-
根系作为植物提供养分的“源”和消耗碳的“汇”,是地下生物量的主要组成部分[1-2],在土壤碳循环过程中发挥着关键作用[3]。在森林生态系统中,植物主要是通过根系实现地上部分和地下部分的关联,每年通过细根向土壤输送的碳和养分甚至超过了叶片[4];根系通过调节植物生长,对陆地生态系统能量和物质循环以及未来气候变化有着重要影响[5]。有研究表明,根系生物量累积贡献了全球净初级生产力的22~33%[4,6]。与木质生物量相比,植物细根直径较小,寿命相对较短,导致其在地下能够快速周转和分解,使其成为森林净初级生产力,养分循环和地下碳分配的重要贡献者[4]。因此,研究植物根系生物量不仅有助于深入了解植物群落组成与功能之间的关系,也是研究全球变化条件下陆地生态系统碳循环的重要内容[7]。
热带森林作为地球上生产力最高的生态系统之一,在全球森林土壤碳库及气候变化中具有举足轻重的作用[8-9]。随着气候变化和人类干扰的增加,热带原始雨林和老龄林面积正在呈现出不断减少的趋势[10]。人为干扰后自然更新的次生林逐渐成为当前热带地区的主要森林植被,因而次生林在缓解气候变化和保护生物多样性方面的作用更加突出[11]。当前热带雨林次生林演替过程研究主要集中在地上生物类群,如植物功能性状[12]、种群动态[13]、物种多样性及乔木生物量等[14],而地下部分的研究则相对较少。热带低地雨林是不同于山地雨林的重要森林类型,对研究全球气候变化[15]及甘什岭地区独有的龙脑香科植物无翼坡垒(Hopea reticulate)等珍贵树种的恢复和保护具有重要意义。海南甘什岭地区热带低地雨林在20世纪80年代曾遭大面积采伐,有大面积由于人为干扰而处于不同恢复阶段的植物群落,这些退化的群落在干扰停止后又通过自然恢复向着进展演替方向发展。郝艳茹等[16]在对国内外不同演替阶段根系生物量数据的研究结果表明,在演替过程中,森林生态系统根系生物量随演替的进行而增加,这在西南山地不同恢复演替阶段的喀斯特常绿阔叶落叶林[17]、中亚热带不同演替阶段的马尾松林[18]以及美国卡罗莱纳州的河滩阔叶林[19]等研究中都有验证,但对于我国热带低地雨林不同演替阶段根系生物量变化还有待进一步研究。演替过程中植物根系生物量的动态变化对于群落恢复演替具有重要意义。随着演替的进行,甘什岭地区植物根系生物量变化及不同演替阶段根系的分布特征是区域生态保护和森林可持续管理中亟待解决的科学问题。本研究基于不同演替阶段的热带低地雨林次生演替过程,分析不同演替阶段根系生物量特征,探讨不同垂直分层状况,以期在未来全球气候变化条件下,为甘什岭热带低地雨林植物群落地下碳素分配及土壤碳库变化奠定基础,增加对热带雨林次生演替地下生态学过程的认识。
HTML
-
研究区域位于海南甘什岭自然保护区(109°37′50″~109°41′36″E,18°19′16″~18°24′12″N),属热带海洋季风气候,年平均气温25.4 ℃,冬季平均气温为20.7 ℃,夏季平均气温为28.3 ℃;旱季、雨季明显,5~10月为雨季,11月至翌年4月为旱季,年降水量约1 800 mm;土壤母质为花岗岩,以酸性红壤为主[15,20]。地表植被在20世纪80年代遭大面积砍伐,经过连续人为干扰后逐渐恢复形成以无翼坡垒为优势种的热带低地雨林[15]。根据2010—2012年的野外调查结果[21],该区域有野生维管植物177科,718属,1334种。保护区主要乔木树种有铁凌(Hopea reticulate)、海南琼楠(Beilschmiedia wangii)、黄杞(Engelhardia roxburghiana)、青梅(Vatica mangachapoi)、岭南山竹子(Garcinia oblongifolia)、长柄琼楠(Beilschmiedia longepetiolata)等, 灌木树种有刺轴榈(Licuala spinosa)、海南龙船花(Ixora hainanensis)、海南狗牙花(Ervatamia hainanensis)、桃金娘(Rhodomyrtus tomentosa)和白茶树(Koilodepas hainanense)等, 草本植物有高秆珍珠茅(Scleria elata)、乌毛蕨(Blechnum orientale)、掌叶海金沙(Lygodium longifolium)、割鸡芒(Hypolytrum nemorum)、芒萁(Dicranopteris pedate)等, 藤本植物有白藤(Calamus tetradactylus)、山鸡血藤(Millettia dielsiana)、锡叶藤(Tetracera sarmentosa)、清香藤(Jasminum lanceolarium)等[15]。
-
笔者在2019年全面调查的基础上,在甘什岭保护区选择4个不同恢复阶段[草本群落(HC)、灌木群落(SC)、40 年次生林(SF40a)和60 年次生林(SF60a)]设置样地。每个恢复阶段设置3个重复样地(灌木群落、40 年次生林和60 年次生林样地为20 m×20 m,草本样地为20 m×10 m),总面积4 200 m2。每块类型相同样地间直线距离不小于1.5 km。参照文献[22]的调查技术规范记录小样方内每株胸径(DBH)≥1 cm的乔木、藤本的种类、萌生或分枝状况、胸径和树高(表1)、生长状态。参照文献[23]测量每个植株的坐标。
样地
Quadrat plot经度
Longitude (E)纬度
Latitude(N)海拔
Altitude/m优势种群
Dominant species平均胸径
Mean diameter at breast height (DBH)/cm平均高度
Mean
height/m密度
Density / (株.·hm−2)HC1 109°40′28.00″ 18°23′20.86″ 196 芒萁 Dicranopteris pedate、
红毛草 Melinis repens0.6±0.16 HC2 109°39′23.69″ 18°22′31.13″ 229 蟛蜞菊 Sphagneticola calendulacea、飞机草 Chromolaena odorata 0.3±0.1 HC3 109°40′48.00″ 18°22′54.84″ 214 芒萁 Dicranopteris pedata 0.9±0.24 SC1 109°41′1.18″ 18°23′2.49″ 232 细叶谷木 Memecylon scutellatum、海南木犀榄
Olea hainanensis2.5±1.7 2.9±0.8 6275 SC2 109°40′20.14″ 18°22′52.42″ 228 海南木犀榄
Olea hainanensis、
银柴 Aporosa dioica2.8±2.1 2.9±0.8 5800 SC3 109°39′52.77″ 18°23′25.51″ 276 银柴 Aporosa dioica、
桃金娘 Rhodomyrtus tomentosa2.2±2.0 2.9±0.9 8425 SF40a1 109°40′32.34″ 18°23′15.05″ 206 青梅 Vatica mangachapoi、
岭南山竹子 Garcinia oblongifolia4.2±3.7 5.4±3.0 11875 SF40a2 109°39′49.41″ 18°21′58.72″ 229 乌柿 Diospyros cathayensis、青梅 Vatica mangachapoi 4.4±4.2 5.3±2.8 14250 SF40a3 109°40′48.75″ 18°22′53.52″ 215 岭南山竹子 Garcinia oblongifolia、海南木犀榄
Olea hainanensis3.9±3.6 5.2±2.4 13625 SF60a1 109°40′22.60″ 18°23′6.69″ 225 海南木犀榄 Olea hainanensis 4.4±4.6 5.8±3.7 11925 SF60a2 109°39′46.02″ 18°21′52.95″ 250 铁凌 Hopea reticulata、
青梅 Vatica mangachapoi5.3±6.4 6.1±3.5 9075 SF60a3 109°39′57.16″ 18°22′42.24″ 221 琼南柿 Diospyros howii、
青梅 Vatica mangachapoi4.2±4.3 5.6±2.7 16275 注:HC:草本群落;SC:灌木群落;SF40a:40年次生林;SF60a:60年次生林。
Note: HC, Herb community; SC, Shrub community; SF40a, Secondary forest with 40 years; SF60a, Secondary forest with 60 years.Table 1. Location and basic vegetation characteristics of tropical lowland rain forest quadrat plots at different restoration stages in Ganshiling Natural Reserve
-
本实验采用平均标准木机械布点法[17,24],根据植被调查数据计算出地上植被的平均树高、胸径和密度等指标,参照文献[17]选择树高、胸径及其密度与植被平均水平接近的群落优势种作为平均标准木。根据上下坡方向,在距离标准木50 cm处确定1、2号土柱位置,沿相同方向在标准木树冠的垂直落水线处确定3、4号土柱,每个土柱的面积为0.25 m2[17,24],分5个垂直层次(0~20 cm、20~40 cm、40~60 cm、60~80 cm和80~100 cm)采集根系样品,对于草本群落则选择4个面积为0.5 m×0.5 m能反映群落特征的草本作为标准草,去除地上部分后分层收集草本根系,不同恢复阶段共收集48个根系样品。将不同径级的根系(活根)分为细根(根径≤2 mm)和粗根(根径>2 mm)[25],在样地进行初步分级,去除死根,带回实验室后进一步详细分级装袋,清洗后置于烘箱中烘干至恒重,测定其干质量。
-
用SPSS 20.0进行实验数据的统计分析,通过对土壤各层根系生物量进行拟合,得到根系生物量随深度变化的近似函数,建立根系生物量与树高(H)、胸径(D)、胸径平方与树高的乘积(D2H)及林分密度(De)之间的多种回归方程,择优选择拟合方程。采用单因素方差分析法(One-way ANOVA)分别判断不同演替阶段及不同深度对根系生物量的影响,根系数据用Origin 2017进行作图。
1.1. 研究区概况
1.2. 典型样地的选择和调查
1.3. 根系生物量获取与估算
1.4. 数据分析
-
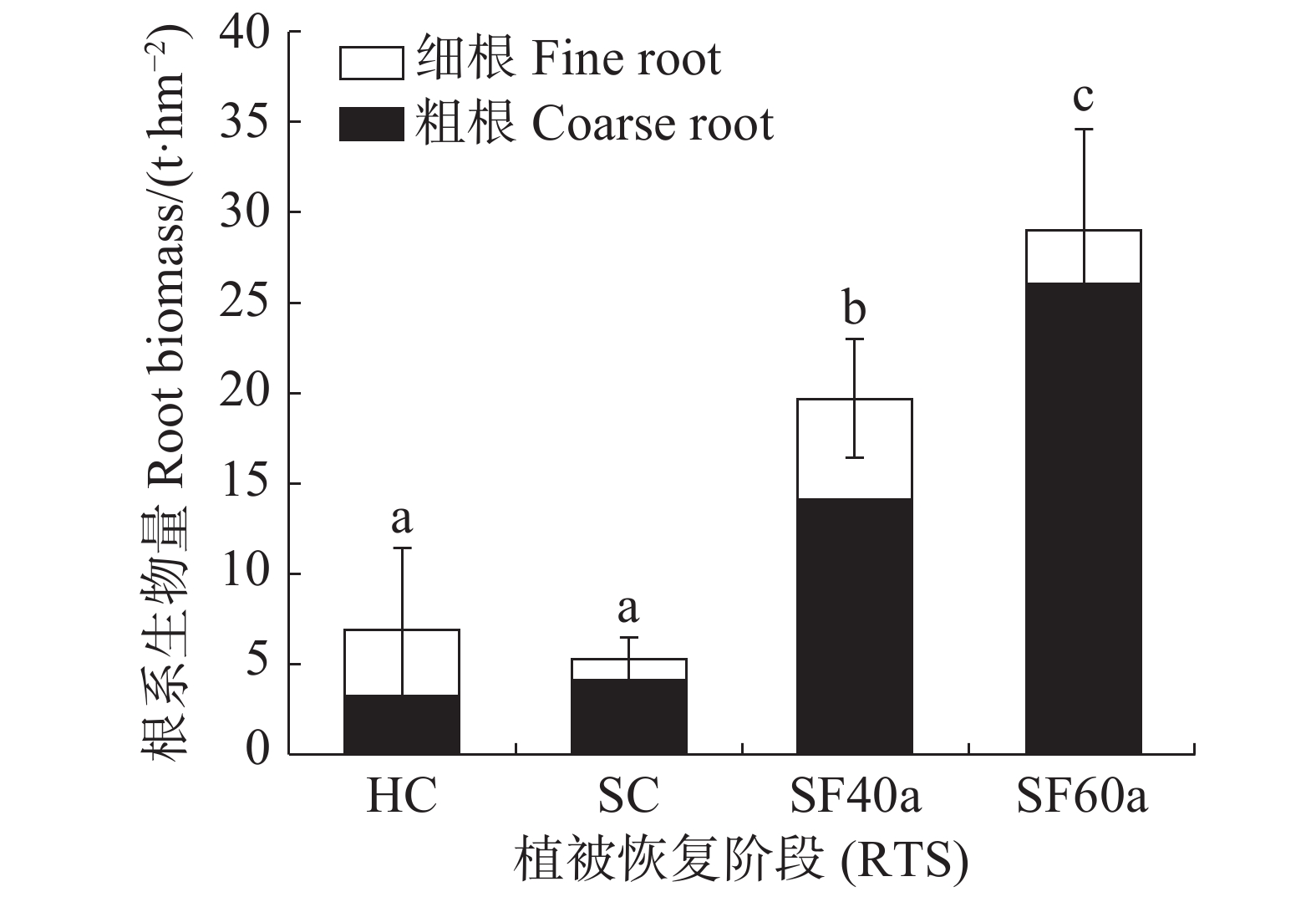
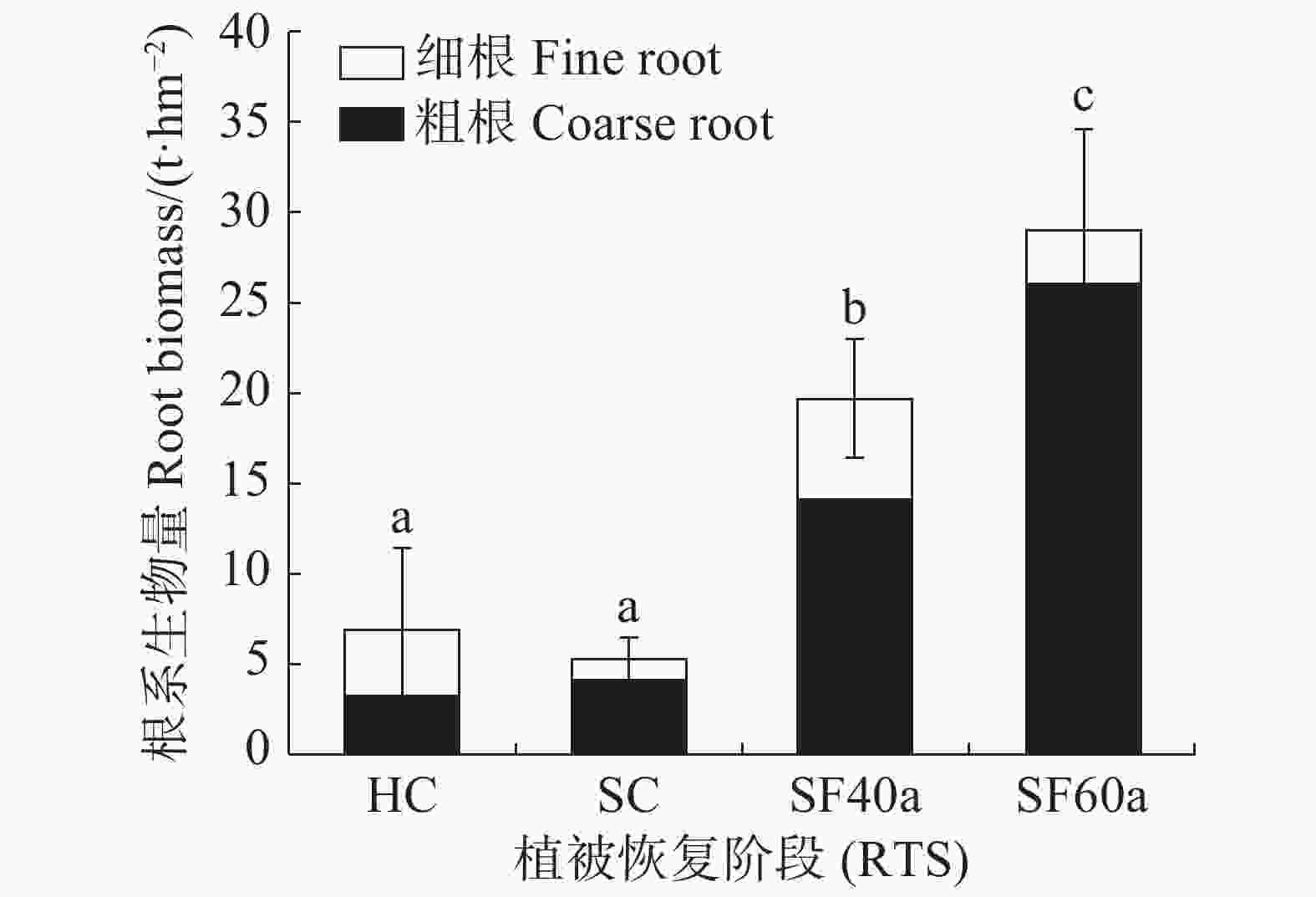
不同植被恢复阶段各径级根系生物量(图1)的方差分析结果表明,甘什岭不同演替阶段的根系总生物量随恢复进程呈显著递增趋势(P < 0.05)(草本与灌木阶段差异不显著,P > 0.05)。根系总生物量从草本群落的6.83 t·hm−2显著增加到60 年次生林群落的28.98 t·hm−2,其中60 年次生林比40 年次生林群落、灌木群落、草本群落分别高47.49%、454.13%和324.45%。粗根生物量占总根系生物量的比例随着演替阶段的进程逐渐增加(灌木到40 年次生林阶段稍有下降外);细根占总根系生物量的比例总体逐渐减少(灌木到40 年次生林阶段稍有上升)。其中草本群落根系粗细组成结构在各演替阶段中相对特殊,细根生物量大于粗根生物量,细根生物量为3.66 t·hm−2,占该阶段根系总生物量的53.53%,粗根生物量为3.17 t·hm−2,占该阶段根系总生物量的46.47%。40 a次生林以及灌木根系粗细组成结构与60 年次生林相同,特点都是粗根生物量大于细根生物量,粗根生物量分别占该阶段根系总生物量的71.41%和77.12%;在60 年次生林中,粗根生物量为26.02 t·hm−2,占60 年次生林根系总生物量的89.76%,是粗根生物量最大的演替阶段。
-
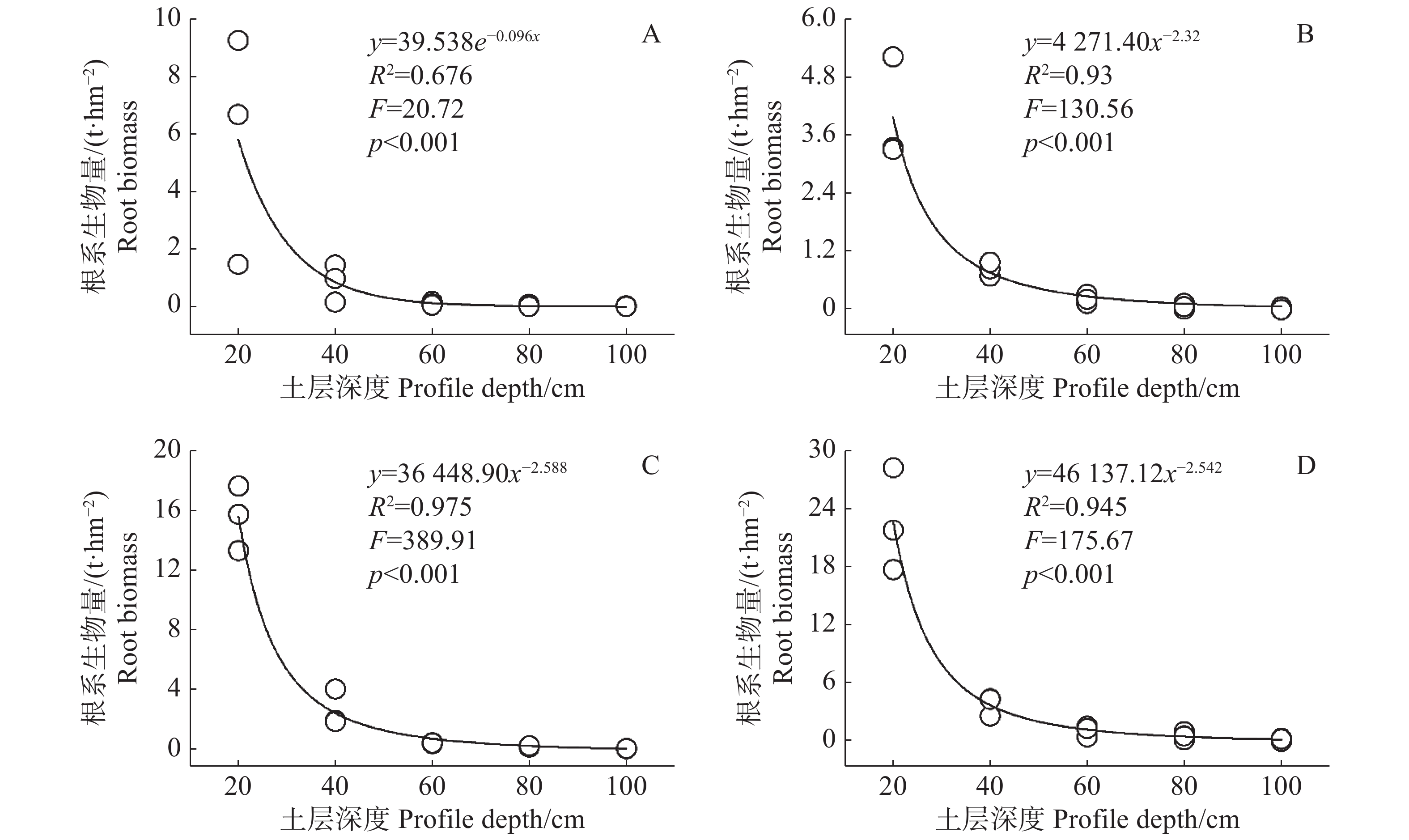
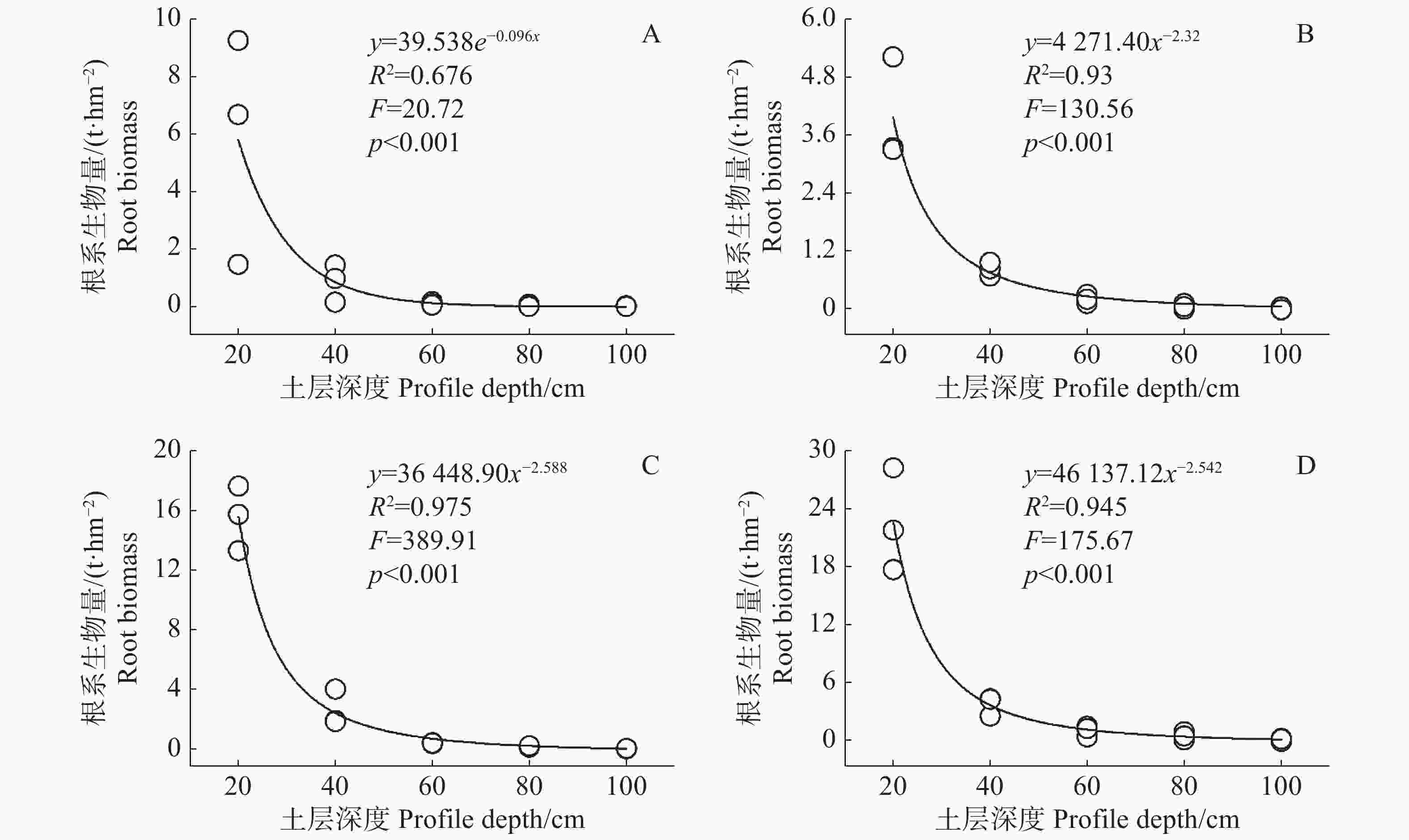
在不同演替阶段,甘什岭热带低地雨林大量根系生物量集中在土壤表层,土层深度0~20 cm的根系生物量平均达到12.03 t·hm−2,占土层深度0~100 cm平均生物量的79.30%,是根系生物量最多的层次(表2)。随着土壤深度的增加,根系生物量大幅度减少(图2),土层深度20~40 cm,根系平均生物量为2.11 t·hm−2,占土层深度0~100 cm生物量的13.91%,土层深度40~60 cm的根系平均生物量为0.56 t·hm−2,仅占土层深度0~100 cm根系生物量的3.69%。在土层深度60 cm以下的植被根系生物量大幅减少,根系生物量为0.24 t·hm−2,仅占土层深度0~100 cm土层根系生物量的1.56%。在土层深度60 cm以下的草地和灌木根系生物量之和还不到土层深度40~60 cm草地灌木根系生物量的二分之一,特别是草本群落,根系生物量已经几乎为零。利用根系生物量在土壤中的垂直分布数据拟合其随土壤深度变化的情况,发现灌木群落、40年次生林、60年次生林根系生物量变化符合幂函数曲线,草本群落根系生物量变化符合指数函数曲线(图2)。不同演替阶段根系生物量在0~60 cm土层随着深度的增加下降迅速,而在60 cm土层以下下降速度逐渐放缓且无限趋近于零。
土壤深度 Soil depth/cm 根系生物量/(t·hm−2) 草本群落 HC 灌木群落 SC 40年次生林 SF40a 60年次生林 SF60a 0~20 5.81±3.97Aa 3.97±1.09Aa 15.63±2.14Ba 22.69±5.29Ca 20~40 0.86±0.66Ab 0.86±0.14Ab 2.79±1.23Bb 3.94±1.01Bb 40~60 0.11±0.06Ab 0.25±0.09Ab 0.60±0.03Ac 1.28±0.58Bb 60~80 0.04±0.04Ab 0.10±0.06Ab 0.38±0.05NSc 0.73±0.41Bb 80~100 0.01±0.02Ab 0.05±0.03Ab 0.24±0.01Bc 0.34±0.15Bb 注:不同大写字母表明不同植被类型根系生物量在同一土层中差异显著;不同小写字母表明同一植被类型根系生物量在不同土层中差异显(P<0.05);NS, 该植被类型根系生物量在同一土层中与其他植被类型无显著差异。
Note: Different capital letters indicate that the root biomass of different vegetation types is significantly different in the same soil layer; different lowercase letters indicate that root biomass of the same vegetation type was significantly different in different soil layers (P<0.05); NS indicates the root biomass of the vegetation type is not significantly different from that of other vegetation types in the same soil layer.Table 2. Distribution of root biomass in the soil profiles under the tropical lowland rain forest in Ganshiling
-
通过分析,发现根系生物量与树高(H)、胸径(D)、胸径平方与树高的乘积(D2H)以及林分密度(De)相关系数F值均达到极显著水平(P<0.01),根系生物量分别与D、H、D2H呈幂函数关系,与D、H和D2H等三因素呈一次函数关系,其中根系生物量与H和D2H拟合效果最好,决定系数(R2)分别达到0.920和0.845(表3)。利用该模型,根据植被的胸径、树高可以来估测甘什岭热带低地雨林根系生物量。
植被因子 Vegetation factor 模型 Models R2 F P 胸径 D y=0.656D2.386 0.780 29.389 <0.001 树高 H y=0.426H2.341 0.920 93.271 <0.001 胸径平方与树高的乘积 D2H y=0.536(D2H)0.804 0.845 44.456 <0.001 胸径、树高、密度 D、H、De y=19.314+3.889D+1.566H+0.001De 0.811 12.471 <0.01 Table 3. Regression model of root biomass
2.1. 甘什岭不同恢复阶段热带低地雨林的根系生物量特征
2.2. 根系生物量在土壤剖面中的垂直分布规律
2.3. 根系生物量回归方程
-
本研究表明,甘什岭热带低地雨林不同演替阶段的根系生物量范围是5.23~28.98 t·hm−2,这与波多黎各热带低地次生林的根系生物量(6.00~22.00 t·hm−2)接近[26],但低于美洲热带低地雨林的根系生物量(36.00~68.00 t·hm−2)[27]。其中40 年次生林和60年次生林根系生物量均大于斯里兰卡热带常绿干旱落叶混交林的根系生物量(17 t·hm−2)[28]和澳大利亚昆士兰北部次生雨林的根系生物量(11.65 t·hm−2)[29],60 年次生林根系生物量与墨西哥热带干旱落叶林的根系生物量(31 t·hm−2)[30]、刚果热带雨林的根系生物量(32 t·hm−2)接近[28],但都小于巴西(69 t·hm−2)、法属圭亚那(42 t·hm−2)、非洲象牙海岸(51 t·hm−2)、苏里南(66 t·hm−2)等热带低地雨林的根系生物量[28],也低于澳大利亚昆士兰北部原始雨林的根系生物量(34.42 t·hm−2)[29]和JACKSON R B等人[31]研究的根系生物量最高的热带常绿林的根系生物量(50 t·hm−2)。甘什岭不同于其他的热带地区环境,虽降水充沛,但由于土壤粗砂高达20%,土壤储水能力低,旱季经常受到干旱胁迫,养分也容易流失,土壤肥力较差,从而导致树种生长速度缓慢,生物量相对较低,生态寿命较短。另外与林分类型、林龄、群落组成和混交度等也有关系[32-33]。
不同植被类型的根系生物量总体表现为随恢复演替的进程而增加的趋势,这与中国西南山地喀斯特常绿阔叶落叶林[17]以及美国卡罗莱纳州河滩阔叶林[19]等国内外所研究的根系生物量随演替的趋势一致。这主要是由于随着植被的恢复进程,木本植物胸径逐渐增大,高度也逐渐增高,与之相对应的植物根系生物量也随之增加。有研究表明,乔木树种根系生物量与胸径(D)或胸径的平方与高度的乘积(D2H)相关系数均达到极显著水平(P<0.01)[34]。本研究发现根系生物量与树高(H)、胸径(D)、胸径平方与树高的乘积(D2H)以及林分密度(De)相关系数均达到极显著水平(P<0.01),其中根系生物量与植被H、D、D2H等单变量存在幂函数关系,所以在演替过程中随着树木胸径及高度的增加,根系生物量也明显增加。甘什岭地区除草本到灌木阶段根系生物量有所下降且差异不显著外,其余各阶段都随恢复进程呈显著递增趋势。这主要是由于在恢复的早期阶段, 草本群落不会受到其它木本植物的影响,受到的光照、水分和热量等气象条件相对充足,草本植物在早期生长迅速,植被密度相对较大,为获取更多的营养支撑自身发展,根系也迅速生长。另外,木本植物的生长会与草本群落形成竞争,影响草本群落的发展,故导致在演替早期两种植被根系生物量不显著。
-
研究发现在甘什岭热带低地雨林不同恢复阶段植被粗根生物量为3.17~26.02 t·hm−2,而细根生物量为1.20~5.62 t·hm−2,稍低于哥斯达黎加热带常绿阔叶林细根生物量(3.69~6.62 t·hm−2)[35],且变化差异也低于哥斯达黎加热带山地森林(3~13 t·hm−2)[26]。其中40 年次生林细根生物量与Jackson R B等人研究的热带落叶林以及热带常绿林平均细根生物量(5.7 t·hm−2)[31]基本一致。灌木群落细根总生物量(1.20 t·hm−2)与马来西亚沙巴州Danum Valley地区热带低地雨林细根生物量(1.70 t·hm−2)[36]较接近。通过分析发现不同演替阶段草本群落植物根系与木本植物群落根系组成结构差异相对较大,60 a次生林粗根生物量占整个生物量的89.76%,草本群落则以细根为主,占其总生物量的53.53%。
本研究结果表明:(1)植被在不同恢复演替阶段的需求不同,各阶段根系生物量组成结构也不同。在演替早期,群落结构相对单一,光照充足,光合作用强烈,植被生长迅速,对养分和水分的需求较高,植被更倾向于分配更多的生物量给根系,尤其是对植物营养起重要作用的细根[25]。所以在演替初期,细根占主要地位,但粗根生物量一般随着树干生长而增加[37]。随着恢复演替的进行,木本植物高度和胸径都迅速增加,受外部环境的影响也随之增加,尤其面对强风的侵扰,此时树木需要大量的水分和养分供应的同时还需要相对粗大的根系来固定和支撑其地上部分生长,因此,随着恢复演替的进行,细根所占的比例也逐渐减小,粗根比例逐渐增加。本研究除灌木至40年次生林阶段细根所占比例稍有上升外,其余阶段均成下降趋势。主要是由于40年次生林阶段植被处于快速生长扩张阶段,其对养分水分的需求大于固定支撑需求,故细根生长迅速,且占比也相对较高。(2)甘什岭热带低地雨林根系生物量主要集中在土壤表层(0~20 cm),随着土壤层次的加深,根系生物量大幅度减少。本研究结果与西南山地不同恢复演替阶段的喀斯特常绿阔叶落叶林[17]、中亚热带不同演替阶段的马尾松林[18]、美洲热带低地雨林[27]等根系分布规律一致。(3)根系生物量在土层中的垂直分布存在一定的规律性,草本群落呈指数函数曲线回归方程,其余各恢复演替阶段呈幂函数曲线回归方程。随着演替的进行,根系在土层中的垂直分布逐渐加深。(4)超过80%以上的根系生物量分布于0~40 cm之间,本结果与JACKSON等[31]研究的全球各生态系统75%根系生物量发生0~30 cm之间的结果一致,且甘什岭热带低地雨林与冻土带、北方针叶林和温带草原等根系剖面最浅的植被类型相同,根系分布都主要集中于土壤表层,0~20 cm根系生物量占到了75%以上。有研究表明,根系在土层中的分布会受到土壤容重、水分、温度、碳氮比、林分密度等因素的影响[38-39]。甘什岭地区土层较薄,基岩分布深度相对较浅,随着深度的增加,石砾逐渐增多,土壤质地趋于紧实,土壤容重相应增加,植被根系难以进入土壤深部,且深层土壤养分含量低,细根更倾向于分布在养分和水分条件更好的土壤表层,以增加竞争优势。热带地区会经常受台风等极端天气的干扰,容易受到台风等强风暴的影响[40],根系集中分布于土壤表层,不利于大型植被的固定,影响其稳定性,对植物的高度产生一定的限制,导致植被平均高度和胸径明显低于其他热带地区,且根系生物量与其他热带、亚热带森林相比也相对较低。因此,本研究对处于该特殊生境下热带雨林的恢复和保护具有指导意义。
3.1. 不同恢复阶段热带低地雨林根系生物量特征
3.2. 根系生物量组成结构及土壤剖面中的垂直变化
-
甘什岭热带低地雨林根系生物量随着演替的进行逐渐增加,且粗根占总根系生物量的比例逐渐增加,细根占总根系生物量的比例逐渐减小;随着土壤剖面深度的增加,根系生物量大幅度减少,草本群落根系生物量在土壤剖面中的垂直变化规律呈指数回归,其余各恢复演替阶段呈幂函数回归;根系生物量模型中树高和D2H与根系生物量拟合程度较高,在实践应用中可依据林木树高和D2H估测甘什岭地区植被根系生物量,为观测不同演替阶段群落的生态恢复和区内珍稀保护物种的发展变化提供数据支持,同时为后续甘什岭热带低地雨林根系生物量及地下碳素分配研究提供参考。






 DownLoad:
DownLoad: