-
内生真菌是一类生活在植物组织内,但不会引起任何外在疾病迹象的真菌[1]。它能促进植物生长、提高植物抗病防御[2-4],对农业及自然生态系统的可持续管理有重要意义[5]。植物的根与叶均是内生真菌生活的热点场所。热带森林中几乎所有的叶都受到内生真菌侵染[6],单株植物的叶内生真菌达30多种[7],而单片叶的内生真菌甚至达20种以上[2]。除叶内生真菌外,植物的根普遍与真菌形成菌根,能提高养分的有效性和促进植物的耐胁迫能力。外生菌根与丛枝菌根是自然界最常见的菌根类型,外生菌根主要长生在壳斗科、松科植物上,真菌主要为担子菌和少量子囊菌;而丛枝菌根涉及80%以上的陆生植物,真菌主要为球囊菌门[8]。在功能上,外生菌根真菌促进植物吸收氮,而丛枝菌根真菌促进植物吸收磷。不同菌根类型在生态生理上的差异,会改变植物营养和土壤过程[9]。植物的菌根类型会影响叶内生真菌群落,如丛枝菌根真菌影响叶内养分的有效性,进而影响叶内生真菌群落组成[10];菌根真菌与叶内生真菌的互作影响植物抗虫性[11]。比较同一片森林中外生菌根植物和丛枝菌根植物的叶内生真菌群落可以更好地理解2种类型植物与真菌之间的相互作用和生态功能。海南岛位于我国南部,地处热带北缘,同时具有热带和亚热带的气候特征,物种丰富,被誉为“热带北缘生物物种基因库”。海南岛的热带山地雨林是当前保存最完整、面积最大的热带森林,具有面积广、物种多样性高、层次结构复杂等特点,是具有国家和国际意义的生物多样性保护的热点地区的代表性植被类型[12-13]。在海南岛尖峰岭的热带山地雨林中,樟科植物重要值最高,其次是壳斗科植物[13],二者的根部真菌群落分别以丛枝菌根真菌和外生菌根真菌为主[14]。目前,基于微生物DNA条形码(如真菌ITS、细菌16S rRNA基因)的高通量测序技术,相对于依赖培养的一代测序技术,具有检测更全面、通量更高的特点,能在短期内高效地获得海量的微生物DNA序列,越来越多地被应用于揭示环境微生物群落的多样性和复杂性[15]。本研究拟采用高通量测序技术检测尖峰岭热带山地雨林樟科和壳斗科植物的叶内生真菌群落的物种组成,旨在揭示植物身份与叶性状在叶内生真菌群落构建中的作用。
-
研究样地位于海南岛西南部尖峰岭国家级自然保护区内(18°23′—18°50′N,108°36′—109° 05′E),地跨乐东、东方两市县。受到热带季风气候的影响,该区域干湿季节明显,干季为11月—翌年4月,湿季为5—10月,年均降雨量介于1 305~3 686 mm之间,属低纬度热带岛屿季风气候。保护区内森林植被类型丰富,植被垂直分布带谱明显,由海边至山顶形成了8个主要植被类型: 包括滨海有刺灌丛、热带稀树草原(或称稀树灌丛)、热带半落叶季雨林、热带常绿季雨林、热带北缘沟谷雨林、热带山地雨林、热带山地常绿阔叶林等,其中热带山地雨林是尖峰岭地区面积最大、物种组成多样的植被类型,它主要分布在海拔600~1200 m的山地丘陵,砖黄壤-黄壤是其主要的土壤类型[13, 16]。样地历年每木调查共记录到439 676株存活的胸径≥ 1.0 cm的乔灌木植株,植株密度为0.732 8株·m−2,在20 m × 20 m尺度上单位面积物种数量平均为80种[13]。在本研究中抽到的样方内,按物种丰富度、相对频度、植株平均胸径三者平均值排序,优势度最高的两科为樟科、壳斗科。
-
样品采集地为中国林业科学研究院尖峰岭森林生物多样性动态监测大样地,简称大样地。样地总面积60 hm2(600 m × 1000 m),西南角原点坐标18°43′41.0″N,108°53′59.6″E,海拔870 m。首先,沿大样地长边将样地划分为2个300 m × 1000 m的子样地。然后,在南侧子样地设置100 m × 100 m的网格,并从中随机抽取5个网格用于叶片采集。在抽取的网格内,随机设置1个20 m × 40 m的样方。接着,先找到样方内樟科和壳斗科植物位置,再使用加长高枝剪从目标植物树冠剪取3条树枝(这些树枝沿水平方向间隔约 120°),同时记录枝条的采样高度。从剪切下的枝条摘取成熟、健康的叶片,并将它们分别装在3个塑料封口袋中。将袋子置于冷藏箱中转运至实验室,并在−20 ℃保存,以备后续DNA提取。
-
测定了10个叶性状,包括叶面积(LA)、鲜质量(FW)、干质量(DW)、比叶面积(SLA)、叶干物质含量(LDMC)、叶含水量(LWC)、叶氮含量(LNC)、叶磷含量(LPC)、叶钾含量(LKC)、叶钙含量(LCaC),这些性状可能会对内生真菌群落组成产生潜在影响[17-18]。其中,氮、磷、钾、钙含量分别用凯氏定氮蒸馏法、钼锑抗比色法、火焰光度法、原子吸收分光光度法进行测定。
-
从每株树的3个枝条分别选取成熟、健康的叶48片,用直径为3 mm打孔器在每片叶的中脉左侧或右侧打孔。将打下的叶片组织块放入2 mL离心管中。先加入400 µLddH2O和1∶1000的表面活性剂Tween 20进行混合,然后放在200 r·min−1的摇床上摇晃15 min。接着用400 µL75%乙醇将样本浸泡并摇晃5 min,最后用800 µL无菌水洗涤5次,每次放入涡旋振荡器中振荡30 s[19-20]。然后每管加入100 µLCTAB提取缓冲液及2颗直径4 mm的钢珠,并放置于−20 ℃的冰箱中冷冻过夜。取出样本后,使用高通量多样品组织研磨仪粉碎叶组织,直到其被充分粉碎为糊状物。接下来,采用改良的CTAB法提取DNA[14]。将同株叶的DNA混合成1个样品,然后委托上海生工生物工程有限公司在Illumina Miseq测序平台下使用引物ITS3F和ITS4R对样品真菌ITS rDNA基因的ITS2区进行测序,再将序列划分为可操作单元(Operational Taxonomic Units,OTUs)。
-
首先将数据整理成植株为行,叶内生真菌为列的植物-真菌矩阵,矩阵中的各单元格为对应的真菌序列数,将矩阵中小于2的值全部转换成0,以去除稀有种和减少PCR扩增或测序过程中引入的错误。然后,绘制稀释曲线以评估本次实验采集的样本大小是否充足;计算每株植物的叶内生真菌Shannon-Wiener、Simpson多样性指数;用Kruskal-Wallis test分析宿主植物对叶内生真菌物种丰富度和多样性指数的影响。使用非度量多维尺度可视化樟科、壳斗科植物叶内生真菌的群落组成差异。进一步采用偏冗余分析法检验宿主身份(哑元变量)、叶性状(数值型变量)对叶内生真菌群落是否有显著影响,以及影响的大小;同时也将单个的叶性状拟合到偏冗余分析的排序图上,以确定各个性状的影响大小。所有分析均在R中进行,用到的软件包为vegan 2.5-7。
-
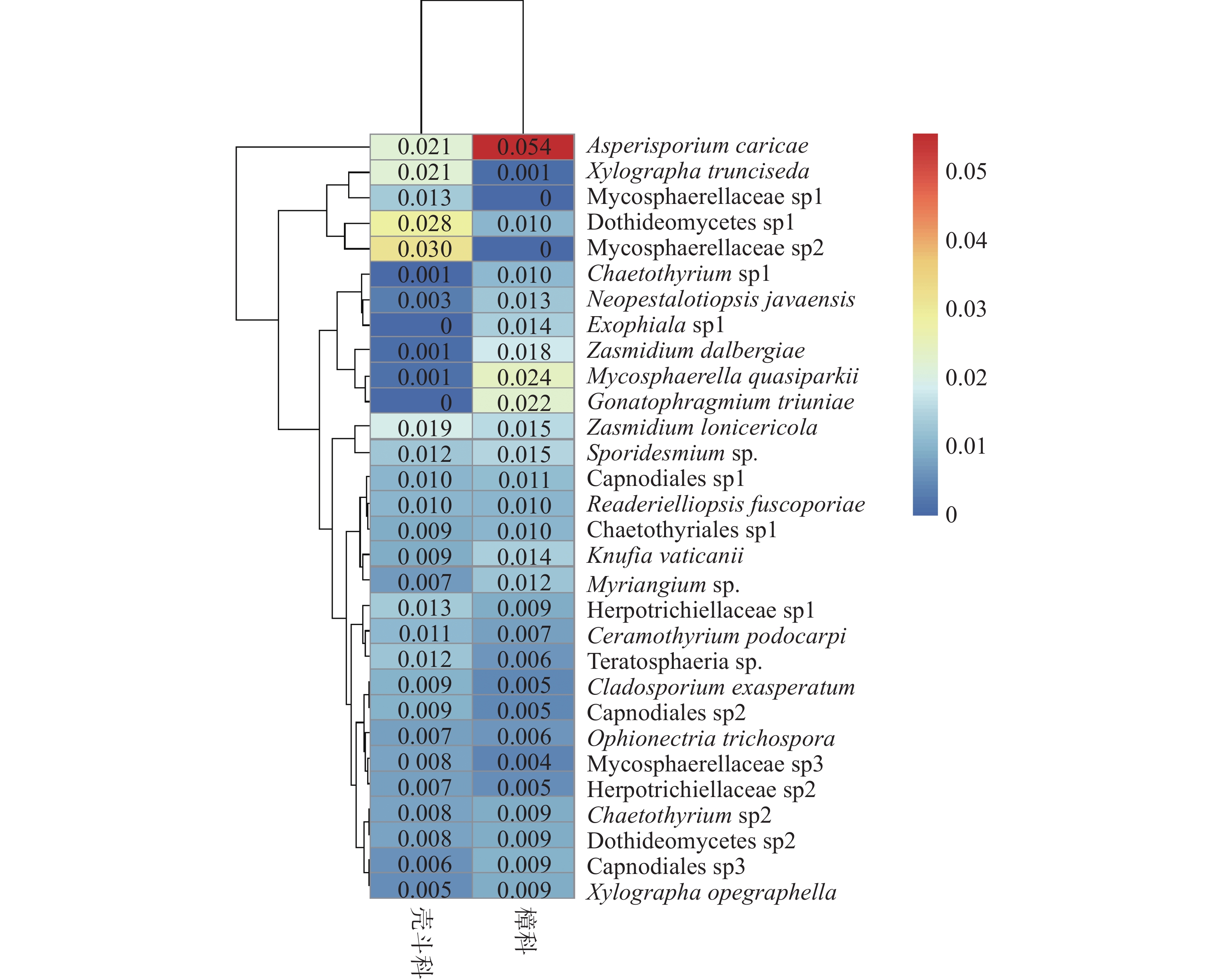
对热带山地雨林中的樟科(26株)和壳斗科(22株)2科植物进行了叶内生真菌ITS2区的高通量测序,共获得叶内生真菌序列数1 557 620 ,经过序列分析被划分为6 805个OTUs。去除仅序列数为1的OTUs后,叶内生真菌序列数仍有1 539 567,5 471个OTUs,每株达( 690 ± 31)个OTUs。这些叶内生真菌隶属于8门、30纲、108目、281科、892属。樟科与壳斗科植物获得叶内生真菌序列数分别为991 193和548 374,平均每株分别为38122 ± 1785和24926 ± 3250 。在樟科与壳斗科中,序列数占比例最高的均是子囊菌门Ascomycota,分别达95.7%和92.4%,其次是担子菌门Basidiomycota,分别占2.5%和3.9%,尚有其他类群的真菌分别占1.8%和3.7%。在纲级分类单元,樟科与壳斗科植物的叶内生真菌序列数占比较高的前四类真菌分别为Dothideomycetes(56.4%和49.5%)、Eurotiomycetes(22.5%和19.3%)、Sordariomycetes (10.4%和11.0%)、Lecanoromycetes (3.2%和8.8%)。此外,Leotiomycetes在樟科植物中占比较高(2.3%),而Agaricomycetes在壳斗科植物中较多(2.2%)。在樟科植物中,序列数占比最高的前5种真菌是Asperisporium caricae(5.4%)、Mycosphaerella quasiparkii(2.4%)、Gonatophragmium triuniae (2.2%)、Zasmidium dalbergiae(1.8%)、Zasmidium lonicericola和Sporidesmium sp.(1.5%),而壳斗科植物中Mycosphaerellaceae sp2(3.0%)、Dothideomycetes sp1(2.8%)、Xylographa trunciseda和Asperisporium caricae(2.1%)、Zasmidium lonicericola (1.9%)较多(图1)。
稀释曲线显示,随着测序深度的增加,真菌物种数增加的趋势变平缓(图2-a),表明当前测序深度能够较为全面地反映热带山地雨林壳斗科、樟科的叶内真菌多样性。整体上单株植物的叶内生真菌达(690 ± 31)个 OTUs,并且樟科植物单株叶内生真菌物种丰富度达(805 ± 32 )个OTUs,显著高于壳斗科植物的(554 ± 41)个 OTUs(Kruskal-Wallis test: χ2 = 16.283,P = 0.000)(图2-b);而Shannon-Wiener多样性指数和Simpson多样性指数在两科植物间均无显著差异(χ2 = 1.028,P = 0.311;χ2 = 0.035,P = 0.852)。
-
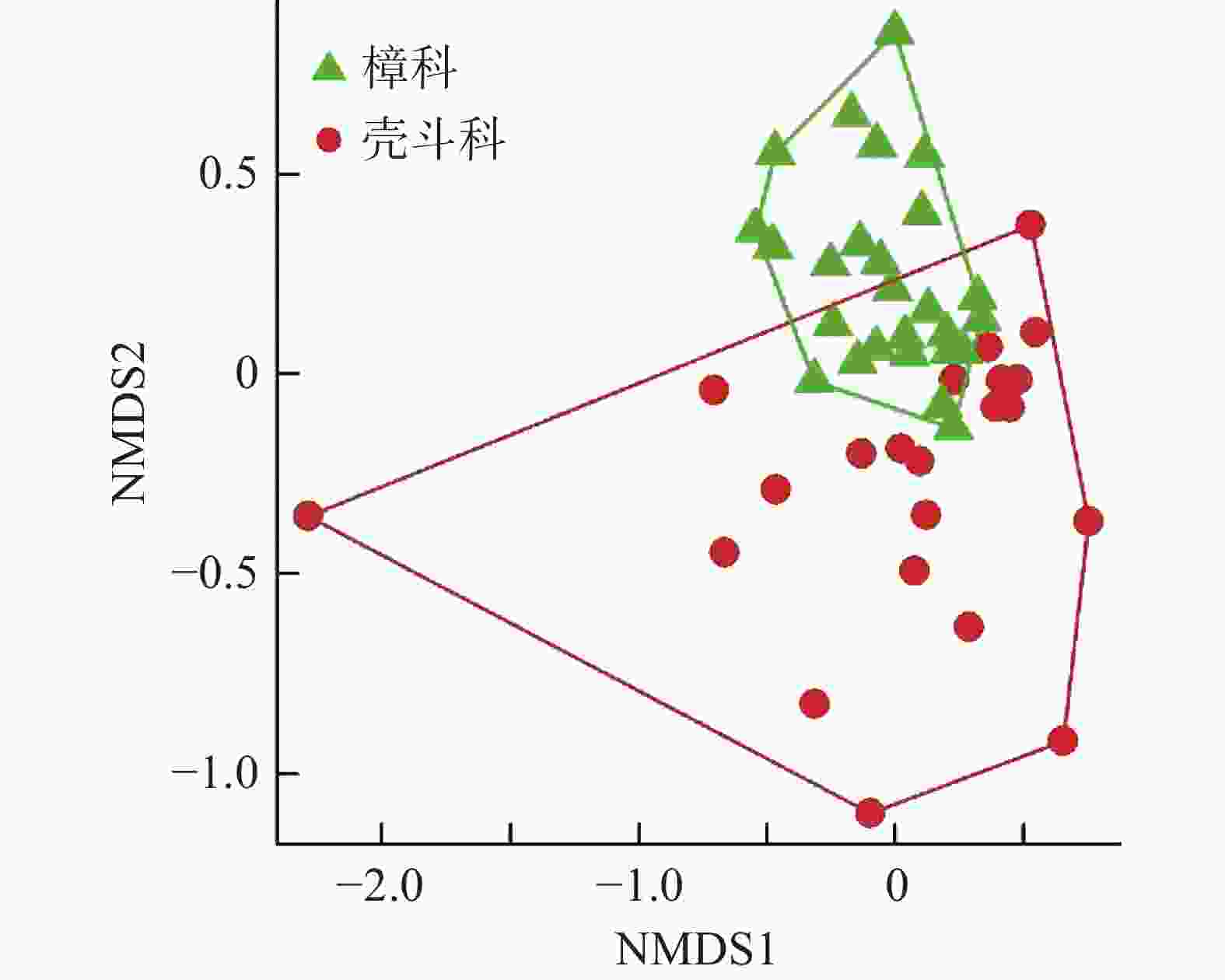
在群落水平,非度量多维尺度分析(NMDS)的结果(图3)显示壳斗科和樟科的叶内生真菌群落组成显著不同(stress = 0.188)。偏冗余分析(RDA)结果表明,宿主科、属、种身份对叶内生真菌群落组成有显著的影响(P = 0.001),去除植物性状的效应后,宿主科、属、种身份能独立解释叶内生真菌群落物种组成的变异量分别达2.9%、15.7%、33.7%。取样高度对叶内生真菌群落组成没有影响(R2 = 0.026,P = 0.081)。
偏冗余分析结果表明,叶性状对叶内生真菌群落结构也有显著影响(R2 = 0.213,P = 0.003)。各性状拟合排序轴的结果表明,对叶内生真菌群落影响程度由高到低依次为:叶钙含量(R2 = 0.572,P = 0.001),比叶面积(R2 = 0.413,P = 0.001),叶氮含量(R2 = 0.181,P = 0.008)和叶钾含量(R2 = 0.137,P = 0.031)(表1)。
表 1 叶性状解释叶内生真菌群落组成的变异量
RDA1 RDA2 R2 P 叶钙含量(LCaC) 0.748 −0.664 0.572 0.001*** 比叶面积(SLA) −0.747 −0.665 0.413 0.001*** 叶氮含量(LNC) −0.763 −0.646 0.181 0.008 ** 叶钾含量(LKC) −0.198 −0.980 0.137 0.031 * 叶磷含量(LPC) −0.724 −0.690 0.0912 0.122 叶面积(LA) −0.472 −0.882 0.060 0.237 鲜质量(FW) −0.196 −0.981 0.012 0.780 叶含水量(LWC) 0.673 −0.740 0.0002 0.995 干质量(DW) 0.315 −0.949 0.018 0.663 叶干物质含量(LDMC) 0.186 0.983 0.009 0.836 注:*:P<0.05,**:P<0.01,***:P<0.001。 -
海南尖峰岭热带山地雨林叶内生真菌的物种丰富度极高,从樟科和壳斗科的48株植物中检测到叶内生真菌达5471 OTUs。单株植物叶内生真菌达(690 ± 31)个 OTUs,远高于目前已有报道的世界其他地区的森林木本植物单株叶内生真菌多样性水平(10 ~ 260种)[1, 7, 20-23],依赖于培养的传统检测方法揭示的单株叶内生菌物种丰富度范围更低至3 ~ 48种[2, 24-25]。不同的采样策略是导致叶内生真菌多样性观察值差异的重要因素之一。如智利温带雨林的优势树种,每株取3片完整叶,单株叶内生真菌物种丰富度为(263 ± 37.6 )个OTUs[22];台湾亚热带山地雨林中的壳斗科、茜草科等10种植物,每株取3片叶,每片叶取样1 cm2,单株叶内生真菌的丰富度最高仅达44 个OTUs[23]。本研究中,每株树采集144片叶,每片叶取样约0.07 cm2,这高度异质性的取样策略可能是单株植物叶内生真菌远高于同类研究的主要原因之一。尖峰岭热带山地雨林的丛枝菌根的樟科植物单株叶内生真菌物种数达(805 ± 32 )个OTUs,显著高于外生菌根的壳斗科植物(554 ± 41)个 OTUs)。樟科植物的叶内生真菌相对较高的α多样性可能是与樟科的AM与壳斗科植物的ECM属性有关。在功能上,外生菌根真菌促进植物吸收氮,而丛枝菌根真菌促进植物吸收磷。不同菌根类型在生态生理上的差异,会改变植物营养和土壤过程[9]。如丛枝菌根真菌影响菊科植物丝路蓟的叶内养分的有效性,进而影响叶内生真菌群落组成[10]。喜马拉雅凤仙花的菌根真菌与叶内生真菌的互作影响其抗虫性[11]。
尖峰岭热带山地雨林中樟科和壳斗科的叶内生真菌群落均以子囊菌门真菌为主。在纲级分类单元,两科植物的优势类群均为Dothideomycetes、Eurotiomycetes、Sordariomycetes、Lecanoromycetes。这些真菌均被普遍报道为木本植物的内生真菌,是亚热带山地雨林[23]、热带红树林[20]、温带雨林[22]中木本植物叶内生真菌群落的常见类群。如本研究中最常见的类群Dothideomycetes,物种丰富度和序列数均最高,也是地球上其他植被类型中报道最广泛、物种最丰富的类群。Dothideomycetes具有多样性的生态功能,通常能为宿主植物分解碳水化合物,对生态系统健康和碳循环有重要作用[26];叶片通常会直接暴露于强太阳辐射之下,研究发现Dothideomycetes类群中的真菌(如Capnodiales spp.)能产生黑色素,有利于提高真菌耐受紫外线辐射的能力[27-28]。相似地,本研究中第二常见的真菌类群Eurotiomycetes,也被报道为可黑色素化真菌,对于提高真菌紫外线耐受性有重要意义[27, 29]。Sordariomycetes真菌作为本研究的第三大类叶内生真菌,也是被高频报道的叶内生真菌,且与纤维素和木质素降解密切相关[30]。
-
植物内生菌群落的构建中,决定群落组成和结构的因素可以分为两大类:确定性和随机性。确定性因素是直接影响植物内生菌群落物种组成与数量的生物与非生物环境因素。非生物确定性因素包括环境条件,如温度,湿度和土壤养分,这些因素可以影响内生真菌的生长和存活[31-32]。生物确定性因素包括宿主植物身份[23, 25, 33],以及宿主植物性状,如植物组织化学成分,形态和物候期[7, 22, 33-34],这些特征可以影响内生真菌的存在和活动。影响植物内生菌群落物种组成和数量的随机因素,即随机事件或过程的因素,包括偶然的定殖事件,扩散限制和生态漂移[35-36]。本研究结果表明,宿主身份对叶内生真菌的群落组成有显著影响。在其他热带和亚热带叶内生真菌群落研究中,得到了相同的结果[23, 25, 33]。例如,在台湾亚热带山地雨林中进行的一项研究表明,宿主身份能够解释叶内生菌群落物种组成的变异量达到42.0%[23];在哥斯达黎加热带森林中,6种常见附生凤梨科植物的可培养叶内生菌群落物种组成具有显著差异,叶内生菌群落物种组成变异的22.30%可以被宿主身份解释[25]。在巴布亚新几内亚雨林中,茜草科、桃金娘科等植物属和种的身份解释的可培养的叶内生真菌群落物种组成变异分别达到16.70%和32.10%[33]。因此,宿主身份是影响叶内生真菌群落组成的重要因素。
较多的报道表明叶性状是叶内生真菌群落的重要影响因子[7, 22, 33-34]。在本研究中,叶性状也是叶内生真菌群落构建的重要驱动因子。去除宿主身份的影响后,涉及的10个叶性状总体上可解释真菌群落组成的变异量达21.3%。西双版纳热带植物园46种榕属植物的叶内生真菌群落试验表明,比叶面积等11个叶性状解释了32.90%的叶内生真菌群落组成的变异量,而比叶面积状也是叶内生真菌群落构建的主要影响因素[7]。在巴拿马热带雨林中,叶氮含量等6种性状可解释其木本植物叶内生菌群落组成28.4%的变异量[34]。叶性状对可培养的叶内生真菌群落也有相似的效应,如巴布亚新几内亚低地雨林中的茜草科、桃金娘科等植物的比叶面积、叶氮含量等3个性状能解释可培养的叶内生真菌群落物种组成变异量达11.2%~22.9%,其中叶氮含量与可培养的叶内生真菌群落组成有显著的相关性[33]。智利温带雨林中优势木本植物的可培养叶内生真菌群落研究也得到类似的结果[22]。
在尖峰岭的热带山地雨林中,常见植物的叶钙含量、比叶面积、叶氮含量和叶钾含量是叶内生真菌群落构建的重要驱动因子。钙是维持细胞壁和细胞膜结构的重要元素[37-38],而细胞壁是微生物在叶片上定殖时必须克服的第一道物理保护屏障[39],因此叶钙含量的差异极可能影响叶片的防御作用,进而影响叶内生真菌的定殖和群落构建。比叶面积、叶氮含量、叶钾含量与植物的光合速率密切相关[17, 18, 40]。其中,比叶面积反映资源在光合组织与结构特征间的分配策略。通常,比叶面积大的叶片倾向于更大的光合速率,而较低的比叶面积值意味着资源优先投入叶片的“防御”和长寿叶[17, 33]。因此,比叶面积大、氮、钾含量高的叶片与具有相反特征的叶片可能会呈现出不同的内生真菌生长环境,从而导致内生真菌群落组成存在差异。由此可见,叶形态结构性状及化学计量性状均是叶内生真菌群落构建中的过滤器。
本研究采用高通量测序技术揭示了海南尖峰岭热带山地雨林优势类群的樟科和壳斗科植物的叶内生真菌群落物种多样性及组成,并进一步揭示了确定性的生物因子,如宿主身份及叶计量化学性状与解剖学性状共同影响叶内生真菌群落物种组成,尤其是叶钙含量、比叶面积、叶氮含量和叶钾含量是尖峰岭热带山地雨林内生真菌群落构建的重要驱动因子。叶内生真菌群落的构建,可能同时受到确定性和随机性因素的影响。未来的研究需要进一步阐明这些因素的相对重要性,以及真菌-真菌和真菌-植物的互作如何影响叶内生真菌群落构建。
宿主身份与叶性状对海南热带山地雨林樟科和壳斗科植物叶内生真菌群落的影响
DOI: 10.15886/j.cnki.rdswxb.20220109
 CSTR: 32425.14.j.cnki.rdswxb.20220109
CSTR: 32425.14.j.cnki.rdswxb.20220109
Effects of host identity and leaf traits on foliar endophytic fungal communities in Lauraceae and Fagaceae plants of tropical montane rainforest of Hainan Island
-
摘要: 为了揭示植物身份与叶性状在叶内生真菌群落构建中的作用,笔者使用Illumina Miseq测序平台检测海南尖峰岭热带山地雨林中优势植物叶内生真菌群落的物种组成,并探讨宿主身份和叶性状对叶内生真菌群落物种组成的影响。本研究共检测到来自8种樟科和7种壳斗科植物的叶内共生真菌的1539567条真菌的ITS2序列,并将它们划分为5471个真菌分类单元(Operational Taxonomic Units,OTUs),隶属于8个门、30个纲、108个目、281个科、892个属。其中,子囊菌是叶内生真菌的最大类群,占所测真菌序列数的94.5%。单株樟科植物叶内生真菌物种数805 ± 32OTUs显著高于壳斗科植物554 ± 41 OTUs。相比之下,Shannon-Wiener、Simpson多样性指数在两科植物的叶内生真菌间均无显著差异。偏冗余分析(partial redundancy analysis)结果表明,宿主科、属、种身份对叶内生真菌群落组成有显著的影响。去除植物性状的效应后,宿主科、属、种身份能够独立解释叶内生真菌群落物种组成的变异量分别达到2.9%、15.7%、33.7%。去除宿主植物身份的影响后,叶性状在总体上能够解释叶内生真菌群落物种组成的变异量达到21.3%(P = 0.003),其中,叶钙含量、比叶面积、叶氮含量和叶钾含量对叶内生真菌群落物种组成有显著影响。上述结果表明,宿主身份和叶性状是热带山地雨林叶内生真菌群落构建的重要驱动因子。Abstract: To investigate the effects of plant identity and leaf traits on foliar endophytic fungal community assembly, the species composition of foliar endophytic fungi in dominant plants in the tropical mountain rainforest in Jianfengling, Hainan Island were determined by using the Illumina Miseq sequencing method, based on which the effects of host identity and leaf traits on the species composition of foliar endophytic fungi were explored. A total of 1,539,567 fungal ITS2 sequences were obtained from the leaves of 8 species of Lauraceae plants and 7 species of Fagaceae plants, which were classified into 5,471 Operational Taxonomic Units (OTUs) belonging to 8 phyla, 30 classes, 108 orders, 281 families, and 892 genera. Ascomycota was the largest group of the foliar endophytic fungi, accounting for 94.5% of all the fungal sequences. The number of endophytic fungal species in the leaves of individual Lauraceae plants (805 ± 32) OTUs was significantly higher than that of Fagaceae plants (554 ± 41) OTUs. However, there was no significant difference in Shannon-Wiener and Simpson diversity indices between the endophytic fungi in the leaves of the plants of these two families. Partial redundancy analysis showed that the host plant identities of family, genus and species significantly affected the composition of endophytic fungal communities. After removing the effect of plant traits, the host plant identities of family, genus, and species could independently explain 2.9%, 15.7%, and 33.7% of the variation in endophytic fungal species composition, respectively. After removing the effect of host plant identity, leaf traits could explain 21.3% of the variation in endophytic fungal species composition (P =0.003), of which leaf calcium content, specific leaf area, leaf nitrogen content, and leaf potassium content had significant effects on the composition of endophytic fungal communities. These findings suggest that host identity and leaf traits are important factors driving the assembly of endophytic fungal communities in the tropical mountain rainforest.
-
Key words:
- foliar endophytic fungi /
- tropical montane rainforest /
- diversity /
- host identity /
- leaf traits
-
表 1 叶性状解释叶内生真菌群落组成的变异量
RDA1 RDA2 R2 P 叶钙含量(LCaC) 0.748 −0.664 0.572 0.001*** 比叶面积(SLA) −0.747 −0.665 0.413 0.001*** 叶氮含量(LNC) −0.763 −0.646 0.181 0.008 ** 叶钾含量(LKC) −0.198 −0.980 0.137 0.031 * 叶磷含量(LPC) −0.724 −0.690 0.0912 0.122 叶面积(LA) −0.472 −0.882 0.060 0.237 鲜质量(FW) −0.196 −0.981 0.012 0.780 叶含水量(LWC) 0.673 −0.740 0.0002 0.995 干质量(DW) 0.315 −0.949 0.018 0.663 叶干物质含量(LDMC) 0.186 0.983 0.009 0.836 注:*:P<0.05,**:P<0.01,***:P<0.001。 -
[1] DARCY J L, SWIFT S O I, COBIAN G M, et al. Fungal communities living within leaves of native Hawaiian dicots are structured by landscape-scale variables as well as by host plants[J]. Molecular Ecology, 2020, 29(16): 3103 − 3116. [2] ARNOLD A E, MEJÍA L C, KYLLO D, et al. Fungal endophytes limit pathogen damage in a tropical tree[J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(26): 15649 − 15654. doi: 10.1073/pnas.2533483100 [3] BUSBY P E, PEAY K G, NEWCOMBE G. Common foliar fungi of Populus trichocarpa modify Melampsora rust disease severity[J]. The New Phytologist, 2016, 209(4): 1681 − 1692. doi: 10.1111/nph.13742 [4] KHAN A L, AL-HARRASI A, AL-RAWAHI A, et al. Endophytic fungi from frankincense tree improves host growth and produces extracellular enzymes and indole acetic acid[J]. PLoS One, 2016, 11(6): e0158207. doi: 10.1371/journal.pone.0158207 [5] GRABKA R, D'ENTREMONT T W, ADAMS S J, et al. Fungal endophytes and their role in agricultural plant protection against pests and pathogens[J]. Plants, 2022, 11(3): 384. [6] ARNOLD A E, MAYNARD Z, GILBERT G S. Fungal endophytes in dicotyledonous neotropical trees: patterns of abundance and diversity[J]. Mycological Research, 2001, 105(12): 1502 − 1507. doi: 10.1017/S0953756201004956 [7] LIU J, ZHAO J, WANG G, et al. Host identity and phylogeny shape the foliar endophytic fungal assemblages of Ficus [J]. Ecology and Evolution, 2019, 9(18): 10472 − 10482. doi: 10.1002/ece3.5568 [8] SMITH S E, READ D J. Mycorrhizal Symbiosis. 3rd Edition[M]. London: Academic Press, 2008 [9] TEDERSOO L, BAHRAM M. Mycorrhizal types differ in ecophysiology and alter plant nutrition and soil processes[J]. Biological Reviews, 2019, 94(5): 1857 − 1880. doi: 10.1111/brv.12538 [10] ESCHEN R, HUNT S, MYKURA C, et al. The foliar endophytic fungal community composition in Cirsium arvense is affected by mycorrhizal colonization and soil nutrient content[J]. Fungal Biology, 2010, 114(11/12): 991 − 998. doi: 10.1016/j.funbio.2010.09.009 [11] RAZAK N A, GANGE A C. Multitrophic interactions between arbuscular mycorrhizal fungi, foliar endophytic fungi and aphids[J]. Microbial Ecology, 2023, 85(1): 146 − 156. doi: 10.1007/s00248-021-01937-y [12] 方精云, 李意德, 朱彪, 等. 海南岛尖峰岭山地雨林的群落结构、物种多样性以及在世界雨林中的地位[J]. 生物多样性, 2004, 12(1): 29 − 43. doi: 10.3321/j.issn:1005-0094.2004.01.005 [13] 许涵, 李意德, 林明献, 等. 海南尖峰岭热带山地雨林 60 ha 动态监测样地群落结构特征[J]. 生物多样性, 2015, 23(2): 192 − 201. doi: 10.17520/biods.2014157 [14] 杨思琪, 张琪, 宋希强, 等. 尖峰岭热带山地雨林根部真菌—植物互作网络结构特征[J]. 生物多样性, 2019, 27(3): 314 − 326. doi: 10.17520/biods.2018339 [15] ZHOU H W, LI D F, TAM N F Y, et al. BIPES, a cost-effective high-throughput method for assessing microbial diversity[J]. The ISME Journal, 2011, 5(4): 741 − 749. doi: 10.1038/ismej.2010.160 [16] 李艳朋, 许涵, 李意德, 等. 海南尖峰岭热带山地雨林物种多样性空间分布格局的尺度效应[J]. 植物生态学报, 2016, 40(9): 861 − 870. doi: 10.17521/cjpe.2015.0400 [17] CORNELISSEN J H C, LAVOREL S, GARNIER E, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide[J]. Australian Journal of Botany, 2003, 51(4): 335. doi: 10.1071/BT02124 [18] WRIGHT I J, REICH P B, WESTOBY M, et al. The worldwide leaf economics spectrum[J]. Nature, 2004, 428(6985): 821 − 827. doi: 10.1038/nature02403 [19] CREGGER M A, VEACH A M, YANG Z K, et al. The Populus holobiont: dissecting the effects of plant niches and genotype on the microbiome[J]. Microbiome, 2018, 6(1): 31. doi: 10.1186/s40168-018-0413-8 [20] YAO H, SUN X, HE C, et al. Phyllosphere epiphytic and endophytic fungal community and network structures differ in a tropical mangrove ecosystem[J]. Microbiome, 2019, 7(1): 57. doi: 10.1186/s40168-019-0671-0 [21] QIAN X, LI H, WANG Y, et al. Leaf and root endospheres harbor lower fungal diversity and less complex fungal co-occurrence patterns than rhizosphere[J]. Frontiers in Microbiology, 2019, 10: 1015. doi: 10.3389/fmicb.2019.01015 [22] GONZÁLEZ-TEUBER M, VILO C, GUEVARA-ARAYA M J, et al. Leaf resistance traits influence endophytic fungi colonization and community composition in a South American temperate rainforest[J]. Journal of Ecology, 2020, 108(3): 1019 − 1029. [23] THOMAS D, VANDEGRIFT R, ROY B A, et al. Spatial patterns of fungal endophytes in a subtropical montane rainforest of northern Taiwan[J]. Fungal Ecology, 2019, 39: 316 − 327. doi: 10.1016/j.funeco.2018.12.012 [24] GONZÁLEZ-TEUBER M. The defensive role of foliar endophytic fungi for a South American tree[J]. AoB PLANTS, 2016, 8: plw050. doi: 10.1093/aobpla/plw050 [25] TELLEZ P H, WOODS C L, FORMEL S, et al. Relationships between foliar fungal endophyte communities and ecophysiological traits of CAM and C3 epiphytic bromeliads in a neotropical rainforest[J]. Diversity, 2020, 12(10): 378. doi: 10.3390/d12100378 [26] SHAHRTASH M, BROWN S P. Drivers of foliar fungal endophytic communities of kudzu (Pueraria montana var. lobata) in the southeast United States[J]. Diversity, 2020, 12(5): 185. doi: 10.3390/d12050185 [27] KEMBEL S W, MUELLER R C. Plant traits and taxonomy drive host associations in tropical phyllosphere fungal communities[J]. Botany, 2014, 92(4): 303 − 311. doi: 10.1139/cjb-2013-0194 [28] EGIDI E, DE HOOG G S, ISOLA D, et al. Phylogeny and taxonomy of meristematic rock-inhabiting black fungi in the Dothideomycetes based on multi-locus phylogenies[J]. Fungal Diversity, 2014, 65(1): 127 − 165. doi: 10.1007/s13225-013-0277-y [29] GEISER D M, GUEIDAN C, MIADLIKOWSKA J, et al. Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae[J]. Mycologia, 2006, 98(6): 1053 − 1064. doi: 10.1080/15572536.2006.11832633 [30] U' REN J M, MIADLIKOWSKA J, ZIMMERMAN N B, et al. Contributions of North American endophytes to the phylogeny, ecology, and taxonomy of Xylariaceae (sordariomycetes, ascomycota)[J]. Molecular Phylogeneticus and Evolution, 2016, 98: 210 − 232. doi: 10.1016/j.ympev.2016.02.010 [31] VORHOLT J A. Microbial life in the phyllosphere[J]. Nature Reviews Microbiology, 2012, 10(12): 828 − 840. doi: 10.1038/nrmicro2910 [32] STONE B W G, JACKSON C R. Seasonal patterns contribute more towards phyllosphere bacterial community structure than short-term perturbations[J]. Microbial Ecology, 2021, 81(1): 146 − 156. doi: 10.1007/s00248-020-01564-z [33] VINCENT J B, WEIBLEN G D, MAY G. Host associations and beta diversity of fungal endophyte communities in New Guinea rainforest trees[J]. Molecular Ecology, 2016, 25(3): 825 − 841. doi: 10.1111/mec.13510 [34] TELLEZ PH. Tropical plants and fungal symbionts leaf functional traits as drivers of plant-fungal interactions (Doctoral dissertation) [D]. Department of Ecology and Evolutionary Biology, Tulane University, 2019. [35] KEMBEL S W, O'CONNOR T K, ARNOLD H K, et al. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(38): 13715 − 13720. doi: 10.1073/pnas.1216057111 [36] DONALD J, ROY M, SUESCUN U, et al. A test of community assembly rules using foliar endophytes from a tropical forest canopy[J]. Journal of Ecology, 2020, 108(4): 1605 − 1616. [37] WHITE P J, BROADLEY M R. Calcium in plants[J]. Annals of Botany, 2003, 92(4): 487 − 511. doi: 10.1093/aob/mcg164 [38] HEPLER P K, WINSHIP L J. Calcium at the cell wall-cytoplast interface[J]. Journal of Integrative Plant Biology, 2010, 52(2): 147 − 160. doi: 10.1111/j.1744-7909.2010.00923.x [39] UNDERWOOD W. The plant cell wall: a dynamic barrier against pathogen invasion.[J]. Frontiers in Plant Science, 2012, 3: 85. [40] JOHNSON R, VISHWAKARMA K, HOSSEN M S, et al. Potassium in plants: growth regulation, signaling, and environmental stress tolerance[J]. Plant Physiology and Biochemistry, 2022, 172: 56 − 69. doi: 10.1016/j.plaphy.2022.01.001 -





 下载:
下载: