-
肾茶[Clerodendranthus spicatus (Thunb.) C.Y.Wu],又名猫须草,为唇形科(Lamiaceae)肾茶属(Clerodendranthus)多年生草本植物,喜高温多湿环境,怕寒,怕旱,忌积水,耐肥。该属植物全世界有5种, 主要分布于福建、台湾、海南、广西、广东和云南等省[1]。肾茶全草均可入药,性凉,味淡、微苦,具有清热祛湿、排石利尿的疗效[2]。对肾结石、胆结石、胆囊炎、尿路结石具有神奇疗效,被誉为“国际利尿化石药”[3]。肾茶除应用于泌尿系统外,还具有抗炎、抗肿瘤、降血糖血脂、保肝、提高机体免疫力及治疗风湿性关节炎等药理活性[4-6]。目前,由于肾茶的药用价值较高,市场需求量越来越大,并且随着环境气候的变化及野生肾茶资源的破坏,野生肾茶已处于濒危状态,无法满足市场需求[7]。据报道,肾茶在我国广东、广西、海南、云南、福建等地已有引种栽培[8],在云南,以思茅和西双版纳地区种植最多。随着肾茶种植面积不断扩大,规模化种植后发现其病害也随之发生,其中肾茶叶枯病发生最严重,导致肾茶严重减产,成为阻碍肾茶规模化生产的重要病害。而国内外对于肾茶的研究多集中在种质资源亲缘关系研究、生药学鉴别研究、传统应用调查研究、化学成分及其生物活性研究和部分高产栽培技术研究[7,9-11]。对于肾茶栽培过程中出现的病虫害少见报道[12]。于旭东等[13]报道了肾茶根结线虫的危害,严珍等[14]报道了肾茶新害虫泡壳背网蝽的危害,关于肾茶叶枯病的研究至今未见报道。笔者针对肾茶叶枯病,进行了病害症状观察,病原菌分离、鉴定和病原菌生物学特性研究,旨在为有效防治肾茶叶枯病提供理论基础。
-
在中国医学科学院药用植物研究所云南分所肾茶种植地里采取肾茶发病叶片。放入无菌采集袋中并编号,运回实验室进行病原菌分离纯化。
-
对中国医学科学院药用植物研究所云南分所肾茶地的肾茶进行病害调查。采集感染叶枯病害肾茶叶片进行症状描述、拍照、镜检。
-
采用组织分离法[15]从采回的肾茶样本上分离病原菌,将叶片的病健交界处病组织,切成5 mm×5 mm小块,用75%乙醇浸泡30 s,再用无菌水漂洗3次,用无菌滤纸置于PDA培养基上25 ℃培养[16],待菌落长出后,挑取菌落组织制片镜检,并进行分离纯化,观察记录菌落颜色和形态等培养特征。
-
用针刺法将分离纯化培养7 d的病原菌饼(直径8 mm)接种于无病健康且长势相近的肾茶叶片上[15],以接种无菌PDA培养基饼(
直径8 mm)作为对照,每个处理3个重复,连续观察7 d记录其病害发生情况。依据Kochʼs法则,再次对发病组织进行病原菌分离和纯化,并与原接种的分离菌株进行比较。 -
将分离纯化得到的菌株接种至PDA培养基上,25 ℃恒温培养7 d。观察菌落的形态和颜色,并挑取菌丝置于显微镜下观察记录分生孢子的形态、大小及有无隔膜等,进行分离菌株的形态学鉴定[17-18]。
-
将分离纯化得到的菌株接种至PDA培养基上进行扩大培养,采用SDS裂解法用DNA提取试剂盒[生工生物工程(上海)股份有限公司]提取基因组DNA。选用真菌ITS序列的通用引物(ITS1/ITS4)进行PCR扩增,扩增反应在15 μL反应体系中进行,Template为1 μL,Primers(5 mmol·L−1)2 μL,10×PCR Buffer为2 μL,dNTP为1.6 μL,Taq酶0.1 μL,加双蒸水至15 μL。热循环反应程序设置: 94 ℃初始变性2 min,接下来为94 ℃变性30 s,55 ℃引物退火30 s,72 ℃延伸2 min,30个循环结束后72 ℃修复延伸10 min。扩增中利用水代替DNA模板为空白对照。用凝胶电泳法检测PCR反应结果,吸取4 μL PCR产物加入1% 琼脂糖凝胶,凝胶置于1×TBE缓冲液( 0.9 mol·L−1 Tris-Borate,0.01 mol·L−1 EDTA,pH8.3 ) 中,在110 V下电泳40 min。将PCR扩增产物测序委托北京优博兰基因技术有限公司完成,将测序结果提交至NCBI的GenBank进行BLAST比对分析,检索出相似性较高的ITS序列,以确定分离菌株的分类地位;再利用MEGA 5.1中的邻接法(Neighbor-Joining,NJ)构建系统发育进化树并分析其亲缘关系[19]。
-
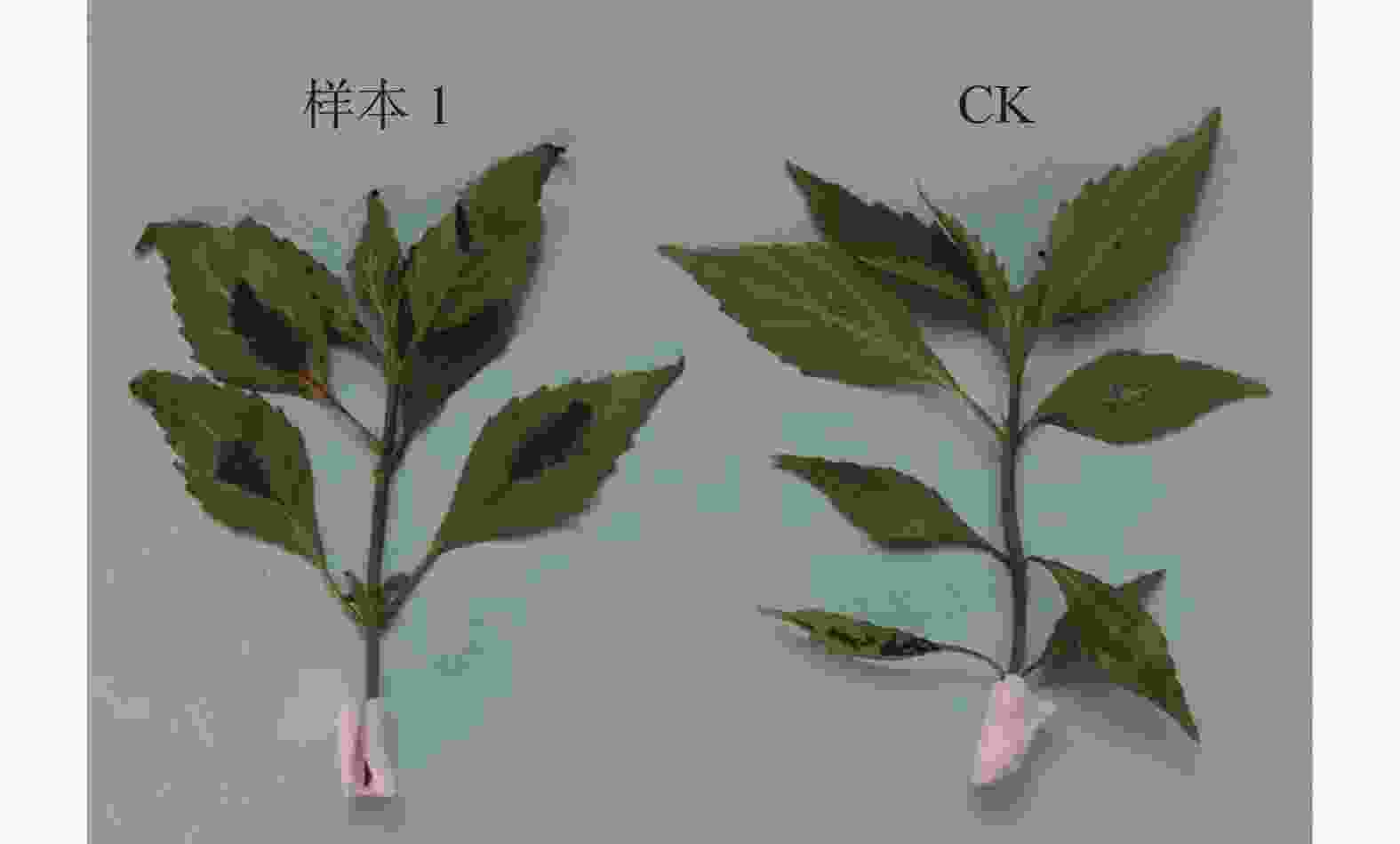
肾茶叶枯病是肾茶种植过程中一种常见的叶片病害,该病一般发生于肾茶叶片的叶缘、叶尖,开始为淡褐色小点,后渐扩大为不规则的大型斑块,若几个病斑连接,1/3~1/2叶片将变干枯,最后使整个叶片坏死脱落。除了为害叶片外,肾茶叶枯病还发生在肾茶嫩梢,嫩梢发病时变黑,枯死(图1)。
-
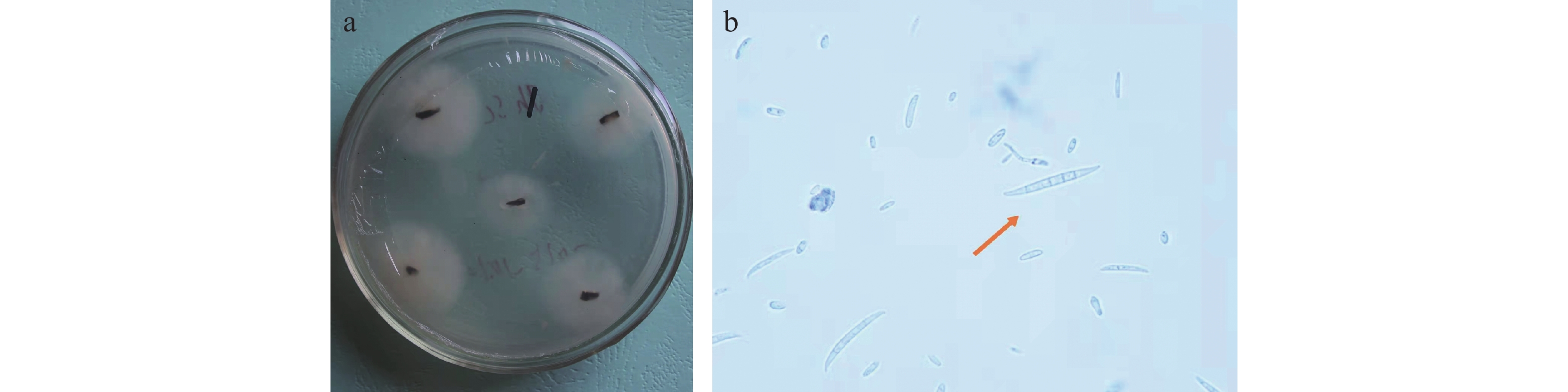
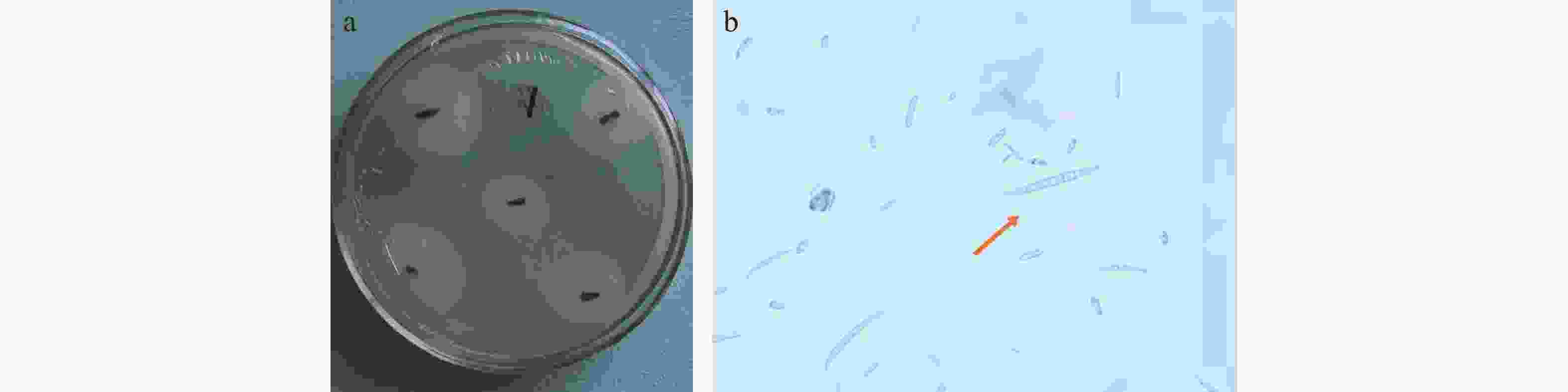
对肾茶叶枯病病斑进行病原菌分离纯化得到7个菌株,于4 ℃保存备用。用针刺法将菌株接种在离体健康的肾茶叶片上,用脱脂棉保湿,持续观察叶片发病情况,其中6个菌株不发病,1号菌株接种的叶片在接种4 d后出现褐色小点,在接种7 d后褐色小点扩大连成片,形成黑褐色病斑,发病症状与田间发病一致(图2)。 将菌株接种到刺伤和未刺伤的肾茶叶片上,结果刺伤的叶片会发病,未刺伤的不发病,证明肾茶叶枯病病原菌主要通过伤口侵入。将1号菌株接种,取病健交界处组织进行再分离,重新分离出相同的菌株。
-
在PDA培养基上生长迅速,菌落呈圆形,菌丝初为白色,气生菌丝发达,菌落初期下部淡粉色,后期为深黄棕色。分生孢子顶胞钩状,成熟的大型分生孢子有3~5个隔膜,多为5个,顶生或顶侧生,分生孢子大小为(16.5~45) μm×(1.6~5.5 )μm(图3),根据形态特征初步鉴定肾茶叶枯病为镰刀菌(Fusarium)。
-
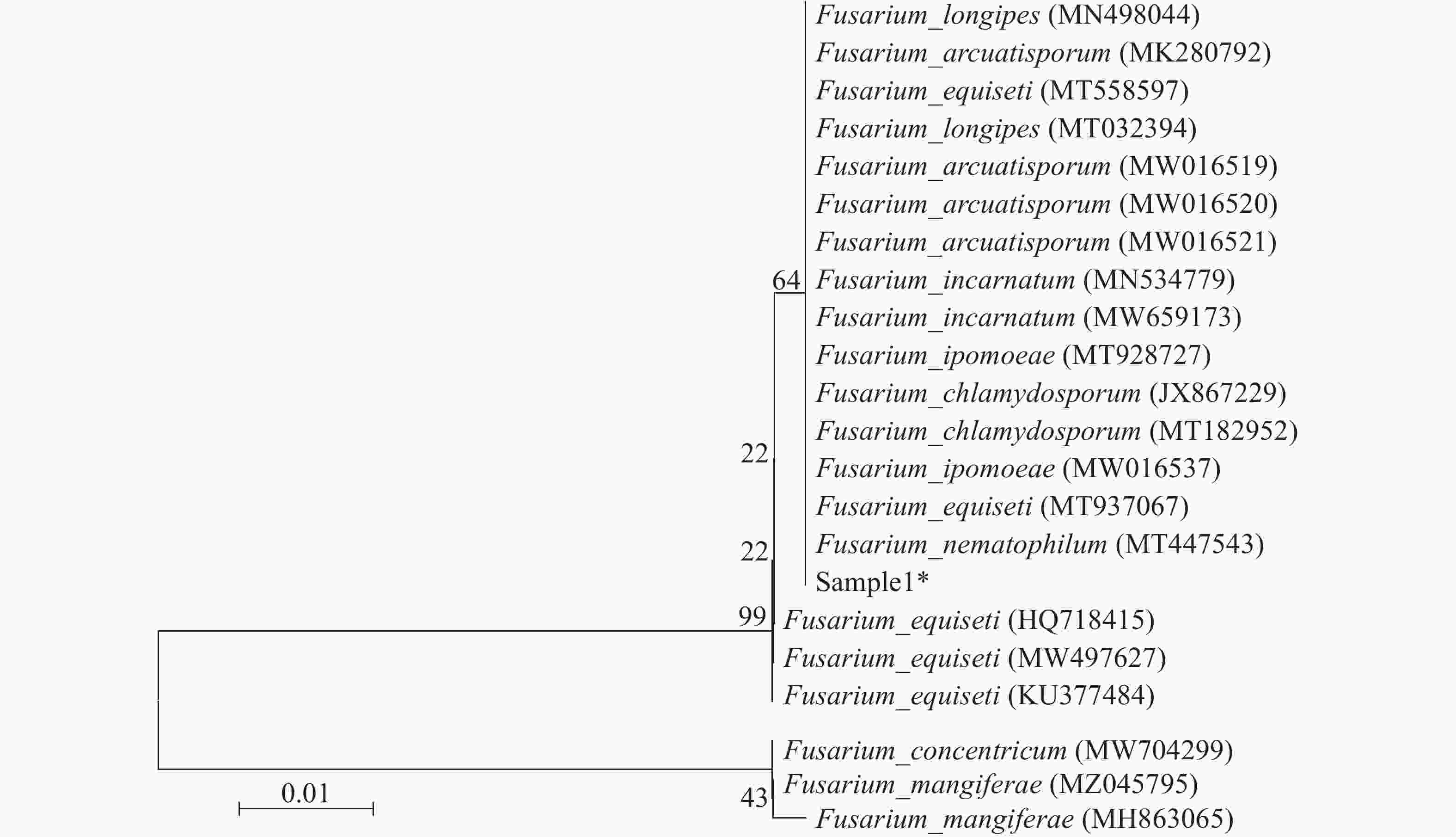
以病原菌基因组DNA为模板,用ITS1/ITS4引物进行PCR扩增目的片段,得到1条500 bp条带,经琼脂糖凝胶回收、克隆测序及BLAST比对,该扩增片段与Fusarium nematophilum (MT447543),Fusarium equiseti (MT937067),Fusarium chlamydosporum (MT182952),Fusarium longipes (MN498044)核酸序列同源性为99.40%~99.60%,结合同源菌株rDNA-ITS序列构建系统发育树(图4),病原菌与Fusarium nematophilum,Fusarium equiseti,Fusarium chlamydosporum,Fusarium longipes聚为一支。
-
肾茶多生长于高温湿热地区,同时也适合病原菌生长繁殖,通过对肾茶在栽培过程中的病害调查,发现叶枯病最严重,造成肾茶大面积减产,是制约肾茶规模化发展的重要瓶颈。本研究对西双版纳肾茶叶枯病病害的症状进行了描述,并分离出病原菌1株,通过致病性测定、形态特征观察及rDNA-ITS序列分析结果,鉴定为镰刀菌。镰刀菌是一种广谱性致病菌,寄主植物种类繁多,已报道铁皮石斛根腐病病原菌(Fusarium chlamydosporum)[20]、北苍术枝枯病致病菌(Fusarium equiseti) [21]、辣木枝枯病病原菌(Fusarium equiseti) [22]、杭白菊叶枯病(Fusarium oxysporum)[23]、黄连根腐病(Fusarium oxysporum)[24]、蓝莓镰孢菌叶枯病(Fusarium asiaticum)[25]、地黄叶枯病(F. acuminatum)[26]等均为镰刀菌引起,但镰刀菌引起肾茶叶枯病尚属首次报道。目前国内外对肾茶叶枯病病害的研究较少,本研究后续将对肾茶叶枯病病原菌的生物学特性、病害循环、发生规律以及综合防治等进行深入研究,为生产上能更好地防控该病害提供理论依据。此外,肾茶叶枯病可参考由镰刀菌引起的其他作物叶枯病的防治技术进行控制,经研究因该病为伤口侵入,在田间管理时要减少机械损伤,加强虫害防治、合理密植、通风透光、合理施肥浇水等,或在发病初期使用25%嘧菌酯和25%蚍唑醚菌酯进行防治[27]。具体药剂种类及综合防治技术尚待室内筛选试验和田间实践验证。
Isolation and identification of leaf blight on Clerodendranthus spicatus
DOI: 10.15886/j.cnki.rdswxb.2023.02.013
- Received Date: 2022-01-20
- Accepted Date: 2022-06-25
- Rev Recd Date: 2022-06-19
- Available Online: 2022-09-06
- Publish Date: 2023-03-25
-
Key words:
- Clerodendranthus spicatu /
- leaf blight /
- pathogen isolation /
- pathogen identification
Abstract: In order to determine the pathogen of leaf blight of kidney plant (Clerodendranthus spicatus (Thunb.) C.Y.Wu), the kidney plant leaves infected with leaf blight were sampled from a kidney plant producing area in Yunnan, and one strain of the pathogen was isolated from the samples and then identified morphologically and molecularly. The results showed that the pathogen cultured on the PDA medium was white in colony, and well developed in aerial mycelia. The colony in the lower part was pale pink initially and then turned yellow-brown. The apical cells of the conidia were uncinate, and the mature macroconidia have 3~5 septa. The pathogen isolated was inoculated onto the healthy plant leaves of C. spicatus. After several days of moist culture, dark-brown lesions appeared at the inoculated sites of the leaves, which were consistent with the field symptoms. Genomic DNA of the pathogen was amplified by using fungal rDNA-ITS universal primers ITS1/ITS4 and analyzed by homology analysis. The results showed that the pathogen isolated was clustered into the same branch with Fusarium nematophilum, F. equiseti, F. chlamydosporum and F. longip, and had a nucleic acid sequence homology of 99.40%−99.60%. The morphological observation, ITS sequence analysis and establishment of Koch’s postulates showed that the pathogen was preliminarily identified as F. oxysporum.
| Citation: | SHANG Rui, MAO Jia, WANG Yanqian, BAI Tingting, YANG Chunyong, WANG Hongwei, WANG Yanfang, GUO Rui, LI Ge. Isolation and identification of leaf blight on Clerodendranthus spicatus[J]. Journal of Tropical Biology, 2023, 14(2): 229-233. doi: 10.15886/j.cnki.rdswxb.2023.02.013 |








 DownLoad:
DownLoad: