-
蜱虫是一种专性吸血的体外寄生虫,它们可以从包括人类在内的哺乳动物、鸟类和爬行动物身上吸血,从而传播各种疾病,被认为是仅次于蚊子的重要传播媒介。它们传播的病原体种类很广,包括病毒、细菌、立克次体、原虫,甚至某些蠕虫(微丝蚴)[1-2]。无形体(Anaplasma)是经由蜱媒传播的一种革兰氏阴性菌,隶属于立克次体目(Rickettsiales)无形体科(Anaplasmataceae),目前常见的无形体有7种,分别是嗜吞噬细胞无形体(Anaplasma pagocytophilum)、牛无形体(Anaplasma bovis)、绵羊无形体(Anaplasma ovis)、山羊无形体(Anaplasma capra)、扁平无形体(Anaplasma platys)、边缘无形体(Anaplasma marginale)及中央无形体(Anaplasma centrale)。
20世纪90年代初,在美国首次发现了人粒细胞无形体病的确诊病例[3]。2006年在我国安徽省也出现了该病例,并发现该病原可通过HGA患者血液或呼吸道分泌物进行传播[4]。牛无形体主要分布在非洲、亚洲和南美洲。牛和水牛被认为是牛无形体的主要宿主,但在我国的犬中也发现了牛无形体的存在[5]。扁平无形体主要侵染犬的血小板,引起犬传染性循环血小板减少症。1978年Harvey和他的同事在美国佛罗里达州的一例犬感染中首次描述了这种疾病[6]。有研究表明血红扇头蜱(Rhipicephalus sanguineus)是扁平无形体的生物媒介[7]。扁平无形体在血蜱和犬中广泛流行,美国、阿根廷、巴西、格林纳达、智利、海地、意大利、葡萄牙、马来西亚等国家和中国台湾省均有报道[8-19];在阿尔及利亚的家畜中[20]、中国的山羊[21]和猫[22]中也检测到了扁平无形体;一些南美和加勒比国家[23-24]也有报道人被扁平无形体感染的案例。由此可见,扁平无形体对包括人类在内的广泛宿主物种构成威胁。
无形体病是一种蜱传疾病,主要在热带和亚热带地区流行,不仅会对家畜的健康和生产力造成严重的影响,还会威胁到人类的健康。该病已被世界动物卫生组织(OIE)列为B类动物传染病,也被我国农业部列为进出口动物检疫项目之一[25]。海南全年暖热,雨量充沛,植被丰富,适合蜱类生长,目前海南省硬蜱有6属20种,并且是斑点热的疫源地[26]。但迄今为止,海南省乃至中国对于犬寄生蜱及其携带的病原报道较少。另外,随着宠物数量的剧增,宠物蜱传人兽共患病的发病率也不断升高,有研究发现,养宠物的人被蜱叮咬的可能性约为不养宠物的人的1.5倍[27],因此,探究海南犬寄生蜱种类及其携带扁平无形体的情况,有助于进一步了解海南犬蜱的分类组成,可为动物寄生蜱种类分布及预防蜱传疾病提供科学依据。
-
样本从海南省白沙、乐东、定安、屯昌4个地区的犬体表获得,主要使用宿主体表检查法,着重检查犬只易被蜱虫叮咬的部位,如犬耳、颈部、腋窝、胸部、腹股沟、肛周、腿跟等易感染部位,发现蜱虫后,使用镊子夹住蜱虫的头部并将其放入干净的离心管中。共采集犬体表寄生蜱虫546只。将采集到的蜱虫用75%酒精漂洗3~5次,洗去表面杂质,再用灭菌蒸馏水冲洗3次后放入75%酒精的样本管中保存。
-
主要试剂:2×Taq Plus Master MixII(Dye Plus)购自南京诺维赞生物科技股份有限公司;Goldview染料购自苏州金唯智生物科技有限公司;琼脂糖、动物基因组DNA提取试剂盒; DNA Marker购自生工生物工程(上海)股份有限公司。
主要仪器:PCR仪(TProfessional)购自德国Biometra公司,小型台式高速冷冻离心机(5415R)购自Eppendorf 公司,体视显微镜购自Saike Digital公司,琼脂糖水平电泳仪(Mini-PROTEAN Tetra)购自北京六一仪器厂,凝胶成像分析系统(GEL DOC XR+ )购自美国 Bio-Rad公司。
-
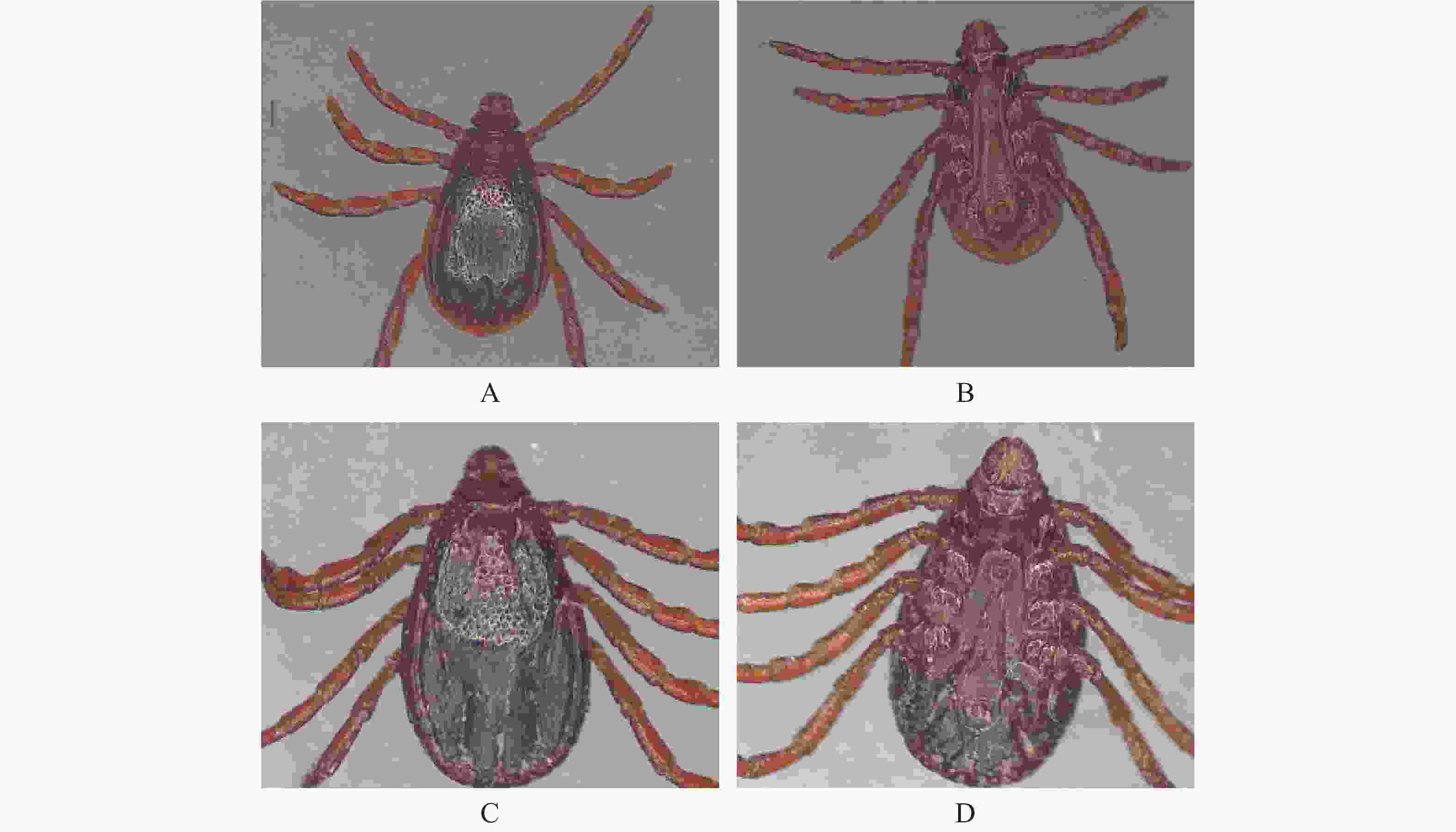
参照中国畜禽外寄生虫形态分类彩色图谱,用体视显微镜对蜱虫进行初步的形态学鉴定,主要观察蜱虫的假头基、盾板、气门板、生殖孔、肛侧板、肛沟和基节等具有形态学鉴定意义的部位,最终确定蜱虫种类并拍摄相应的图片。
-
取出上述经过形态学鉴定的蜱虫,每只放入1.5 mL离心管中,再用ddH2O反复冲洗,去除表面杂质,干燥后将蜱虫磨碎,再参照动物基因组DNA提取试剂盒说明书提取蜱虫DNA,并于−20 ℃保存。
-
查找蜱种鉴定及无形体检测相关的引物序列送至广州天一辉远基因科技有限公司合成,本研究使用的引物信息见表1。嗜吞噬细胞无形体和牛无形体使用巢氏PCR进行扩增。扁平无形体和牛无形体使用常规PCR进行扩增。PCR反应体系25 μL:2×Taq PCR Master Mix 12.5 μL,ddH2O 10.5 μL,上游引物(浓度10 pmol·μL−1)0.5 μL,下游引物(浓度10 pmol·μL−1)0.5 μL,基因组DNA(~20 ng)1 μL。PCR反应扩增条件见表2。反应结束后,取5μL PCR产物于1.5%琼脂糖凝胶电泳,紫外灯下观察是否有目的条带,并拍照保存。将所获得的阳性产物送往广州天一辉远基因科技有限公司进行测序。
基因 引物名称 引物序列(5´-3´) 片段大小/bp 参考文献 16S rDNA 16SF CTGCTCAATGATTTTTTAAATTGCTGTGG 460 [28] 16SR CCGGTCTGAACTCAGATCAAGT gltA gltAF GACCTACGATCCGGGATTCA 580 [29] gltAR CCGCACGGTCGCTGTT 16S rRNA EE1 TCCTGGCTCAGAACGAACGCTGGCGGC 1433 [30] EE2 AGTCACTGACCCAACCTTAAATGGCTG 16S rRNA SSAP2F GCTGAATGTGGGGATAATTTAT 641 [31] SSAP2R ATGGCTGCTTCCTTTCGGTTA 16S rRNA AB1F CTCGTAGCTTGCTATGAGAAC 551 [31] AB1R TCTCCCGGACTCCAGTCTG 种类 引物名称 扩增条件 预变性 变性 退火 延伸 循环/次 再延伸 A. bovis EE1 EE2 94 ℃ 94 ℃ 55 ℃ 72 ℃ 35 72 ℃ 5 min 30 s 30 s 30 s 5 min AB1f AB1r 94 ℃ 94 ℃ 58 ℃ 72 ℃ 40 72 ℃ 5 min 30 s 30 s 30 s 10 min A. phagocytophilum EE1 EE2 94 ℃ 94 ℃ 55 ℃ 72 ℃ 35 72 ℃ 5 min 30 s 30 s 30 s 5 min SSAP2f SSAP2r 94 ℃ 94 ℃ 58 ℃ 72 ℃ 40 72 ℃ 5 min 30 s 30 s 30 s 10 min A. platys gltAF gltAR 94 ℃ 94 ℃ 60 ℃ 72 ℃ 35 72 ℃ 3 min 1 min 1 min 1 min 5 min 16S rDNA 16SF 16SR 95 ℃ 95 ℃ 58 ℃ 72 ℃ 35 72 ℃ 5 min 30 s 30 s 45 s 5 min -
使用NCBI中BLAST在线比对工具将蜱种鉴定及扁平无形体的测序结果与GenBank中已知序列进行比对分析,利用DNAMAN 8.0基因分析软件进行同源性分析,使用Mega X软件,选择Neighbor-joining(NJ)算法中的Kimura-2-parameter参数模型,Bootstrop值选择1 000进行系统发育进化树的构建。
-
经形态学鉴定,546只犬寄生蜱均属于扇头蜱属中的血红扇头蜱,其中雌蜱265只,雄蜱281只。蜱虫外部形态观察结果见图1。
-
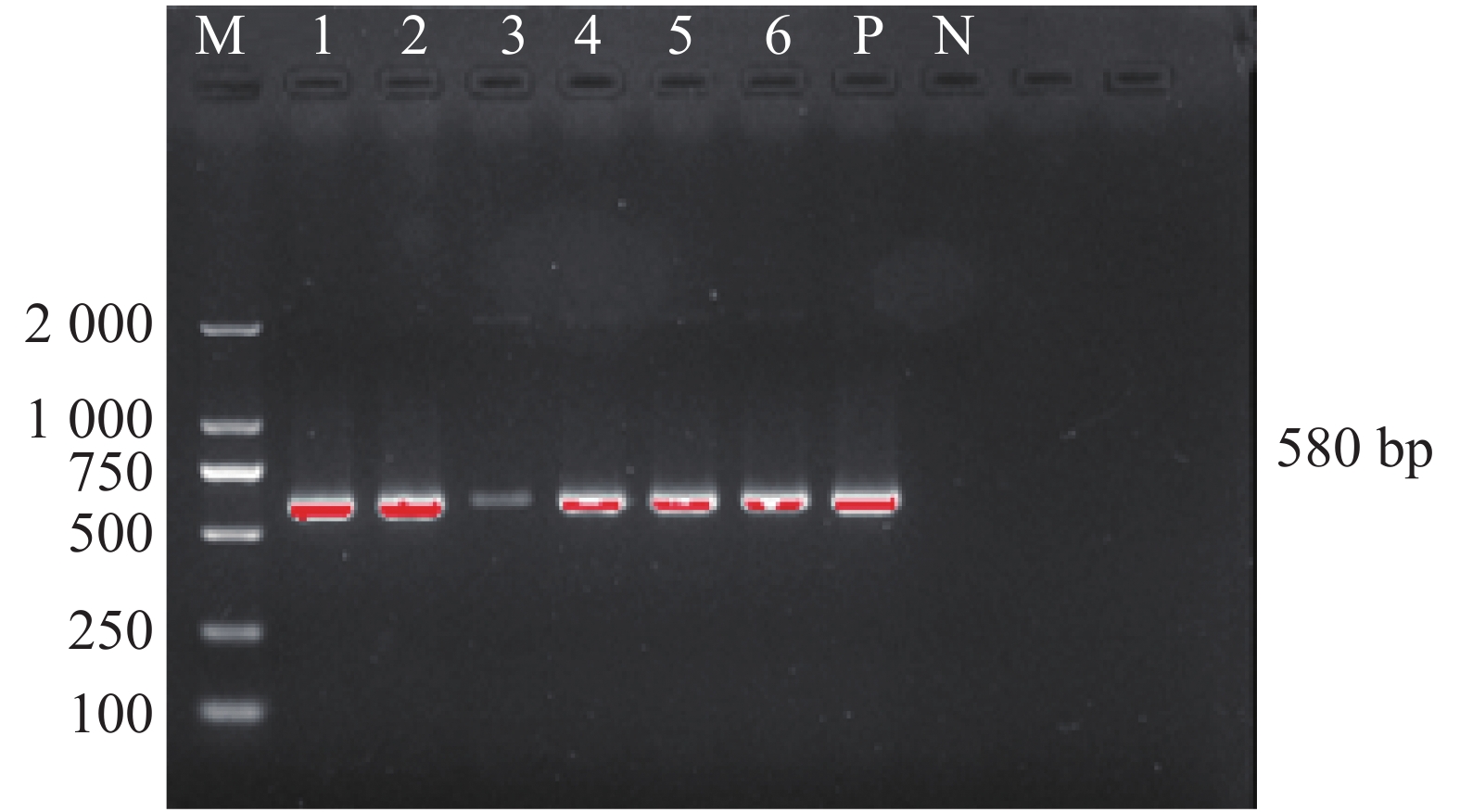
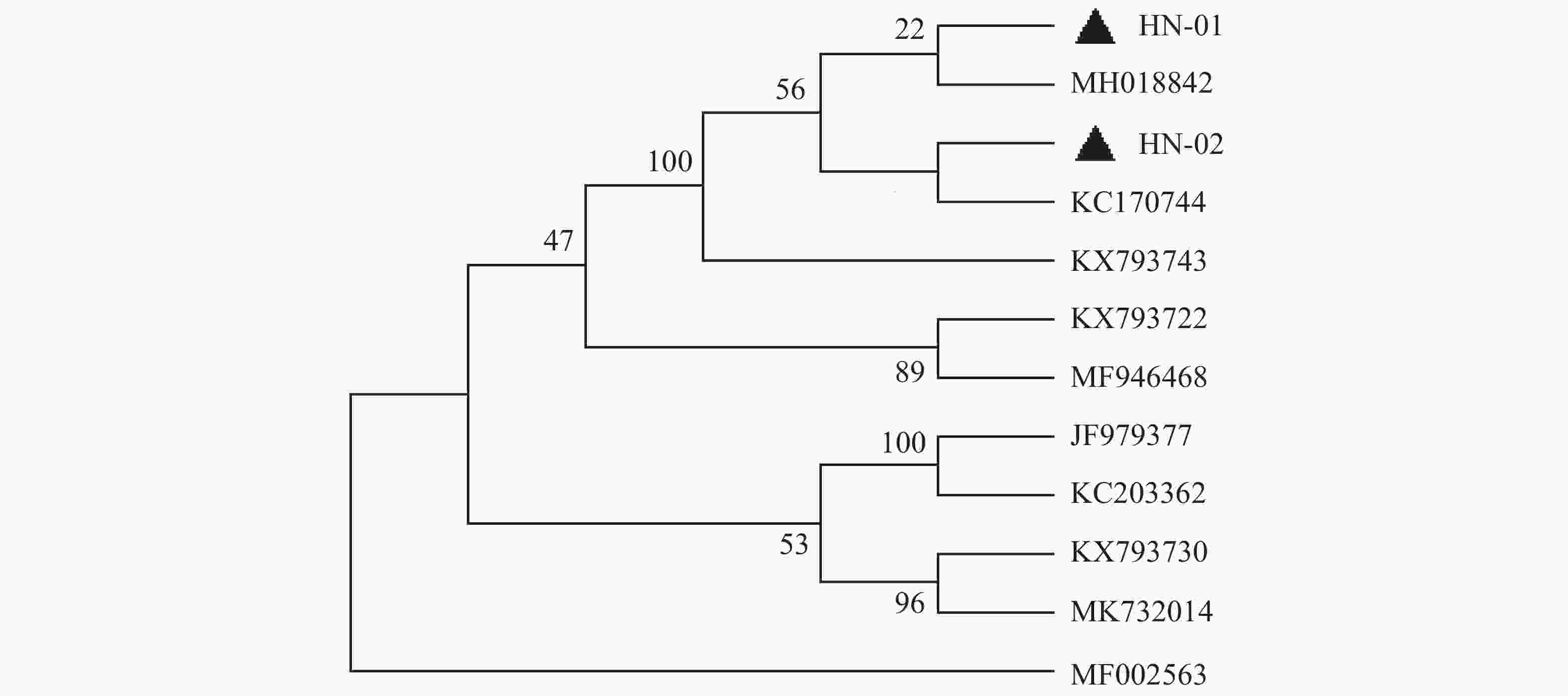
犬寄生蜱的16S rDNA基因PCR扩增产物琼脂糖凝胶电泳见图2,扩增片段为460 bp,与预期片段长度一致。通过序列比对之后,发现本实验中所有蜱虫均为血红扇头蜱,与Genbank中已有的蜱种参考株Rhipicephalus sanguineus(登录号为MG651947)的同源性为100%,与形态学鉴定结果相符。用MEGA-X软件选择本实验中获得的代表性血红扇头蜱线粒体16S rDNA基因序列构建系统发育树(图3),分析结果表明序列HN-01和HN-02处于不同的遗传进化分支,HN-01与已知的来自美国的血红扇头蜱(MH018842)处于同一分支,HN-02与来自泰国的血红扇头蜱(KC170744)处于同一分支,与我国甘肃省、北京市所获得的血红扇头蜱(JF979377、KC203362)序列亲缘关系较远。
-
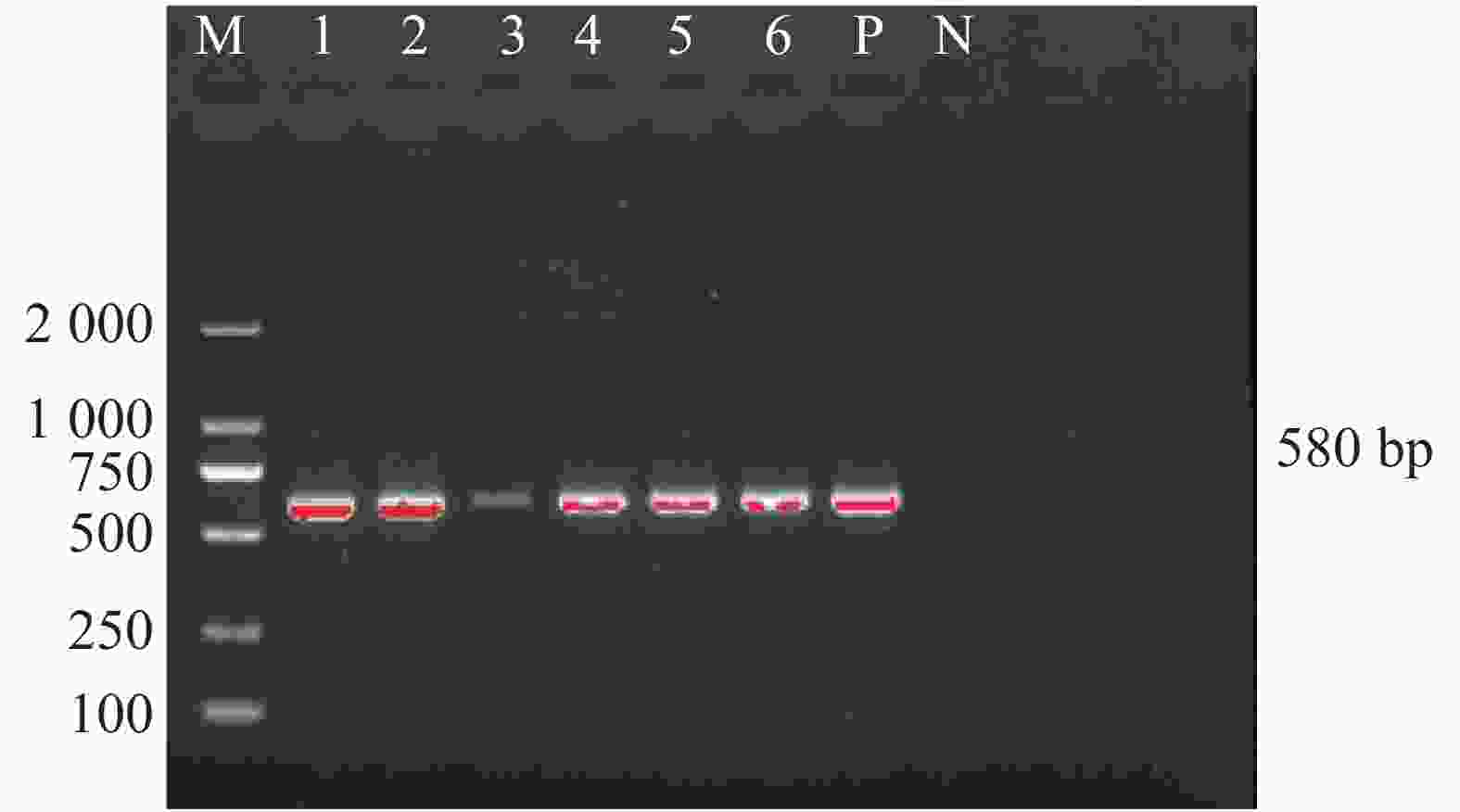
笔者在犬体表蜱中未检测到嗜吞噬细胞无形体和牛无形体,只发现了犬蜱中携带有扁平无形体。犬体表蜱3种无形体感染率见表3。犬蜱携带扁平无形体gltA基因的PCR扩增产物琼脂糖凝胶电泳见图4,结果显示扩增产物在580 bp处出现目的片段,与预期片段大小相符。扩增序列测序后经Blast比对后与GenBank上已有的扁平无形体序列同源性为100%。
种类 A. pagocytophilum A. bovis A. platys 阳性 0 0 6 感染率/% 0 0 1.1 -
蜱虫是犬只常见的外寄生虫,研究报道,有多种蜱类可以寄生在犬的体表,如血红扇头蜱(Rhipicephalus sanguineus)、长角血蜱(Haemaphysalis Iongicornis)、微小牛蜱(Boophilus microplus)、草原血蜱(Haemaphysalis verticalis)、图兰扇头蜱(Rhipicephalus turanicus)、铃头血蜱(Haemaphysalis campanulata)等,但由于各地地理环境不同,其优势蜱种也不同。宋胜男等[32]在2021年通过PCR检测的方法鉴定出石河子地区宠物犬体表436只图兰扇头蜱(Rhipicephalus turanicus),它也是新疆地区的优势蜱种。张艳等[33]在2021年通过PCR的方法鉴定出河南犬体表2只长角血蜱(Haemaphysalis Iongicornis),1只褐黄血蜱(Haemaphysalis flava)。张俭伟等[34]在2017年统计了我国中东地区20个城市宠物犬体表蜱虫种类,其中杭州、福州、广州、厦门、深圳、南宁、青岛、太原和长沙地区为血红扇头蜱;郑州、石家庄、济南和重庆地区为长角血蜱;上海、北京、天津、西安和宁波地区则均存在血红扇头蜱与长角血蜱。陈绍基等[35]在2020年通过PCR检测出重庆市荣昌区中华田园犬体表有长角血蜱。本研究通过形态学和分子生物学相结合的方法鉴定出犬体表寄生蜱均为血红扇头蜱,与国内大部分地区犬体表蜱虫鉴定结果一致[36-38]。血红扇头蜱对环境适应性强,广泛分布于世界各地[39],它也是犬最常见的寄生蜱种。本次调查结果显示蜱种种类分布较为单一,这也证明了血红扇头蜱是犬的优势蜱种,这一方面是因为血红扇头蜱本身属于热带物种,海南岛炎热的气候条件为其提供了良好的生存环境;另一方面可能是由于采样点的局限性造就了单一蜱种,因此,要获得更丰富的犬寄生蜱种类资料,还需要扩大采样范围及样本数量。
基于蜱虫16S rDNA基因构建的系统发育树表明,本试验中所得到的HN-01和HN-02序列处于同一大的分支上,但分别聚集在不同的小分支上,HN-01序列与美国所得到的血红扇头蜱同源性较高,HN-02序列与泰国的血红扇头蜱同源性较高,然而2个序列与国内甘肃、河北等地的血红扇头蜱亲缘关系较远。这可能是由于海南独特的地理位置造成蜱种地域差异性。
-
在国内外,关于蜱虫感染无形体的报道有很多。Hosseini等[40]在伊朗不同的地理区域分别鉴定出亚洲璃眼蜱(Hyalomma asiaticum)、单峰驼璃眼蜱(H. dromedarii)、血红扇头蜱(Rhipicephalus sanguineus)和牛蜱(Boophilus),并在牛蜱中获得一条边缘无形体序列和在血红扇头蜱中获得2条绵羊无形体序列。Qin等[41]调查了我国山东省胶南市长角血蜱中无形体的感染率,结果发现嗜吞噬细胞无形体、牛无形体和山羊无形体的感染率分别为0.10%、1.55%和0.33%。然而,在我国集中于犬体表寄生蜱携带无形体的调查研究较少。本试验通过对采集的所有犬体表蜱虫进行无形体16S rRNA和gltA基因的PCR检测结果发现扁平无形体阳性率为1.1%(6/546),未检测到嗜吞噬细胞无形体和牛无形体。2018年韩宏晓等[36]通过PCR的方法鉴定出上海地区宠物犬体表的血红扇头蜱,并对蜱虫携带嗜吞噬细胞无形体的情况进行了调查,结果也未检测到,与本试验结果一致。Yuasa[19]在2017年用PCR检测的方法调查了台湾省犬体表寄生血红扇头蜱携带扁平无形体的阳性率为3.6% (11/306),这一结果高于本实验调查得到的扁平无形体阳性率。尽管本试验中检测到的犬蜱感染扁平无形体的阳性率较低,但是这也间接反应了犬只本身感染扁平无形体的情况,这也需要后续对犬只进行更加深入的调查。
此外,我国养宠家庭逐年上升,犬作为一种伴侣动物并且与人类的活动息息相关,二者均存在被蜱叮咬并感染蜱传疾病的危险。虽然扁平无形体还未被公认为是一种人兽共患病原,但已有感染人的记录。据报道,在委内瑞拉的2名女性患者中检测到扁平无形体的病原[23]。为了避免蜱虫叮咬及感染蜱传疾病的危险,犬主应加强对犬只的行为约束,避免带犬只去野外或草地玩耍,另外也应定期对犬只进行卫生管理,定期驱虫,做好防蜱灭蜱工作。
-
本试验对海南部分地区犬体表寄生蜱进行形态学和分子生物学鉴定,结果全为血红扇头蜱。并通过PCR的方法对蜱虫进行了无形体的检测,结果表明犬体表蜱携带扁平无形体的阳性率为1.1%(6/546),没有检测到嗜吞噬细胞无形体和牛无形体。
Identification of tick species and investigation of anaplasma spp. in some areas of Hainan
DOI: 10.15886/j.cnki.rdswxb.2023.02.004
- Received Date: 2021-11-19
- Accepted Date: 2022-12-14
- Rev Recd Date: 2022-06-01
- Available Online: 2023-03-21
- Publish Date: 2023-03-25
-
Key words:
- dog /
- Rhipicephalus sanguineus /
- Anaplasma platys /
- molecular identification
Abstract: Ticks are arthropods that transmit more types of pathogens globally than any other vector, and they can transmit a variety of diseases by sucking blood from mammals, birds and reptiles, including humans. Anaplasma spp are an important tick-borne pathogens, which have a significant impact on human and animal health and the development of animal husbandry. In order to investigate the species of ticks on canine body surface and the situation of ticks carrying Anaplasma spp. in some areas of Hainan province, morphological observation, molecular biological identification and evolutionary analysis were performed on canine ticks from four regions of Hainan province, and 16S rRNA and gltA genes were used to detect Anaplasma spp in canine ticks. Morphological observation and molecular biological identification showed that all the ticks were Rhipicephalus sanguineus. The phylogenetic tree established based on tick mitochondrial 16S rDNA gene indicated that the HN-01 and HN-02 gene sequences obtained were in different genetic evolutionary clade, and that HN-01 was in the same clade as the known tick from the United States (MH018842). HN-02 was in the same branch as R. sanguineus from Thailand (KC170744), and was far related to R. sanguineus from Gansu Province (JF979377) and Beijing (KC203362). PCR results based on the gltA gene showed that A. platys was carried by canine ticks with a positive rate of 1.1% (6/546). PCR detection based on 16S rRNA gene indicated that the canine body surface ticks obtained did not carry Anaplasma pagocytophilum and Anaplasma bovis.
| Citation: | ZHOU Sa, LIN Yang, ZU Haiyue, WANG Jinhua. Identification of tick species and investigation of anaplasma spp. in some areas of Hainan[J]. Journal of Tropical Biology, 2023, 14(2): 159-165. doi: 10.15886/j.cnki.rdswxb.2023.02.004 |








 DownLoad:
DownLoad: