-
普通大蓟马(Megalurothrips usitatus)是豆类植物上的一种重要害虫[1-3],尤其对豇豆属植物有强烈偏好性[4]。该虫的发生与危害严重影响豆类作物的产量和品质[5-6]。在我国海南岛,普通大蓟马对多种常用杀虫剂产生了抗药性,成为最难对付的豇豆(Vigna unguiculata)害虫[5, 7]。普通大蓟马是典型的单倍−二倍体生物(Haplodiploidy),其繁殖方式包括两性生殖和产雄孤雌生殖[1, 8]。性比是昆虫种群结构特征之一[9],可用来预测蓟马种群增长趋势、确定防治时间[10]。性比和繁殖力是影响蓟马种群动态的最敏感因子[11]。蓟马的性比受多种非生物因子和生物因子的影响,例如,温度、食物、蓟马的生物学和生态学特性和防控因子等[8, 12-15]。寄主植物质量的变化不仅能改变植食性昆虫的繁殖力,还能影响某些昆虫的性别分配,导致性比偏雌或偏雄[16]。氮(N)是影响昆虫寄主植物营养质量的关键因子之一[17]。然而,迄今为止,有关N对普通大蓟马繁殖的影响国内外尚无报道。因此,本研究拟研究不同施氮水平对豇豆普通大蓟马繁殖及其子代性比的影响,以期为普通大蓟马的绿色防控提供技术支撑。
-
豇豆(品种:‘纯丰长豇’)种植于网室大棚内,按“无公害食品豇豆生产技术规程”进行栽培管理[18],不施用农药。选取健康的幼嫩豆荚作为饲养普通大蓟马的食物。
-
在海南大学海甸校区植物保护学院教学实验基地豇豆种植区(110°32′ E,20°05′ N),采集豇豆花内的普通大蓟马成虫,带回室内,以幼嫩的豇豆豆荚饲养,适时更换食物。饲养环境条件:温度(26±1)℃、相对湿度(60±5)%、光周期14∶10(光∶暗)。子代化蛹后将蛹挑入试管(12 mm×100 mm)内,进行单头饲养。当成虫羽化后,立即按性比1∶1 配对饲养24 h。最后,挑选出健康的已交配雌虫作为试虫。
-
试验处理:按尿素(含N 46%,中海石油化学股份有限公司)的施用水平设置3个处理:低N、中N和高N,其施N量分别为0、160和320 kg·hm−2[18]。
盆栽豇豆:采用盆栽法种植豇豆。盆栽土来自海南大学海甸校区植物保护教学基地,其基本理化特征值为:全氮 0.371 g·kg-1−2,全磷0.181 g·kg-1−2,全钾0.952 g·kg-1−2;碱解氮75.3 mg·kg−1,有效磷5.3 mg·kg−1,速效钾44.7 mg·kg−1,有机质4.62 g·kg−1;PH为5.93。3个处理施用的肥料还包括15 t·hm−2羊粪(内蒙古吉人化肥有限公司)、146.25 kg·hm−2过磷酸钙(含P2O5 16%,广东丰泽化工有限公司)和243.75 kg·hm−2硫酸钾(含量99%,西陇科学股份有限公司)。上述肥料的施肥量换算成每盆1 kg土壤的施肥量,均以基肥形式混施于盆栽土中。采用分期播种豇豆。每个花盆(17.2 cm×17 cm)保留5株幼苗,适时浇水。所有花盆放置于网室大棚内。选取苗龄为13~15 d、健康的第1张叶片供试。
接虫与饲养:用直径为12 mm的打孔器在豇豆叶片上切取叶盘。每支玻璃试管(12 mm×150 mm)内放置1个叶盘,叶背面朝上,然后接入1头已交配的1日龄雌虫。每24 h更换叶盘,直至雌虫死亡。在体视显微镜(XTS20,北京泰克仪器公司)下对已更换出来的叶盘进行镜检,检查和记录卵粒数。然后将已着卵的叶盘转移到新的试管中,用幼嫩豇豆豆荚继续饲养,直至成虫羽化。所有试管均放置于(26±1)℃、相对湿度(60±5)%、光周期L光∶D暗= 14∶10条件下。各处理的亲代雌虫数为40~51头。每天观察亲代雌虫的繁殖与存活状况、子代的数量与性别。计算亲代雌虫的存活时间、产卵前期、产卵期、产卵后期、产卵量和日产卵量,以及亲代雌虫所产子代成虫的性比(即雄性占成虫比例)和日性比、子代从卵至成虫羽化时的存活率。
-
采用Excel 2010进行试验数据的汇总与绘图;利用SPSS 19.0软件进行数据统计分析。子代性比和存活率均经反正弦转换后,进行方差分析和多重比较(Duncan多重比较法)。
-
亲代雌虫的存活天数和产卵期均以高N处理最长,分别长达24.48 d和23.13 d,且显著大于另2个处理(P<0.05);低N和中N处理的存活天数相似,其值为19 d左右;低N和中N处理的产卵期差异不大,大约为17 d。对产卵前期而言,高N处理仅有0.15 d,显著小于低N处理(0.92 d)和中N处理(0.91 d)(P<0.05)。低N、中N和高N处理的产卵量分别为46.51、63.30和100.13粒·雌−1,其中,高N处理与低N和中N处理之间的差异都达到了显著水平(P<0.05)。同样地,高N处理的日产卵量[4.38粒·(雌·d)−1]也显著大于低N处理[2.82粒·(雌·d)−1]和中N处理[3.36粒·(雌·d)−1](P<0.05)(表1)。说明已交配普通大蓟马雌虫的存活天数、产卵前期、产卵期、产卵量和日产卵量均受施N处理的影响,但产卵后期不随施N水平而变化。
参数 低N 中N 高N 存活天数/d 18.86±0.67b 19.70±0.95b 24.48±1.29a 产卵前期/d 0.92±0.21a 0.91±0.21a 0.15±0.10b 产卵期/d 16.20±0.79b 17.16±1.10b 23.13±1.26a 产卵后期/d 1.75±0.26a 1.64±0.27a 1.20±0.19a 产卵量/(粒·雌−1) 46.51±4.38b 63.30±8.25b 100.13±5.99a 日产卵量/[粒·(雌·d)−1] 2.82±0.19b 3.36±0.29b 4.38±0.15a 注:表中数值为平均数±标准差,同一行数据后标有不同字母表示在P<0.05上差异显著。 -
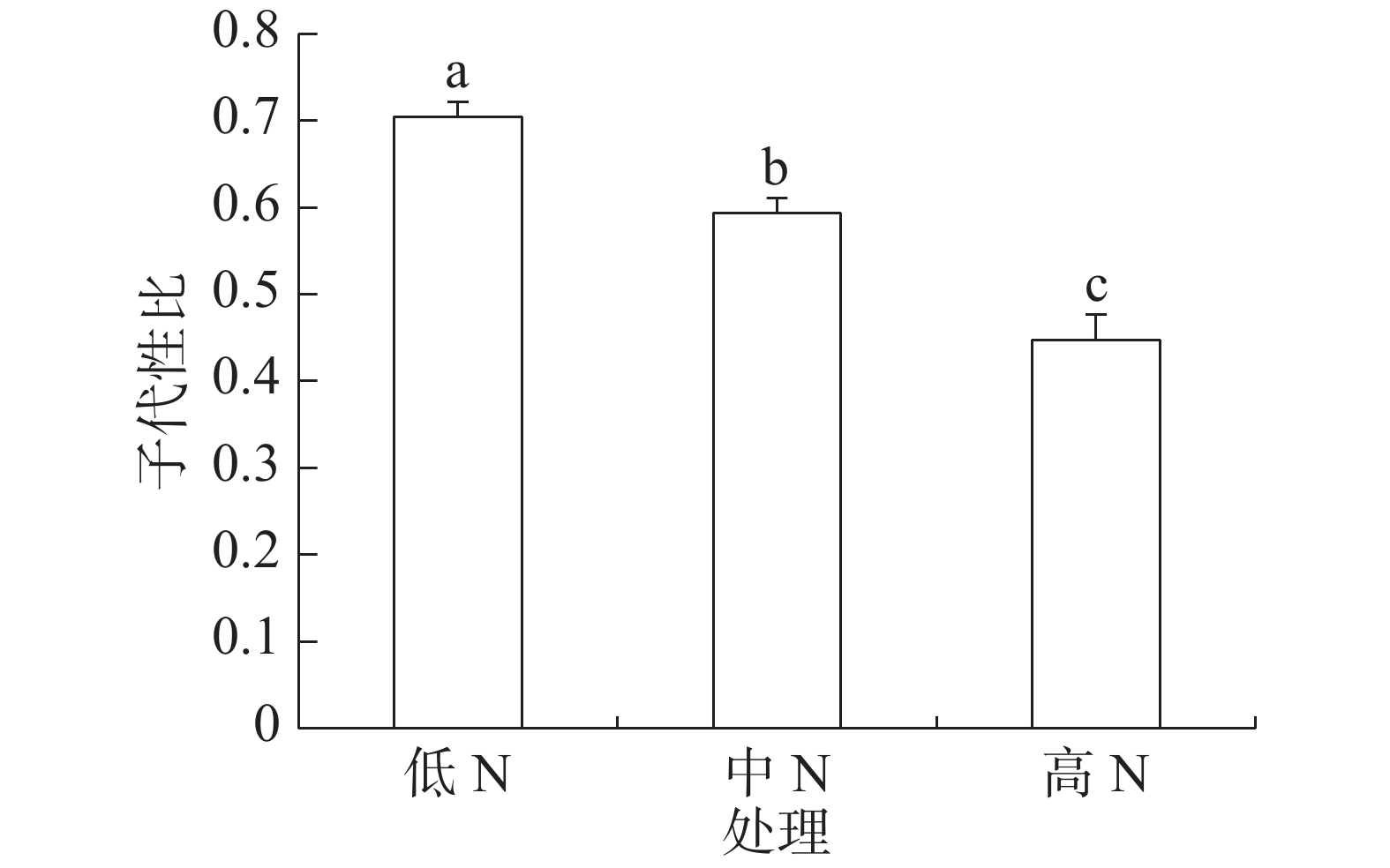
豇豆3种施N水平下,普通大蓟马亲代雌虫在存活期内所产子代的性比见图1。从图1可知,子代性比随豇豆施N水平的增加而下降,从偏雄性逐步转向偏雌性;低N、中N和高N处理的子代性比分别为0.70、0.59和0.45,彼此之间的差异都达到了显著水平(P<0.05)。
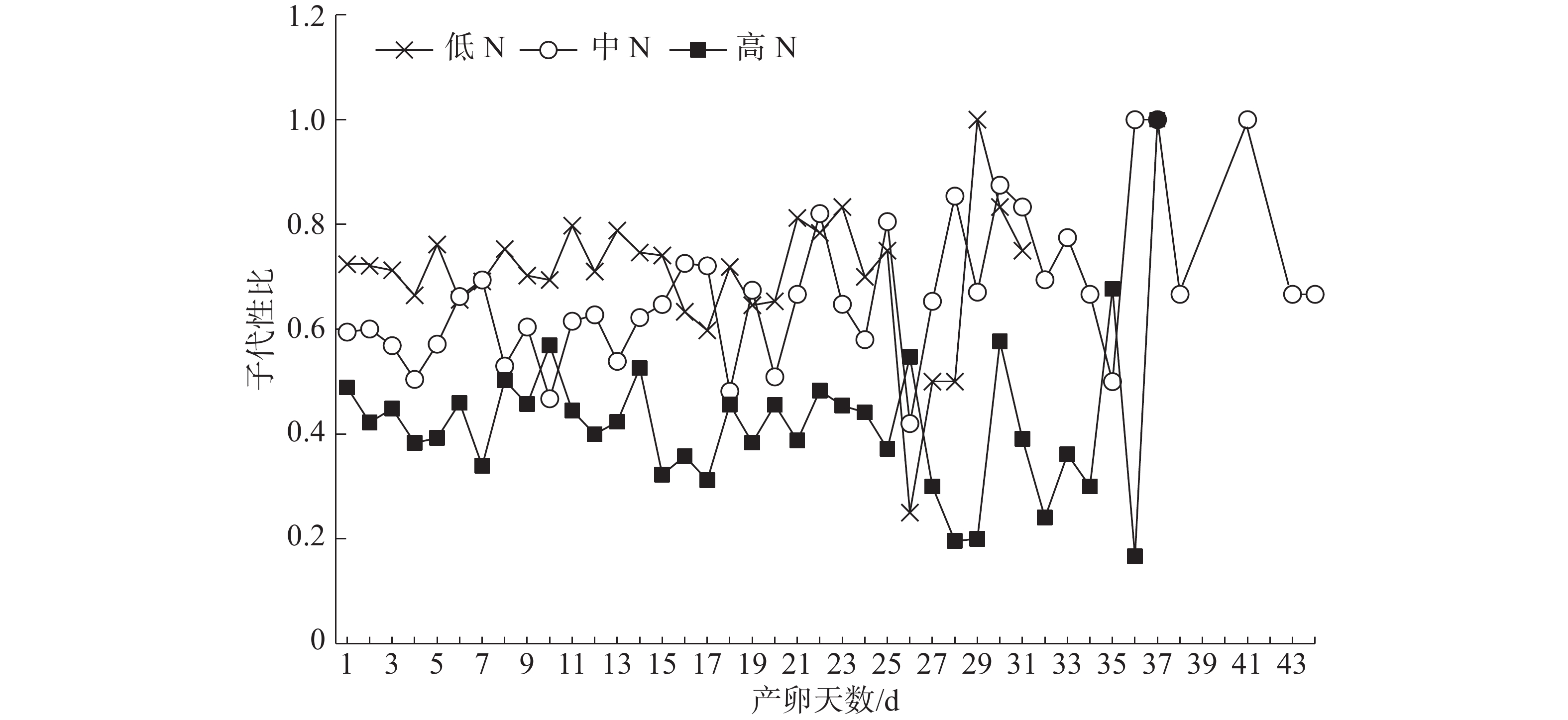
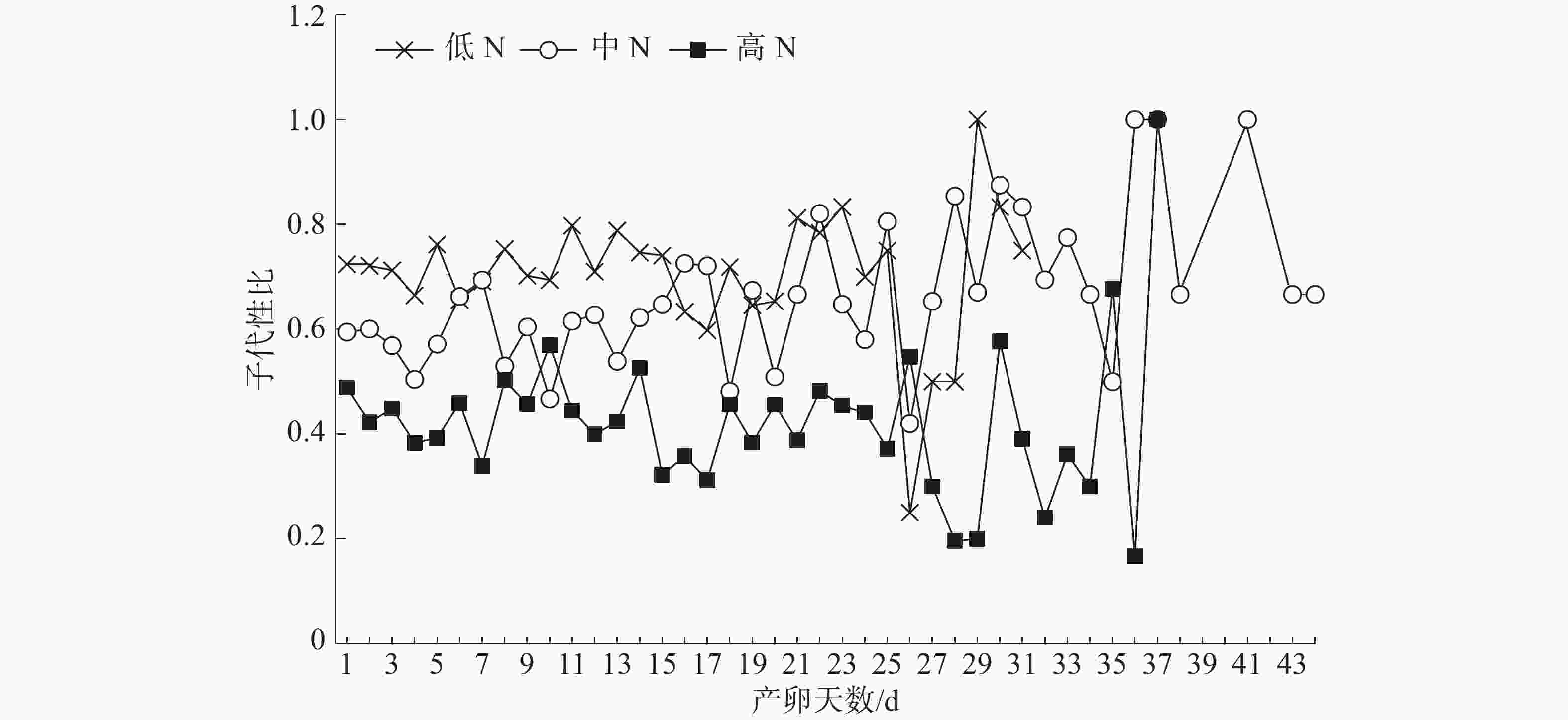
不同施N条件下普通大蓟马所产子代的日性比展现出了波动性(图2)。但是,在雌虫产卵的前25 d内,子代日性比的波动相对较小;低N处理的日性比全部偏雄,其值介于0.60~0.83;同样,中N处理的日性比基本偏雄,仅有2 d的性比小于0.5;对高N处理而言,日性比多数偏雌,其值在0.31~0.57之间变化,日性比等于或大于0.5的情形仅出现了3次。
-
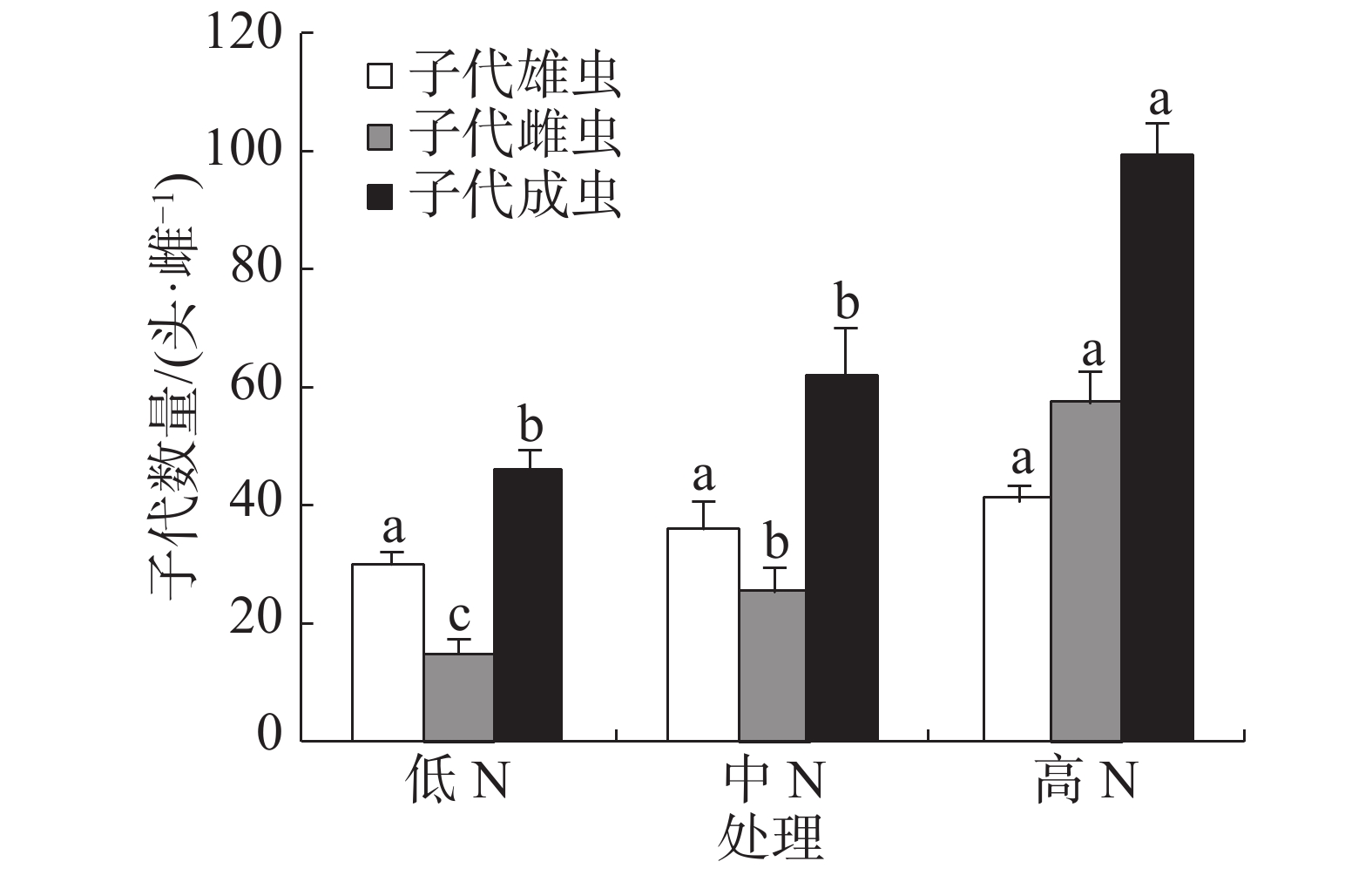
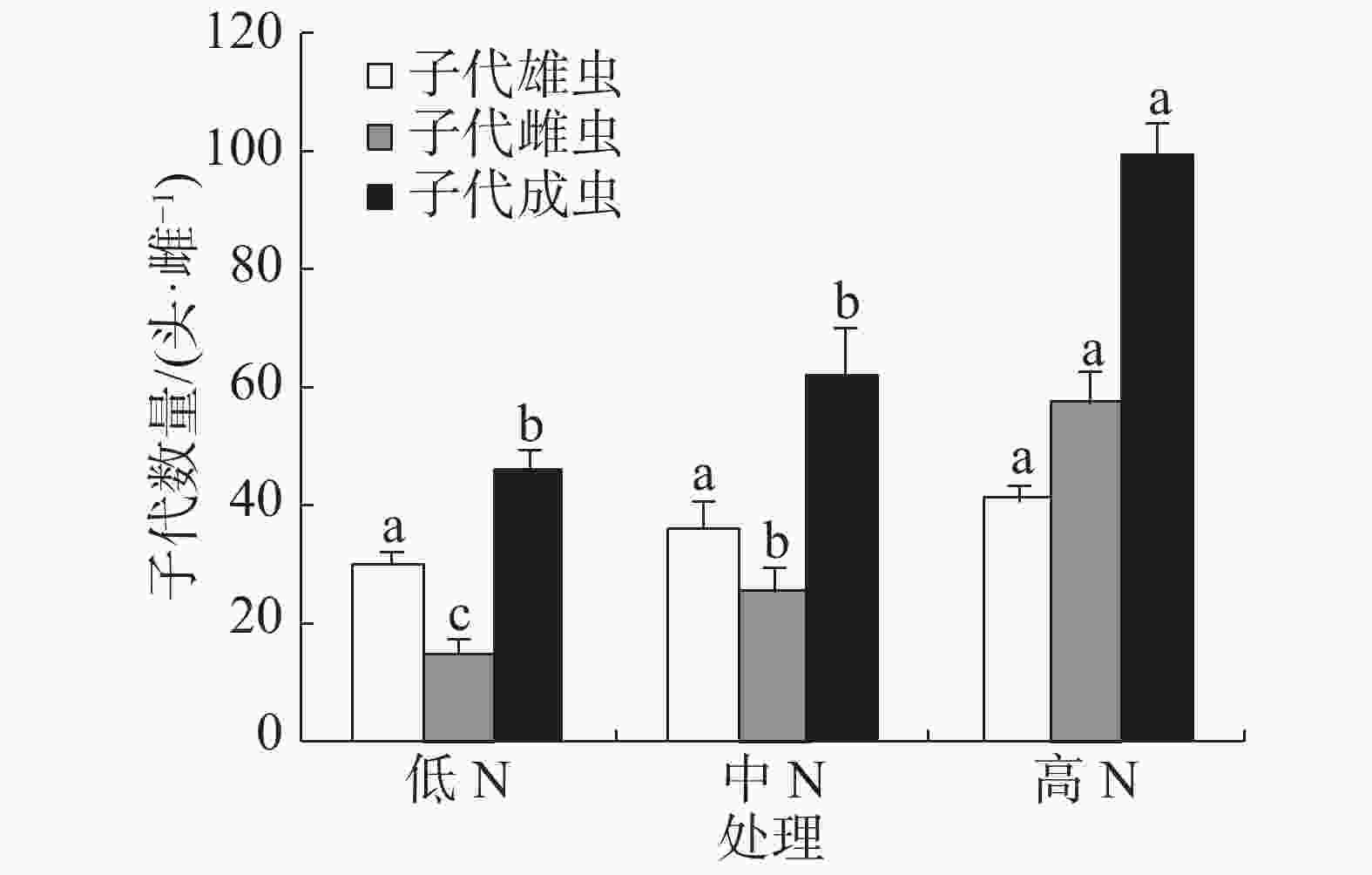
豇豆施N水平对普通大蓟马所产子代的雄虫数没有显著影响,3个处理的子代雄虫数介于30.22~41.45头·雌−1(图3)。子代雌虫数量则随施N水平的提高而增加,低N、中N和高N处理的子代雌虫数分别为15.51、26.02和57.93头·雌−1,彼此之间差异显著(P<0.05)。子代成虫数以高N处理最多,其值高达99.38头·雌−1,同另2个处理的差异均达到了显著水平(P<0.05),但低N处理(45.73头·雌−1)和中N处理(62.32头·雌−1)之间没有显著差异。
-
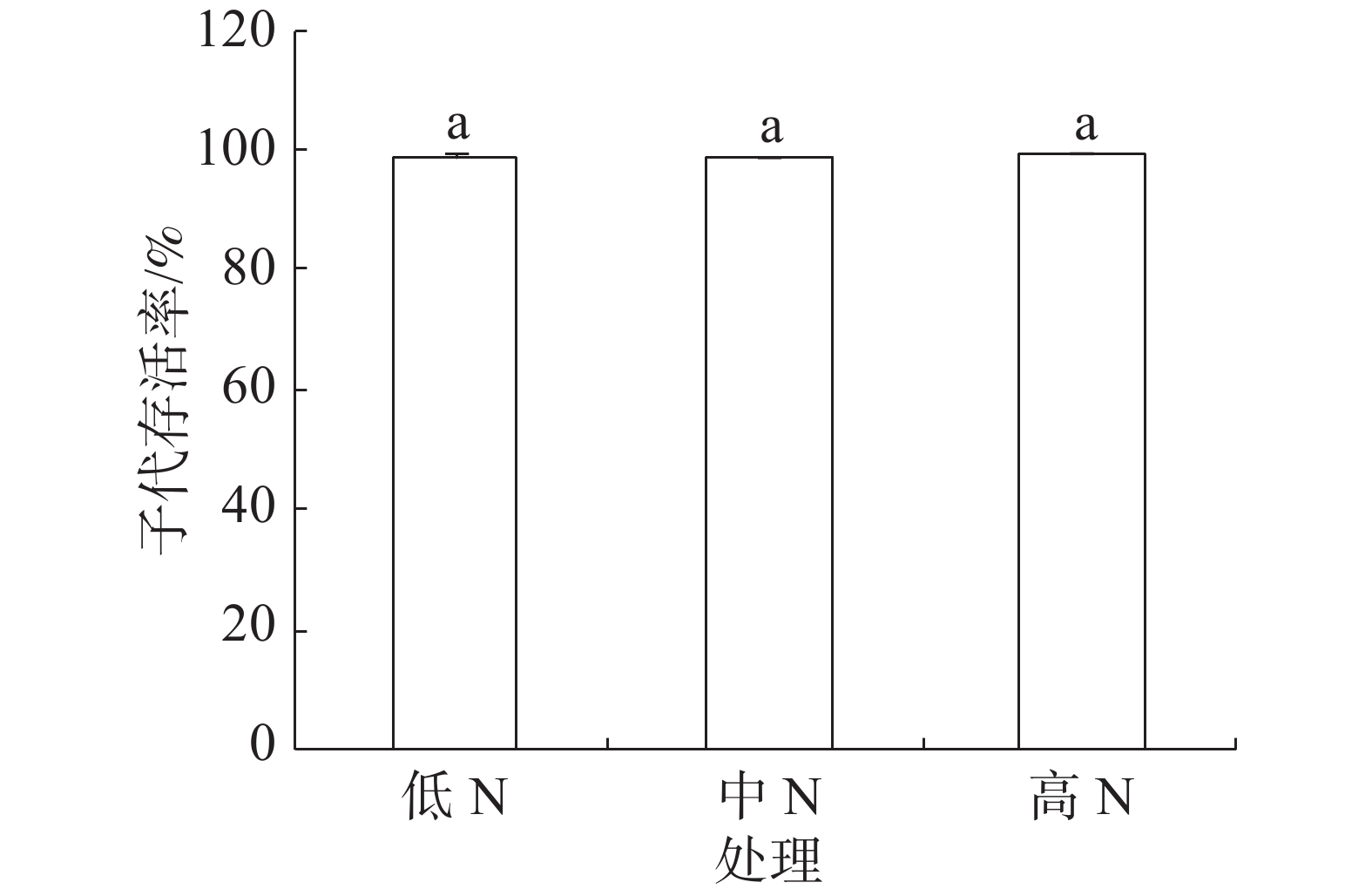
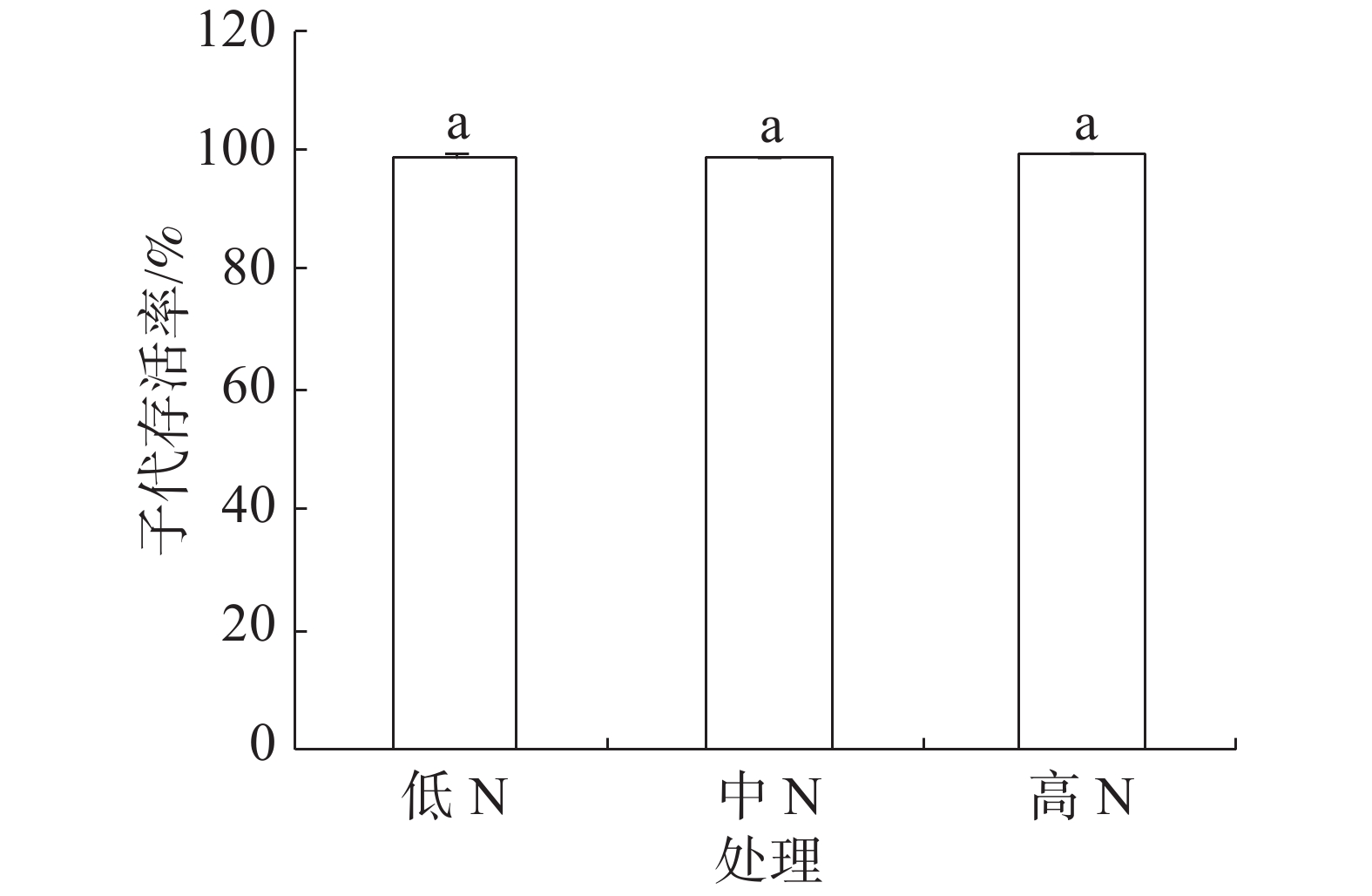
豇豆3种施N条件下,普通大蓟马所产子代的存活率介于98.68%~99.24%之间(图4),彼此间差异不显著。
-
本研究首次在室内评价了豇豆3种施N水平对普通大蓟马繁殖及其子代性比的影响。试验结果表明,高N显著延长已交配普通大蓟马雌虫的存活天数和产卵期,并缩短产卵前期,同时还提高其产卵量和日产卵量。但是,亲代雌虫的这些特征参数在低N和中N处理之间没有显著差异。由此可见,偏施N肥能促进普通大蓟马的繁殖,而少施N肥对该虫的繁殖没有明显影响。已交配普通大蓟马雌虫所产子代的性比随豇豆施N水平的增加而显著下降,由偏雄性比逐步转向偏雌性比,这归功于雌性子代数的增加。高N处理还能显著提高子代成虫的数量。普通大蓟马子代存活率不受豇豆施N水平的影响,所以,豇豆施N水平对该虫的性别分配具有调控作用,施N愈多,产雌愈多。
寄主植物施N水平影响害虫的生长与繁殖[16]。Hunt等[19]研究发现,施以中等N水平的黄瓜(Cucumis sativus)更有利于西花蓟马(Frankliniella occidentalis)的生长发育、存活和繁殖。Baez等[20]曾报道,施用N肥促使甜椒(Capsicum annuum)和番茄(Solanum lycopersicum)花朵上西花蓟马的性比更加偏雌。施N后番茄花朵苯丙氨酸等芳香族氨基酸的含量上升,对西花蓟马雌虫有利[21-22]。二斑叶螨(Tetranychus urticae)也属单倍二倍体生物[23],高N处理能导致该螨的产卵前期缩短、产卵期和寿命延长、繁殖力提高[24]。雌螨比率随叶片氮含量的增加而增大[25]。本研究也得到了类似的结果,豇豆偏施N肥有利于普通大蓟马的繁殖,促使子代性比偏雌。
蓟马的发生与危害跟N肥的施用有密切的关系[26-27]。对西花蓟马的研究表明,在多种寄主植物上的成虫数量随施N水平的增加而增加,例如,番茄[20-21, 28]、菊花(Dendranthema grandiflora)[29-31]和月季(Rosa hybrida)[32]。施用N肥对蓟马种群的这种促进作用可能跟植株体内氨基酸含量的上升有关。在氨基酸含量高的植株上,蓟马往往产下更多的卵[33]。西花蓟马对生菜(Lactuca sativa)、番茄、甜椒和黄瓜等4种蔬菜的危害跟叶片蛋白质中芳香族氨基酸含量呈极显著正相关[34]。Agbahoungba等[35]发现,非洲豆蓟马(Megalurothrips sjostedti)对不同豇豆品种的危害程度与豇豆可溶性氨基酸含量有极显著的正相关性。同样地,蓟马在花生(Arachis hypogea)不同品种上的发生与危害同叶片游离氨基酸含量也呈极显著正相关[36]。因此,在未来的研究中,有必要评价氨基酸在普通大蓟马繁殖和性别分配中的作用。
Effects of nitrogen fertilization on reproduction and offspring sex ratio in Megalurothrips usitatus (Bagrall) (Thysanoptera: Thripidae)
DOI: 10.15886/j.cnki.rdswxb.20230061
- Received Date: 2023-04-16
- Accepted Date: 2023-05-10
- Rev Recd Date: 2023-05-03
- Available Online: 2024-01-16
- Publish Date: 2024-01-16
-
Key words:
- Megalurothrips usitatus /
- sex ratio /
- oviposition /
- Vigna unguiculata /
- nitrogen fertilizer
Abstract: In order to evaluate the influences of nitrogen fertilization on reproduction and sex ratio of Megalurothrips usitatus (Bagrall), an economically important pest of legumes, the 1-day-old mated females were provided daily with leaf disks cut from the first leaves of potted cowpea at the age of 13 to 15 days, which were fertilized as a single pre-plant application with three different levels of urea at rates of 0, 160, and 320 N kg·hm−2, respectively. After being checked the number of eggs, each leaf disk was transferred into a glass tube containing a section of young bean pod of cowpea, which was harvested from the field plants. Oviposition days of the focal females were recorded, and their offspring adult were sexed and counted. Sex ratios of offspring were calculated as proportion of males. The results showed that the females reared on leaf disks from plants fertilized with high N had significantly prolonged survival duration and oviposition duration, and shortened pre-oviposition period, and significantly greater oviposition rate and daily oviposition rate, compared to low N and medium N treatments. Offspring sex ratios for low N, medium N and high N treatments were 0.70, 0.59, and 0.45, respectively, which were significantly different among each other. The number of offspring females produced by the females increased as N application rate increased. The number of offspring adults for high N treatment was significantly higher than those for low N and medium N treatments. N application level had no effect on immature survival of the offspring. In conclusion, nitrogen fertilization impacts on development, reproduction, and sex ratio of M. usitatus. Nitrogen overfertilization increases offspring production of this thrips, with a female-biased sex ratio.
| Citation: | ZHANG Zhong, ZOU Youxing, DAN Jianguo. Effects of nitrogen fertilization on reproduction and offspring sex ratio in Megalurothrips usitatus (Bagrall) (Thysanoptera: Thripidae)[J]. Journal of Tropical Biology, 2024, 15(1): 79-84. doi: 10.15886/j.cnki.rdswxb.20230061 |







 DownLoad:
DownLoad: