肝簇虫属已经达到300多种,其中至少有46种肝簇虫属能感染哺乳动物[1]。肝簇虫可以通过不同种类的节肢动物进行传播,比如蜱(Ixodida),虱(Phthiraptera),蚊(Culicidae),螨(Acariformes and Parasitiformes),白蛉(Phlebotomidae),采采蝇(Glossina),锥蝽(Triatominae),水蛭(Whitmania pigra Whitman)[1-3]。
目前已知能感染犬和其他野生犬科动物的肝簇虫有2种,分别是犬肝簇虫(Hepatozoon canis)[4]和美洲肝簇虫(Hepatozoon americium)[5]。2种肝簇虫都是通过犬和其他野生犬科动物吞食含有成熟卵囊的蜱虫而进行传播的[6-7]。也有包括犬肝簇虫通过胎盘屏障感染胎儿,或新宿主猎食了作为美洲肝簇虫的原宿主等其他途径感染的报道[8]。血红扇头蜱(Rhipicephalus sanguineus),一种以犬为主要宿主,以多种哺乳动物血液为食的棕色犬蜱,在世界范围内广泛分布[9-10],已被证明是犬肝簇虫的主要传播媒介[11]。最近,也有实验研究证实,对犬肝簇虫也有传播作用的还有在意大利南部发现的图兰扇头蜱(Rhipicephalus turanicus) [12];其他蜱种如巴西的微小扇头蜱(牛蜱)[Rhipicephalus (Boophilus) microplus][13]、卵圆花蜱(Amblyomma ovale)[14]、日本的长角血蜱(Haemaphysalis longicornis)和褐黄血蜱(Haemaphysalis flava)[15]、意大利的蓖籽硬蜱(Ixodes ricinus)[16]、匈牙利的嗜群血蜱(Haemaphysalis concinna)[17]等也可能是犬肝簇虫的潜在载体。
犬肝簇虫可广泛感染世界各地的肉食性宿主,如狗、豺、狐狸、负鼠和家猫[16, 18-22]。家养犬对犬肝簇虫的感染表现出良好的适应性,大多数犬在感染后的疾病状态处于亚临床到轻度之间,而犬被美洲肝簇虫感染后往往是致命的[5, 23]。虽然美洲肝簇虫和犬肝簇虫在犬科动物感染中都有报道,但犬肝簇虫的传播更广泛,也是犬肝簇虫病的主要原因[9]。
犬肝簇虫的诊断常用镜检方法,即在染色的血涂片中检测出犬肝簇虫的配子体[24]。酶联免疫吸附试验和间接荧光抗体试验等工具也被用于犬肝簇虫抗体的血清学诊断[25]。还可以通过PCR技术检测犬体内的犬肝簇虫,这是检测犬感染犬肝簇虫最准确的诊断方法[7, 26]。
犬肝簇虫广泛分布,欧洲[16, 19]、非洲[27-28]、美洲[29]、亚洲[4, 30]、澳洲[31]等地都有犬肝簇虫的报道;在中国的邻国也有发现,如柬埔寨[32],印度[33],日本[34],韩国[35],巴基斯坦[36],泰国[37],马来西亚半岛[38]等,而美洲肝簇虫只在北美地区有报道[5, 39]。目前我国犬只数量估计在1.5亿到2亿[40]之间。国内对犬肝簇虫的研究却非常有限,仅有北京、河南、江苏、陕西、新疆[41]、广西[42]、广东[43]、台湾[44]等地区有关于犬肝簇虫的报道。
在过去的几年里,世界范围内影响家畜和野生动物的肝簇虫流行率有所增加,因此,对肝簇虫的研究在兽医领域具有重要意义。本研究旨在通过PCR和测序方法检测出海南岛犬体内携带肝簇虫的情况,可为海南岛犬蜱传疾病的流行和分布格局提供信息。
-
2017年10月至2022年5月,在海南岛18个市县共采集了1039份犬血,血液保存于含有EDTA的EP管中,放入−80℃冻存。并对每只犬的信息建立档案记录。
-
Ezup柱式血液基因组DNA提取试剂盒、琼脂糖、DL 2000 DNA Marker均购自生工生物工程(上海)股份有限公司;Goldview染料,购自苏州金唯智生物科技有限公司;2 X Taq Plus Master Mix II(Dye Plus),购自南京诺维赞生物科技股份有限公司。
-
PCR仪,TProfessional,德国Biometra公司产品;小型台式高速冷冻离心机,5415R,Eppendorf 公司产品;琼脂糖水平电泳仪,Mini-PROTEAN Tetra,北京六一仪器厂产品;凝胶成像分析系统,GelDoc XR+,美国Bio-Rad公司产品。
-
取出100 μL上述采集到的血液,分别加入到1.5 mL EP管中,参照DNA提取试剂盒说明书步骤提取犬血中DNA,并于−20 ℃保存。
-
本实验采用的引物序列参照文献[45],上游引物为HEP-F:5′-ATACATGAGCAAAATCTCAAC-3′;下游引物为HEP-R:5′-CTTATTATTCCATGCTGCAG-3′。PCR具体反应体系:2 X Taq PLus Master Mix II 12.5 μL,特异性引物F和特异性引物R(10−8 moL·L−1)各 0.5 μL,血液基因组DNA(约20 ng) 1 μL。PCR反应程序:95 ℃,预变性5 min;95 ℃,变性时间60 s;58 ℃,退火时间60 s;72 ℃,延伸时间60 s;共35个循环;最后72 ℃延伸5 min,4 ℃保存。反应结束后,取5 μL PCR产物加入1.5%琼脂糖凝胶加样孔中进行电泳,然后在凝胶成像分析系统下观察结果是否出现目的条带。将出现目的条带所对应的阳性产物送往生工生物工程(上海)股份有限公司进行测序。
-
使用NCBI中BLAST在线比对工具将肝簇虫的测序结果进行比对,选出代表性的犬肝簇虫序列与GenBank中已知序列进行比对,利用软件进行同源性分析(DNAMAN 8.0),使用Mega XI软件,选择Neighbor-joining(NJ)算法中的Kimura-2-parameter参数模型,Bootstrop值选择1000进行支序图构建,以刚地弓形虫(Toxoplasma gondii)的18S rRNA序列(登录号L37415)为外群。
-
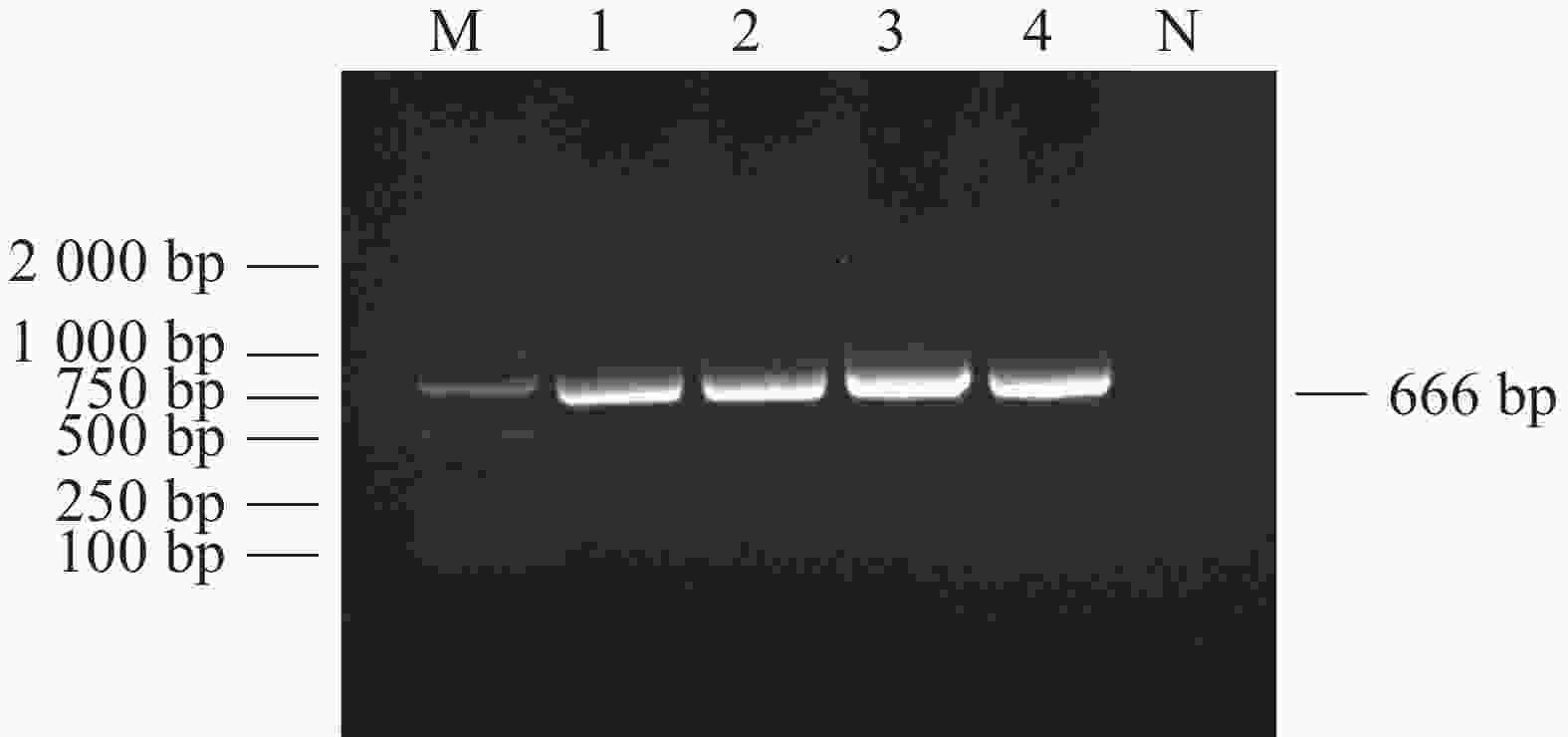
对采集到的1 039份犬全血DNA进行犬肝簇虫18S rRNA基因扩增,电泳结果显示扩增产物在666 bp处出现目的片段(图1),与犬肝簇虫18S rRNA基因预期片段大小相符。扩增序列测序拼接后经BLAST比对与GenBank上已有的犬肝簇虫序列AF176835同源性为99.85%。
-
在海南岛1 039份犬血中发现犬肝簇虫阳性血液135份,感染率为13.0%。海南岛18个市县犬肝簇虫感染率见表1。海口(20.7%)、三亚(20.6%)、屯昌(33.3%)等地犬肝簇虫感染率较高。文昌市(8.7%)、定安县(5.6%)、澄迈县(6.3%)、儋州市(5.0%)、琼中县(15.8%)、乐东县(18.8%)、昌江县(6.7%)等地均发现有犬感染犬肝簇虫。
表 1 海南岛18个市县犬肝簇虫感染率
测序方法 市/县 犬肝簇虫
样品数量/阳性数量
(感染率/%)18S rRNA 海口市 435/90(20.7) 18S rRNA 文昌市 46/4(8.7) 18S rRNA 琼海市 34/0(0) 18S rRNA 定安县 232/13(5.6) 18S rRNA 澄迈县 16/1(6.3) 18S rRNA 临高县 12/0(0) 18S rRNA 儋州市 60/3(5.0) 18S rRNA 琼中黎族苗族自治县 19/3(15.8) 18S rRNA 保亭黎族苗族自治县 24/0(0) 18S rRNA 万宁市 18/0(0) 18S rRNA 五指山市 12/0(0) 18S rRNA 三亚市 34/7(20.6) 18S rRNA 乐东黎族自治县 16/3(18.8) 18S rRNA 陵水黎族自治县 4/0(0) 18S rRNA 白沙黎族自治县 11/0(0) 18S rRNA 昌江黎族自治县 15/1(6.7) 18S rRNA 东方市 21/0(0) 18S rRNA 屯昌县 30/10(33.3) 18S rRNA 总数 1039/135(13.0) -
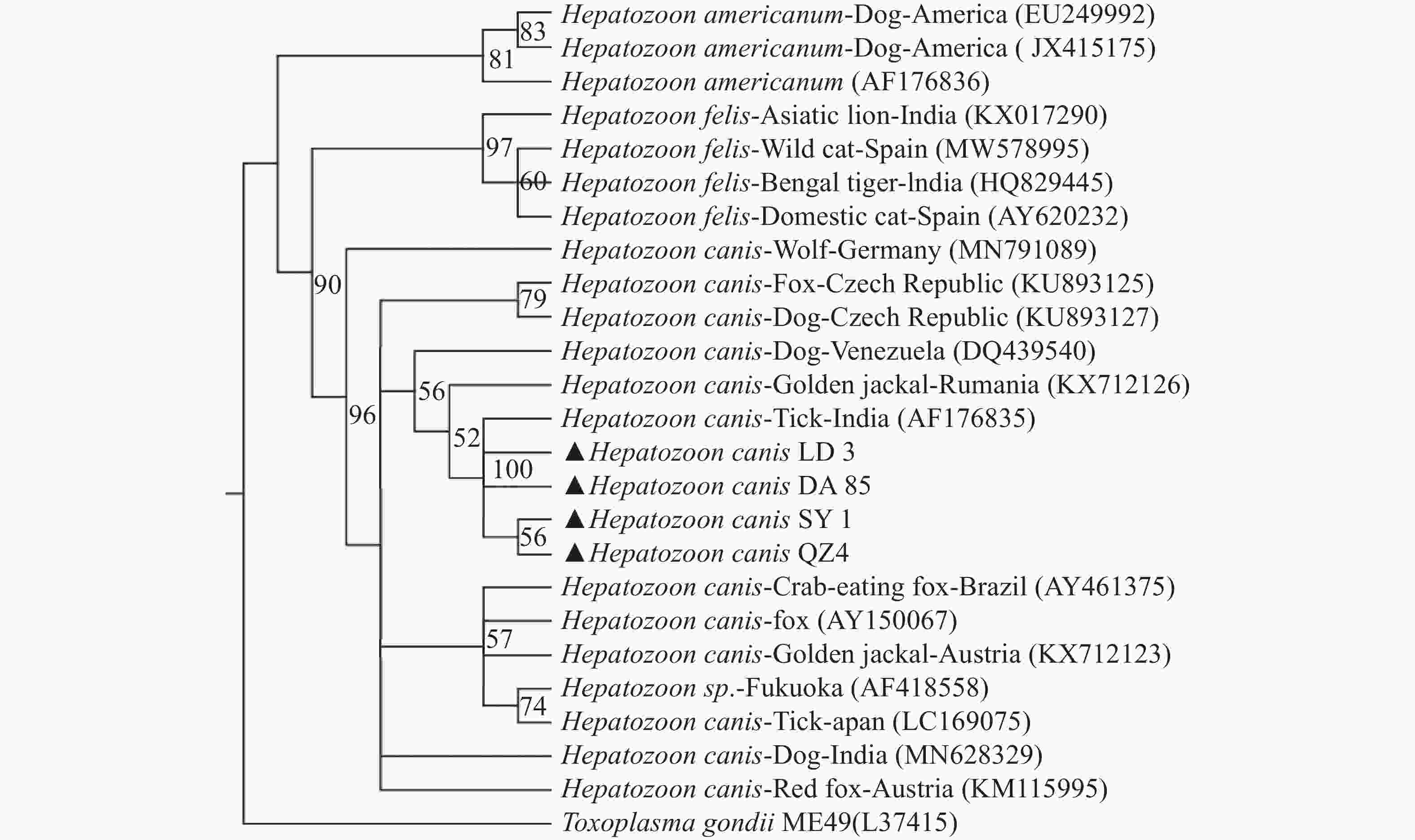
BLAST分析表明,海南的犬肝簇虫的同源性为99.4%~100%,用MEGA-X软件选择本实验中获得的代表性犬肝簇虫18S rRNA基因序列构建支序图(图2)(三亚(OP699205)、乐东(OP699206)、定安(OP699207)、琼中(OP699208)的阳性样品)。从图2中发现海南的犬肝簇虫(Hepatozoon canis LD3、Hepatozoon canis DA85、Hepatozoon canis SY1、Hepatozoon canis QZ4)与印度的犬肝簇虫(AF176835)均位于bootstrap支持度100的同一分支中,并在bootstrap支持度90的情况下与该属其他分支分离[猫肝簇虫(Hepatozoon felis ),美洲肝簇虫( Hepatozoon americium )]
-
犬肝簇虫是蜱传病原虫中分布最广的一种,主要由血红扇头蜱[46]传播。本研究采集的样本来自于海南岛18个市县,海南岛属于热带季风气候,全年温度和湿度相对较高,年平均温度在22~27 ℃。海南岛气候适合热带物种血红扇头蜱的发展和生存。目前针对犬肝簇虫对不同宿主或宿主特异性的情况尚未有较多的研究[6, 11]。因此,无法消除犬肝簇虫通过其他蜱虫物种传播的可能性[19]。
自1905年犬肝簇虫首次被提出以来[4],犬肝簇虫已在美洲、欧洲、亚洲、非洲和澳大利亚的几个国家被报道。大多数的报道通常是通过组织病理学或显微镜诊断[35]的方法来确定肝簇虫的感染,缺乏对其种群的遗传结构和基因变化的理解。本实验采用PCR技术检测犬体内的犬肝簇虫,并通过测序和建立支序图的方式对该血液寄生虫的物种进行鉴定[47],使调查结果的准确性更高。
本研究使用了一套肝簇虫属的特异性引物对海南岛1039份犬血样进行检测,琼脂糖凝胶电泳结果显示,其中135份样品(13 .0%)为阳性。通过对获得的阳性扩增子进行测序分析来确定肝簇虫的种类。
本研究结果支持并证实了海南犬体内存在犬肝簇虫,也是海南岛犬肝簇虫的首次分子生物学报道。据报道,犬肝簇虫在非洲国家流行率如苏丹42.3%[48]、尼日利亚41.4%[49]和赞比亚13%[50]。犬肝簇虫在欧洲国家流行率如法国1%[51]、西班牙3%[52] 捷克50%[53]、意大利南部50.6%[7]。犬肝簇虫在北美洲流行率如古巴47.5%[54]、美国犬肝簇虫与美洲肝簇虫同时出现,感染率为2.5%[55]。犬肝簇虫在南美洲流行率如巴西(53.3%)[56],犬肝簇虫在亚洲流行率如印度(30%)[33]、日本(42.9%)[34]。
文献报道我国犬肝簇虫感染率为河南登封市4.08%[43],河南郑州4.9%,北京4.5%,江苏南京2.3%,陕西杨凌8.9%,新疆乌鲁木齐1.2%[41],中国农业大学动物医院有确诊一例犬肝簇虫阳性病例[57],广州也有疑似犬肝簇虫的病例报道[58]。
海南岛犬肝簇虫的感染率为13.0%,与其他省市感染率相比偏高,比国外其他国家相比感染率偏低,这些犬肝簇虫感染率的差异可能是由多种因素造成的,如目标犬的种群特征、采样季节、社会因素(犬类饲养、使用药物杀蜱)、地理位置和气候条件等,这些因素最终会影响病媒蜱物种的数量和分布[59]。因犬肝簇虫的传播需要通过吞食含有成熟卵囊的蜱虫进行,使得犬肝簇虫病的感染率不高。但目前没有可用的犬肝簇虫疫苗,使得犬肝簇虫病的治疗比较困难。防治这种疾病的最佳方法是采取适当的蜱虫控制策略来预防感染。
支序图分析结果表明,来自海南岛的犬肝簇虫与其他地区的犬肝簇虫属于同一支系,与海南样品同源性最接近的序列是来自印度的犬肝簇虫(AF176835)。这一发现可能表明,该病原体可能是由于旅游业、自然灾害和社会因素等,人与宠物的流动性日益增加的原因而带入海南。
此外,本研究中犬肝簇虫基因型与猫肝簇虫、美洲肝簇虫分类不同。由于该类群的成员已在全球广泛分布的犬科动物和家养犬中被报道,因此可能对进一步研究该类群的新类群划定提供一定的帮助。
犬肝簇虫病在海南岛的流行病学调查及其遗传关系的研究
DOI: 10.15886/j.cnki.rdswxb.20220114
 CSTR: 32425.14.j.cnki.rdswxb.20220114
CSTR: 32425.14.j.cnki.rdswxb.20220114
Epidemiological investigation and genetic relationship of canine hepatozoonosis in Hainan Island
-
摘要: 为了解近几年犬肝簇虫病在海南岛的流行情况,对其防控措施进行评估,便于更好地对海南岛的犬肝簇虫病进行防治。采用PCR方法对2017年10月至2022年5月海南岛18个市县的1 039份犬血样品进行犬肝簇虫(Hepatozoon canis)的检测。共检测出135份犬血样品呈阳性,感染率为13.0%,其中,海口(20.7%)、三亚(20.6%)、屯昌(33.3%)等地犬肝簇虫感染率较高;文昌市(8.7%)、定安县(5.6%)、澄迈县(6.3%)、儋州市(5.0%)、琼中县(15.8%)、乐东县(18.8%)、昌江县(6.7%)等地均发现有犬感染犬肝簇虫。以三亚(OP699205)、乐东(OP699206)、定安(OP699207)、琼中(OP699208)的阳性样品基于犬肝簇虫18S rRNA基因构建支序图,结果发现海南岛的犬肝簇虫与印度的犬肝簇虫(AF176835)同源性(99.85%)最接近,并与猫肝簇虫(Hepatozoon felis),美洲肝簇虫(Hepatozoon americium)的分支分离。说明在海南岛的犬群中犬肝簇虫的感染率较高,需采用定期驱除犬肝簇虫的传播媒介血红扇头蜱(Rhipicephalus sanguineus)方式进行防治。Abstract: In order to understand the prevalence of Hepatozoon canis disease in Hainan in recent years, the prevention and control measures were evaluated for better control of Canine hepatozoonosis in Hainan. There were 1039 dog blood samples collected from 18 cities and counties in Hainan Province from October 2017 to May 2022, and they were detected for H. canis by PCR. Among the dog blood samples collected, 135 samples were found to be positive for H. canis, with an infection rate of 13.0%. Of the samples higher infection rates of H. canis were found in Haikou (20.7%), Sanya (20.6%), Tunchang (33.3%), and some infections of H. canis were also found in Wenchang (8.7%), Dingan (5.6%), Chengmai (6.3%), Danzhou (5.0%), Qiongzhong (15.8%), Ledong (18.8%) and Changjiang (6.7%). The positive samples from Sanya (OP699205), Ledong (OP699206), Dingan (OP699207) and Qiongzhong (OP699208) were selected to construct a phylogenetic tree based on the 18S rRNA of the H. canis. It was found that the homology of H. canis in Hainan was the closest to that of H. canis (AF176835) in India, which was 99.85%, and was also separated from that of the branches of H. felis and H. americanum. This shows that the dogs in Hainan Island has a high infection rate of H.canis. The prevention and treatment of H.canis requires regular elimination of the transmission vector Rhipicephalus sanguineus .
-
Key words:
- Hepatozoon canis /
- PCR /
- infection rate /
- epidemiology /
- Hainan
-
表 1 海南岛18个市县犬肝簇虫感染率
测序方法 市/县 犬肝簇虫
样品数量/阳性数量
(感染率/%)18S rRNA 海口市 435/90(20.7) 18S rRNA 文昌市 46/4(8.7) 18S rRNA 琼海市 34/0(0) 18S rRNA 定安县 232/13(5.6) 18S rRNA 澄迈县 16/1(6.3) 18S rRNA 临高县 12/0(0) 18S rRNA 儋州市 60/3(5.0) 18S rRNA 琼中黎族苗族自治县 19/3(15.8) 18S rRNA 保亭黎族苗族自治县 24/0(0) 18S rRNA 万宁市 18/0(0) 18S rRNA 五指山市 12/0(0) 18S rRNA 三亚市 34/7(20.6) 18S rRNA 乐东黎族自治县 16/3(18.8) 18S rRNA 陵水黎族自治县 4/0(0) 18S rRNA 白沙黎族自治县 11/0(0) 18S rRNA 昌江黎族自治县 15/1(6.7) 18S rRNA 东方市 21/0(0) 18S rRNA 屯昌县 30/10(33.3) 18S rRNA 总数 1039/135(13.0) -
[1] SMITH T G. The genus Hepatozoon (Apicomplexa: Adeleina) [J]. The Journal of Parasitology, 1996, 82(4): 565 − 585. doi: 10.2307/3283781 [2] BANETH G. Perspectives on canine and feline hepatozoonosis [J]. Veterinary Parasitology, 2011, 181(1): 3 − 11. doi: 10.1016/j.vetpar.2011.04.015 [3] SCHäFER I, KOHN B, VOLKMANN M, et al. Retrospective evaluation of vector-borne pathogens in cats living in Germany (2012–2020) [J]. Parasites & Vectors, 2021, 14(1): 1 − 9. [4] JAMES S. A new Leucocytozoon of dogs [J]. British Medical Journal, 1905, 1(2320): 1361. [5] VINCENT-JOHNSON N A, MACINTIRE D K, LINDSAY D S, et al. A new Hepatozoon species from dogs: description of the causative agent of canine hepatozoonosis in North America [J]. The Journal of Parasitology, 1997, 83(6): 1165 − 1172. doi: 10.2307/3284379 [6] BANETH G, SAMISH M, ALEKSEEV E, et al. Transmission of Hepatozoon canis to dogs by naturally-fed or percutaneously-injected Rhipicephalus sanguineus ticks [J]. Journal of Parasitology, 2001, 87(3): 606 − 611. doi: 10.1645/0022-3395(2001)087[0606:TOHCTD]2.0.CO;2 [7] OTRANTO D, DANTAS-TORRES F, WEIGL S, et al. Diagnosis of Hepatozoon canis in young dogs by cytology and PCR [J]. Parasites & Vectors, 2011, 4(1): 1 − 6. [8] JOHNSON E M, ALLEN K E, PANCIERA R J, et al. Experimental transmission of Hepatozoon americanum to New Zealand white rabbits (Oryctolagus cuniculus) and infectivity of cystozoites for a dog [J]. Veterinary Parasitology, 2009, 164(2/3/4): 162 − 166. doi: 10.1016/j.vetpar.2009.05.028 [9] ALLEN K E, LI Y, KALTENBOECK B, et al. Diversity of Hepatozoon species in naturally infected dogs in the southern United States [J]. Veterinary Parasitology, 2008, 154(3/4): 220 − 225. doi: 10.1016/j.vetpar.2008.03.027 [10] CORONA B, DÍAZ-SÁNCHEZ A, MELI M, et al. Occurrence of tick-borne pathogens in stray dogs from Havana, Cuba [J]. Acta Biomedica Scientifica, 2018, 3(4): 158 − 159. doi: 10.29413/ABS.2018-3.4.25 [11] BANETH G, SAMISH M, SHKAP V. Life cycle of Hepatozoon canis (Apicomplexa: Adeleorina: Hepatozoidae) in the tick Rhipicephalus sanguineus and domestic dog (Canis familiaris) [J]. Journal of Parasitology, 2007, 93(2): 283 − 299. doi: 10.1645/GE-494R.1 [12] GIANNELLI A, LIA R P, ANNOSCIA G, et al. Rhipicephalus turanicus, a new vector of Hepatozoon canis [J]. Parasitology, 2017, 144(6): 730 − 737. doi: 10.1017/S003118201600250X [13] DE MIRANDA R L, DE CASTRO J R, OLEGÁRIO M M M, et al. Oocysts of Hepatozoon canis in Rhipicephalus (Boophilus) microplus collected from a naturally infected dog [J]. Veterinary Parasitology, 2011, 177(3/4): 392 − 396. doi: 10.1016/j.vetpar.2011.01.044 [14] FORLANO M, SCOFIELD A, ELISEI C, et al. Diagnosis of Hepatozoon spp. in Amblyomma ovale and its experimental transmission in domestic dogs in Brazil [J]. Veterinary Parasitology, 2005, 134(1/2): 1 − 7. doi: 10.1016/j.vetpar.2005.05.066 [15] MURATA T, INOUE M, TAURA Y, et al. Detection of Hepatozoon canis oocyst from ticks collected from the infected dogs [J]. Journal of Veterinary Medical Science, 1995, 57(1): 111 − 112. doi: 10.1292/jvms.57.111 [16] GABRIELLI S, KUMLIEN S, CALDERINI P, et al. The first report of Hepatozoon canis identified in Vulpes vulpes and ticks from Italy [J]. Vector-borne and Zoonotic Diseases, 2010, 10(9): 855 − 859. doi: 10.1089/vbz.2009.0182 [17] HORNOK S, TÁNCZOS B, DE MERA I G F, et al. High prevalence of Hepatozoon-infection among shepherd dogs in a region considered to be free of Rhipicephalus sanguineus [J]. Veterinary Parasitology, 2013, 196(1/2): 189 − 193. doi: 10.1016/j.vetpar.2013.02.009 [18] DA SILVA M R L, FORNAZARI F, DE CASTRO DEMONER L, et al. Didelphis albiventris naturally infected with Hepatozoon canis in southeastern Brazil [J]. Ticks and Tick-borne Diseases, 2017, 8(6): 878 − 881. doi: 10.1016/j.ttbdis.2017.07.005 [19] FARKAS R, SOLYMOSI N, TAKÁCS N, et al. First molecular evidence of Hepatozoon canis infection in red foxes and golden jackals from Hungary [J]. Parasites & Vectors, 2014, 7(1): 1 − 7. [20] GIANNELLI A, LATROFA M S, NACHUM-BIALA Y, et al. Three different Hepatozoon species in domestic cats from southern Italy [J]. Ticks and Tick-borne Diseases, 2017, 8(5): 721 − 724. doi: 10.1016/j.ttbdis.2017.05.005 [21] JARQUÍN-DÍAZ V H, BARBACHANO-GUERRERO A, MALDONADO-RODRÍGUEZ R, et al. First molecular evidence of Hepatozoon canis in domestic dogs and ticks in fragmented rainforest areas in Mexico [J]. Veterinary Parasitology:Regional Studies and Reports, 2016, 6: 4 − 8. doi: 10.1016/j.vprsr.2016.11.001 [22] NAJM N-A, MEYER-KAYSER E, HOFFMANN L, et al. Hepatozoon canis in German red foxes (Vulpes vulpes) and their ticks: molecular characterization and the phylogenetic relationship to other Hepatozoon spp [J]. Parasitology Research, 2014, 113(7): 2679 − 2685. doi: 10.1007/s00436-014-3923-8 [23] BANETH G, MATHEW J S, SHKAP V, et al. Canine hepatozoonosis: two disease syndromes caused by separate Hepatozoon spp [J]. Trends in Parasitology, 2003, 19(1): 27 − 31. doi: 10.1016/S1471-4922(02)00016-8 [24] IBRAHIM N D, RAHAMATHULLA P M, NJOKU C O. Neutrophil myeloperoxidase deficiency associated with canine hepatozoonosis [J]. International Journal for Parasitology, 1989, 19(8): 915 − 918. doi: 10.1016/0020-7519(89)90119-7 [25] GONEN L, STRAUSS-AYALI D, SHKAP V, et al. An enzyme-linked immunosorbent assay for antibodies to Hepatozoon canis [J]. Veterinary Parasitology, 2004, 122(2): 131 − 139. doi: 10.1016/j.vetpar.2004.03.021 [26] VOJTA L, MRLJAK V, ĆURKOVIĆ S, et al. Molecular epizootiology of canine hepatozoonosis in Croatia [J]. International Journal for Parasitology, 2009, 39(10): 1129 − 1136. doi: 10.1016/j.ijpara.2009.02.007 [27] EZEOKOLI C, OGUNKOYA A, ABDULLAHI R, et al. Clinical and epidemiological studies on canine hepatozoonosis in Zaria, Nigeria [J]. Journal of Small Animal Practice, 1983, 24(7): 455 − 460. doi: 10.1111/j.1748-5827.1983.tb00385.x [28] MCCULLY R, BASSON P, BIGALKE R, et al. Observations on naturally acquired hepatozoonosis of wild carnivores and dogs in the Republic of South Africa [J]. The Onderstepoort Journal of Veterinary Research, 1975, 42(4): 117 − 133. [29] O’DWYER L H, MASSARD C L, DE SOUZA J C P. Hepatozoon canis infection associated with dog ticks of rural areas of Rio de Janeiro State, Brazil [J]. Veterinary Parasitology, 2001, 94(3): 143 − 150. doi: 10.1016/S0304-4017(00)00378-2 [30] RAJAMANICKAM C, WIESENHUTTER E, ZIN F M, et al. The incidence of canine haematozoa in Peninsular Malaysia [J]. Veterinary Parasitology, 1985, 17(2): 151 − 157. doi: 10.1016/0304-4017(85)90101-3 [31] GREAY T L, BARBOSA A D, REES R L, et al. An Australian dog diagnosed with an exotic tick-borne infection: should Australia still be considered free from Hepatozoon canis? [J]. International Journal for Parasitology, 2018, 48(11): 805 − 815. doi: 10.1016/j.ijpara.2018.05.002 [32] INPANKAEW T, HII S F, CHIMNOI W, et al. Canine vector-borne pathogens in semi-domesticated dogs residing in northern Cambodia [J]. Parasites & Vectors, 2016, 9(1): 1 − 6. [33] ABD RANI P A M, IRWIN P J, COLEMAN G T, et al. A survey of canine tick-borne diseases in India [J]. Parasites & Vectors, 2011, 4(1): 1 − 8. [34] EL-DAKHLY K M, GOTO M, NOISHIKI K, et al. Prevalence and diversity of Hepatozoon canis in naturally infected dogs in Japanese islands and peninsulas [J]. Parasitology Research, 2013, 112(9): 3267 − 3274. doi: 10.1007/s00436-013-3505-1 [35] KWON S-J, KIM Y-H, OH H-H, et al. First case of canine infection with Hepatozoon canis (Apicomplexa: Haemogregarinidae) in the Republic of Korea [J]. The Korean Journal of Parasitology, 2017, 55(5): 561 − 564. doi: 10.3347/kjp.2017.55.5.561 [36] AHMAD A S, SAEED M A, RASHID I, et al. Molecular characterization of Hepatozoon canis from farm dogs in Pakistan [J]. Parasitology Research, 2018, 117(4): 1131 − 1138. doi: 10.1007/s00436-018-5790-1 [37] PIRATAE S, PIMPJONG K, VAISUSUK K, et al. Molecular detection of Ehrlichia canis, Hepatozoon canis and Babesia canis vogeli in stray dogs in Mahasarakham province, Thailand [J]. Annals of Parasitology, 2015, 61(3): 183 − 187. [38] PRAKASH B K, LOW V L, TAN T K, et al. Detection of Hepatozoon canis in the brown dog tick and domestic dogs in Peninsular Malaysia [J]. Journal of Medical Entomology, 2018, 55(5): 1346 − 1348. [39] BANETH G, BARTA J R, SHKAP V, et al. Genetic and antigenic evidence supports the separation of Hepatozoon canis and Hepatozoon americanum at the species level [J]. Journal of Clinical Microbiology, 2000, 38(3): 1298 − 1301. doi: 10.1128/JCM.38.3.1298-1301.2000 [40] 马大君, 丁晓鳞, 曹锦和, 等. 中国犬资源现状调查[J]. 中国工作犬业, 2012, 201(1): 45 − 50. [41] XU D, ZHANG J, SHI Z, et al. Molecular detection of vector-borne agents in dogs from ten provinces of China [J]. Parasites & Vectors, 2015, 8(1): 1 − 7. [42] 张羽. 南宁市本地犬三种血液原虫的初步调查与犬伊氏锥虫病例人工复制实验研究 [D]. 南宁: 广西大学, 2016. [43] 班超平. 河南部分地区三种蜱传疾病的流行病学调查 [D]. 郑州: 河南农业大学, 2019. [44] CHAO L-L, LIAO H-T, SHIH C-M. First detection and genetic identification of Hepatozoon canis in Rhipicephalus sanguineus sensu lato ticks collected from dogs of Taiwan [J]. Ticks and Tick-borne Diseases, 2019, 10(4): 929 − 934. doi: 10.1016/j.ttbdis.2019.04.020 [45] INOKUMA H, OKUDA M, OHNO K, et al. Analysis of the 18S rRNA gene sequence of a Hepatozoon detected in two Japanese dogs [J]. Veterinary Parasitology, 2002, 106(3): 265 − 271. doi: 10.1016/S0304-4017(02)00065-1 [46] NETHERLANDS E C, STROEBEL C, DU PREEZ L H, et al. Molecular confirmation of high prevalence of species of Hepatozoon infection in free-ranging African wild dogs (Lycaon pictus) in the Kruger National Park, South Africa [J]. International Journal for Parasitology: Parasites and Wildlife, 2021, 14: 335 − 340. doi: 10.1016/j.ijppaw.2021.03.002 [47] SOLANO-GALLEGO L, BANETH G. Babesiosis in dogs and cats:expanding parasitological and clinical spectra [J]. Veterinary Parasitology, 2011, 181(1): 48 − 60. doi: 10.1016/j.vetpar.2011.04.023 [48] OYAMADA M, DAVOUST B, BONI M, et al. Detection of Babesia canis rossi, B. canis vogeli, and Hepatozoon canis in dogs in a village of eastern Sudan by using a screening PCR and sequencing methodologies [J]. Clinical and Vaccine Immunology, 2005, 12(11): 1343 − 1346. doi: 10.1128/CDLI.12.11.1343-1346.2005 [49] KAMANI J, BANETH G, MUMCUOGLU K Y, et al. Molecular detection and characterization of tick-borne pathogens in dogs and ticks from Nigeria [J]. PLoS Neglected Tropical Diseases, 2013, 7(3): e2108. doi: 10.1371/journal.pntd.0002108 [50] WILLIAMS B M, BERENTSEN A, SHOCK B C, et al. Prevalence and diversity of Babesia, Hepatozoon, Ehrlichia, and Bartonella in wild and domestic carnivores from Zambia, Africa [J]. Parasitology Research, 2014, 113(3): 911 − 918. doi: 10.1007/s00436-013-3722-7 [51] CRIADO-FORNELIO A, BULING A, PINGRET J, et al. Hemoprotozoa of domestic animals in France: prevalence and molecular characterization [J]. Veterinary Parasitology, 2009, 159(1): 73 − 76. doi: 10.1016/j.vetpar.2008.10.012 [52] TABAR M D, FRANCINO O, ALTET L, et al. PCR survey of vectorborne pathogens in dogs living in and around Barcelona, an area endemic for leishmaniosis [J]. Veterinary Record, 2009, 164(4): 112 − 116. doi: 10.1136/vr.164.4.112 [53] MITKOVÁ B, HRAZDILOVÁ K, STEINBAUER V, et al. Autochthonous Hepatozoon infection in hunting dogs and foxes from the Czech Republic [J]. Parasitology Research, 2016, 115(11): 4167 − 4171. doi: 10.1007/s00436-016-5191-2 [54] DÍAZ-SÁNCHEZ A A, HOFMANN-LEHMANN R, MELI M L, et al. Molecular detection and characterization of Hepatozoon canis in stray dogs from Cuba [J]. Parasitology International, 2021, 80: 102200. doi: 10.1016/j.parint.2020.102200 [55] LI Y, WANG C, ALLEN K E, et al. Diagnosis of canine Hepatozoon spp. infection by quantitative PCR [J]. Veterinary Parasitology, 2008, 157(1/2): 50 − 58. doi: 10.1016/j.vetpar.2008.06.027 [56] RUBINI A S, PADUAN K D S, LOPES V V A, et al. Molecular and parasitological survey of Hepatozoon canis (Apicomplexa: Hepatozoidae) in dogs from rural area of Sao Paulo state, Brazil [J]. Parasitology research, 2008, 102(5): 895 − 899. doi: 10.1007/s00436-007-0846-7 [57] 张兆霞, 张海霞, 刘洋, 等. 犬肝簇虫感染的病例报告[J]. 中国兽医杂志, 2018, 54(3): 45 − 46. [58] 谢静匀, 沈丽婵. 一例疑似犬肝簇虫病的诊断报告[J]. 中国畜牧兽医文摘, 2016(1): 206 − 207. [59] DE MIRANDA R, O'DWYER L, DE CASTRO J, et al. Prevalence and molecular characterization of Hepatozoon canis in dogs from urban and rural areas in Southeast Brazil [J]. Research in Veterinary Science, 2014, 97(2): 325 − 328. doi: 10.1016/j.rvsc.2014.06.015 -



 下载:
下载: