-
茚虫威是由美国杜邦公司开发的含有手性分子的恶二嗪类杀虫剂,其中(+)-S-茚虫威具有杀虫活性,对鳞翅目害虫有较好的防效[1]。由于单一纯异构体开发成本和技术工艺要求高、成本高,目前国内外市面上销售的茚虫威杀虫剂多数为(+)-S-茚虫威和(−)-R-茚虫威两种对映体的混合剂型,其中杜邦公司生产的茚虫威产品主要有3种对映体混合剂型:DPX-JW062(S∶R =1∶1)、DPX-MP062(S∶R=3∶1)、DPX-KN128(S∶R=1∶0)[2],中国市场上茚虫威产品主要是富含S异构体的产品(S∶R =2.2∶1和3∶1) [3-4]。茚虫威广泛应用于对棉花、玉米、水稻和果树等的害虫防治[5-8]。由于桑园往往与农田、果园等呈交错分布,对桑园附近农田及果园施药时,杀虫剂会随空气漂移落在桑树上对桑叶产生污染[9-10]。家蚕(Bombyx mori)进食被杀虫剂污染的桑叶后后果严重,甚至可导致蚕茧绝收,不仅给养蚕业造成巨大的经济损失,而且对丝绸产业产生影响[11-12]。目前对环境生物家蚕的毒性报道中使用的多为茚虫威商品药,其中陈伟国等[13]测定30%茚虫威悬浮剂对3龄期蚕的24 h LC50为0.3604 mg ·L−1,俞瑞鲜等[14]和陈丽萍等[15]测定15%茚虫威悬浮剂对家蚕96 h LC50分别为0.449 mg ·L−1和0.439 mg ·L−1,毒性为剧毒。(+)-S-茚虫威、(−)-R-茚虫威及茚虫威原药对家蚕毒性研究较少,仅有石丽红[16]采用浸叶法测定了(+)-S-茚虫威(>98%)、(−)-R-茚虫威(>98%)和S-茚虫威富集体(2.33S∶1R)对家蚕96 h的急性毒性报道,LC50分别为1.96、108和6.89 mg L−1。
有研究发现茚虫威手性异构体对斑马鱼的毒性具有对映选择性[17-18],诱导斑马鱼产生氧化应激毒性且表现出对映选择性[19]。然而茚虫威原药、(+)-S-茚虫威和(−)-R-茚虫威对家蚕的氧化应激的影响尚未见报道,因此本研究以家蚕为生物模型,采用喷雾法测定茚虫威原药(3S:1R)、(+)-S-茚虫威和(−)-R-茚虫威对家蚕的急性毒性,测定其抗氧化酶超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和过氧化物酶(POD)的活性变化来评价茚虫威原药(3S:1R)、(+)-S-茚虫威和(−)-R-茚虫威对家蚕的毒性效应,为后续茚虫威的实际应用及其对于环境生物的安全性评价提供理论依据。
-
试验所用家蚕品种为‘秋风’ב月白’,蚕卵购于诸暨市鉴宝农业开发有限公司。实验室自行催青和饲养,在实验室经2 d孵化,幼虫用新鲜嫩桑叶喂养,恒温养虫室设置温度条件为(27±1)℃,相对湿度为70%~85%,光照黑暗比为16 h:8 h,以二龄起蚕为供试生物,挑选活性良好、身体健康、体态大小一致、起蚕时间一致的二龄起蚕用作试验。桑叶品种选用‘云桑二号’,购于诸暨市鉴宝农业开发有限公司。超氧化物歧化酶(SOD)测定试剂盒、过氧化氢酶(CAT)测定试剂盒和过氧化物酶(POD)测定试剂盒均购于南京建成生物工程研究所有限公司。茚虫威原药(3S:1R,98%)购于南京乐邦化工有限公司,(+)-S-茚虫威(98%)和(−)-R-茚虫威(98%)由上海大赛路手性公司拆分所得。
-
参照《化学农药家蚕慢性毒性试验准则》(NY/T 3087—2017)附录B中农药家蚕急性毒性试验—喷雾法,使用溶剂DMF和助剂Tween-80将茚虫威原药、(+)-S-茚虫威和(−)-R-茚虫威配制成100 mg ·L−1的茚虫威母液,将母液梯度稀释,分别为14.600 、7.300 、3.650 、1.825 和0.915 mg ·L−1,同时设置空白对照组CK和助溶剂对照组SC。选取桑树顶端大小和质量尽量一致的新鲜有光泽的嫩桑叶,每个处理的桑叶用量为(2.5±0.1)g。将桑叶用清水清洗干净并晾干表面水渍,使用喷雾塔设备将试验药液均匀喷洒到桑叶的背面,以初始喷雾体积为5 mL,喷雾压力为0.048 MPa,沉降时间为20 s作为标准参数。将桑叶自然风干至其表面看不到药液,再将染毒桑叶置于培养装置中。选择健康、大小一致的二龄起蚕转到桑叶上。每处理20头二龄起蚕,重复3次。每结束1个处理组的喷雾操作后均对喷雾塔内壁进行清洗擦拭,以防止内壁形成水滴滴落。喷药前用万分之一天平称量桑叶的质量,喷药后再次立即称量桑叶质量,进而可测定每片桑叶上喷施茚虫威的准确含量。计算所得的试验浓度为2.920 、1.460 、0.730 、0.365和0.183 mg·kg−1,在药剂处理后24、48、72及96 h观察并记录家蚕的中毒症状和死亡情况。用移虫笔轻触家蚕,家蚕无反应视作死亡。当毒性测定试验中溶剂对照组和空白对照组的死亡率小于10%视为有效试验。
-
参照国家农药室内生物测定试验准则NY/T1154.7-2006进行共毒系数(CTC)计算。若CTC≤80,表明2种药剂混用为拮抗作用;若80<CTC<120,则为相加作用;若CTC≥120,则为增效作用。
-
使用喷雾法按照急性毒性试验浓度处理24 h,其中各浓度处理组设置多组重复以满足试验需求,从每个浓度的处理组中各取10头活蚕分别装入离心管中,用液氮处理后加入2 mL的1×PBS溶液进行冰浴研磨,然后使用Z216MK冷冻离心机4 ℃ 3 500 r·min−1离心10 min后,取上清液作为粗酶液。
-
蛋白质含量的测定采用考马斯亮蓝G-250染色法进行。首先用0.15 mol·L−1 的NaCl配制出100 mL浓度为1 000 mg · L−1的牛血清白蛋白,备用。分别向编号0 ~ 8的离心管中加入0.01、0.02、0.06、0.08、0.1、0.12、0.14和0 mL牛血清白蛋白(1 000 mg · L−1),然后依次加入0.19、0.18、0.14、0.12、0.10、0.08、0.06和0.2 mL蒸馏水,每管中加入5 mL考马斯亮蓝G-250染剂后用离心机涡旋混匀后静置5 min,再将其移到比色皿内置于752紫外分光光度计中,波长调至595 nm,测定吸光值绘制蛋白标准曲线,8号管用于调零,每个处理重复3次。
-
向编号1~7号的离心管中分别加入0.2 mL上述步骤制备的粗酶液,在8号管中加入0.2 mL蒸馏水,向8个离心管中分别加入5 mL考马斯亮蓝G-250染剂,再将离心管中液体转移至比色皿内,将比色皿置于紫外分光光度计中,波长调至595 nm,测定吸光值,8号管用于调零,每个处理重复3次。
-
SOD、CAT和POD酶活力的测定使用752紫外分光光度计进行测定,具体试验步骤操作参照南京建成试剂盒进行。
-
使用软件IBM SPSS statistics 21.0软件进行统计分析,用GraphPad Prism 9进行数据分析与绘图。
-
参照《化学农药家蚕慢性毒性试验准则》使用桑叶浸渍修正系数对农药对家蚕的急性毒性等级划分标准的LC50进行修正后可知,茚虫威原药对家蚕96 h的毒性等级为高毒,(+)-S-茚虫威和(−)-R-茚虫威对家蚕96 h的毒性等级为剧毒,毒性大小依次为(−)-R-茚虫威> (+)-S-茚虫威>茚虫威原药。主要中毒症状为吐黄水、蚕体发黑、蚕体缩短现象,随着药液浓度的升高,中毒家蚕越多。(+)-R-茚虫威对家蚕的LC50是(−)-S-茚虫威的2.75倍,茚虫威的2种手性对映体对家蚕表现出对映选择性毒性。茚虫威原药(3S:1R)的共毒系数为12.868≤80,(+)-S-茚虫威和(−)-R-茚虫威2种药剂混合表现为拮抗作用(表1)。
药剂名称 96 h致死中浓度LC50
/(mg ·kg−1)95%置信区间
/(mg ·kg−1)回归方程 R2 共毒系数 茚虫威原药 0.379 0.182~0.616 y=1.14+2.57x 0.911 12.868 (+)-S-茚虫威 0.041 0.002~0.093 y=2.25+1.55x 0.895 (−)-R-茚虫威 0.113 0.008-0.232 y=1.75+1.81x 1.000 -
以牛血清白蛋白为标准蛋白绘制蛋白质标准曲线,标准曲线方程为y=0.044x+0.048,相关系数R2为0.971。
-
从图1-A可知茚虫威原药诱导家蚕体内的超氧化物歧化酶(SOD)活性上升,和对照组SC相比,5个处理组SOD活性均显著上升。在图1-B中(+)-S-茚虫威处理组中0.730、1.460和2.920 mg ·kg−13个浓度诱导SOD活性显著上升,其余2个浓度SOD活性显著下降。在图1-C中(−)-R-茚虫威处理组中0.183和0.365 mg ·kg−1浓度下诱导SOD活性显著降低,SOD活性在0.730、1.460和2.920 mg ·kg−1浓度下显著上升。
在0.183和0.365 mg ·kg−1浓度下,茚虫威原药诱导SOD酶活性和对照相比上升8.577%和23.585%。(+)-S-茚虫威诱导SOD酶活性和对照相比下降28.536%和23.317%,(−)-R-茚虫威诱导SOD酶活性和对照相比下降14.735%和8.170%。在0.730、1.460和2.920 mg ·kg−1浓度下,茚虫威原药诱导SOD酶活性和对照相比上升34.129%、20.626%和32.948%。(+)-S-茚虫威诱导SOD酶活性和对照相比上升66.079%、25.723%和38.650%,(−)-R-茚虫威诱导SOD酶活性和对照相比上升77.247%、32.006%和54.537%。0.183和0.365 mg ·kg−1(+)-S-茚虫威对SOD的抑制效果更好。
-
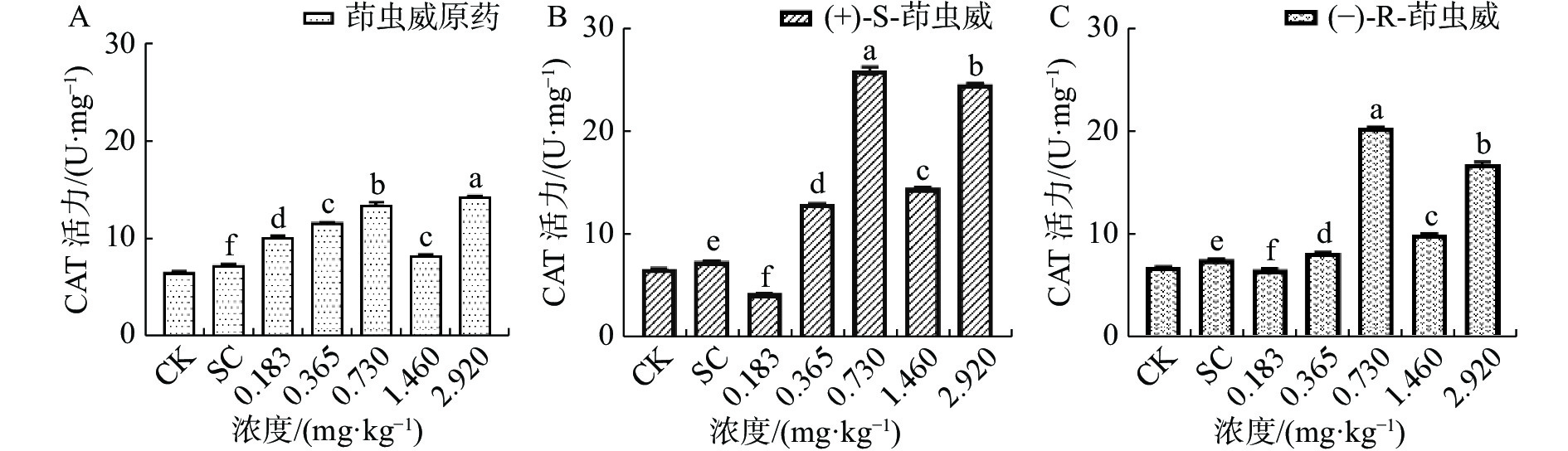
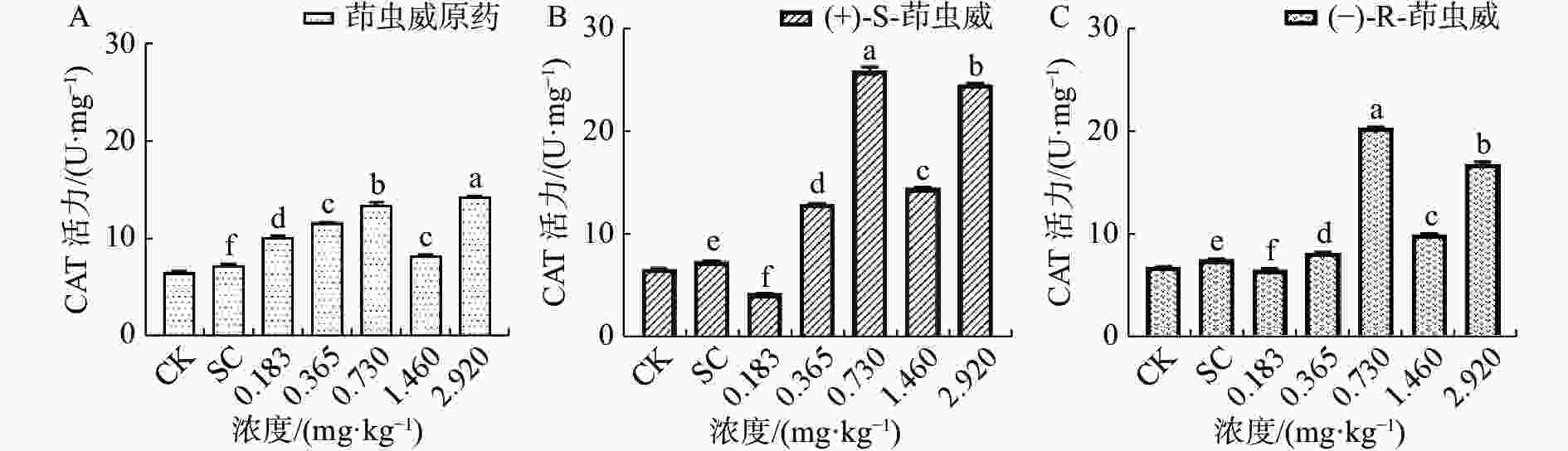
由图2-A可知茚虫威原药诱导家蚕体内的过氧化氢酶(CAT)活性整体呈上升趋势,和对照相比,5个处理组CAT活性均显著上升。由图2-B可以看出(+)-S-茚虫威处理组中除0.183 mg ·kg−1浓度下CAT活性显著下降,其余处理组CAT活性均显著上升。由图2-C可知(−)-R-茚虫威处理组中除0.183 mg ·kg−1浓度下CAT活性下降,其余4个浓度CAT活性均显著上升。
和对照相比,在0.183 mg ·kg−1浓度下茚虫威原药诱导CAT活性上升39.054%,(+)-S-茚虫威和-)-R-茚虫威诱导CAT活性下降42.420%和13.169%。在0.365、0.730、1.460和2.920 mg ·kg−1浓度下,茚虫威原药诱导CAT活性上升59.065%、84.756%、33.526%和95.957%,(+)-S-茚虫威诱导CAT活性上升76.001%、251.725%、34.229%和234.280%,(−)-R-茚虫威诱导CAT活性上升8.590%、173.678%、30.407%和125.553%。在0.183 mg ·kg−1(+)-S-茚虫威对CAT的活性抑制效果最好。
-
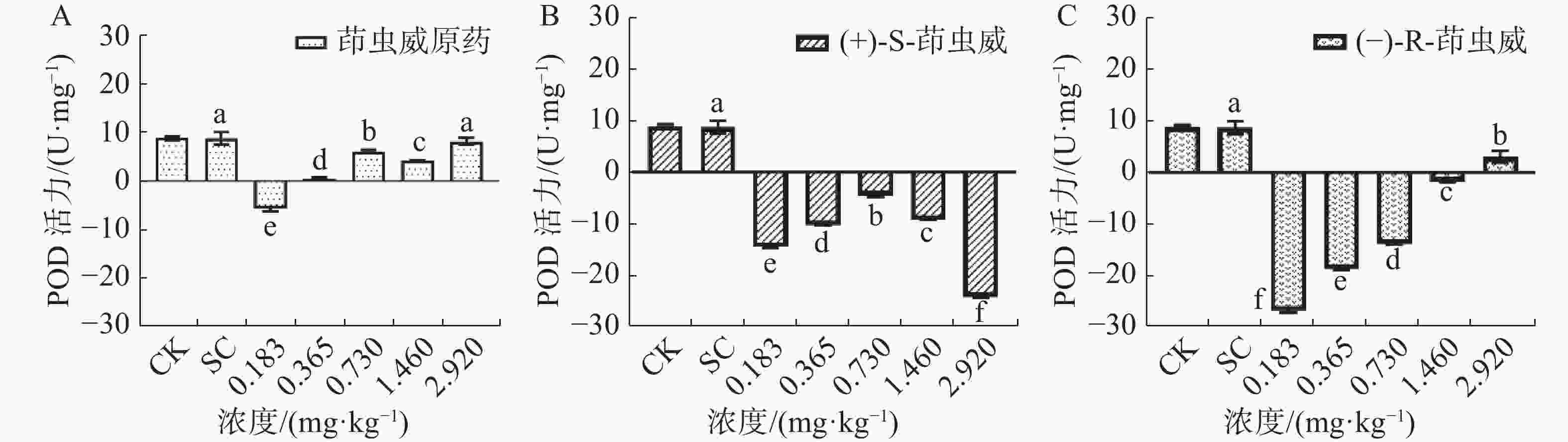
由图3-A可知,茚虫威原药处理组中5个浓度均诱导家蚕体内POD活性下降。图3-B结果表明,和对照相比,(+)-S-茚虫威诱导POD活性均显著下降。由图3-C可知,(−)-R-茚虫威诱导POD活性下降,和对照相比,5个浓度家蚕POD活性均显著下降。
和对照相比,在0.183、0.365、0.730、1.460和2.920 mg ·kg−1浓度下茚虫威原药诱导POD活性下降165.885%、93.751%、30.108%、52.268%和6.206%;(+)-S-茚虫威诱导POD活性下降266.722%、217.385%、151.490%、205.520%和378.033%;(−)-R-茚虫威诱导POD活性下降406.287%、314.239%、258.185%、119.468%和64.830%。(−)-R-茚虫威在0.183、0.365、0.730 mg·kg−1对POD活性的抑制效果最好,(+)-S-茚虫威在0.183和2.920 mg ·kg−1 2个浓度对POD的抑制效果最好。
-
对于大多数手性农药而言,其一种手性异构体对同种生物的急性毒性往往高于其外消旋体,且2种手性异构体可能在同种生物中表现出不同的毒性[20-21]。石丽红[16]采用浸叶法测定的(+)-S-茚虫威和(−)-R-茚虫威96 h的LC50分别为1.96 和108 mg·L−1,用桑叶浸渍修正系数修正后为0.901 6和49.680 0 mg·kg−1。本试验家蚕毒性测定方法中喷雾法可通过试验前后称量桑叶重量对农药进行定量,和浸叶法相比,该方法定量更精准、喷雾更均匀[22-23],本研究结果表明,(+)-S-茚虫威和(−)-R-茚虫威对家蚕96 h的LC50分别为0.041和0.113 mg·kg−1,茚虫威的2种手性异构体对家蚕的毒性存在对映选择性的差异。
据报道,斑马鱼成鱼暴露于不同浓度的(−)-R-茚虫威和(+)-S-茚虫威96 h后,(+)-S-茚虫威在96 h对斑马鱼胚胎的毒性是(−)-R-茚虫威的5.637倍;斑马鱼幼鱼暴露于不同浓度的(−)-R-茚虫威和(+)-S-茚虫威96 h后,(+)-S-茚虫威在96h对斑马鱼幼鱼的毒性是(−)-R-茚虫威的3.352倍[24]。本研究结果表明,(−)-R-茚虫威对家蚕的毒性是(+)-S-茚虫威的2.75倍,茚虫威的2种手性异构体对家蚕的毒性存在对映选择性的差异。
生物体中存在多种抗氧化酶系包括过超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)等,SOD、CAT和POD三者协调一致,处于动态平衡,从而使自由基维持在一个较低水平,防止自由基产生毒害[25]。微量农药诱导昆虫体内自由基(ROS)积累发生氧化应激[26-27],钩虾暴露于茚虫威24、48、96 h后,SOD和CAT活性随暴露时间增加而增加[28],低剂量的茚虫威诱导鲤鱼肾脏中的SOD和CAT酶基因表达均发生上调[29]。本试验结果表明,和对照相比,在(+)-S-茚虫威和(−)-R-茚虫威处理组中SOD和CAT活性在0.730、1.460和2.920 mg·kg−1 3个浓度时显著上升,SOD和CAT活性上升可能参与了家蚕氧化应激中自由基的清除过程[30],以减轻茚虫威对家蚕造成的损害。在茚虫威原药、(+)-S-茚虫威和(−)-R-茚虫威处理组中POD活性均下降,可能影响自由基清除过程中对H2O2的催化[31]。
杀虫剂混配后可能产生增效、相加或拮抗的联合作用效果[32-33]。三唑磷和氯氟氰菊酯混合剂对蚯蚓的联合毒性表现为协同作用,该混合剂对蚯蚓体内POD酶活性的诱导作用大于单独使用三唑磷或氯氟氰菊酯的效果[34]。阿维菌素和氟虫脲混合剂联合毒性表现为协同作用,混合农药处理的舞毒蛾幼虫SOD、POD和CAT活性均高于单独使用阿维菌素或氟虫脲处理组[35]。有研究表明,敌敌畏和溴氰菊酯及其混合剂均能抑制大鼠肝脏的SOD和CAT的活性,单独使用时溴氰菊酯抑制效果更好,敌敌畏与溴氰菊酯混配剂对SOD和CAT的抑制效果低于二者单独作用的效果总和,敌敌畏与溴氰菊酯混合使用对大鼠肝脏氧化应激的诱导具有拮抗作用[36]。二嗪农和溴氰菊酯混合对大鼠血液中MDA、GST、SOD的活性具有拮抗作用[37]。本试验结果表明,(+)-S-茚虫威和(−)-R-茚虫威)之间的毒性效应为拮抗作用,和二者联合毒性作用效果一致。
The acute toxic effects of chiral indoxacarb on Bombyx mori
DOI: 10.15886/j.cnki.rdswxb.20220035
- Received Date: 2022-05-30
- Accepted Date: 2023-04-18
- Rev Recd Date: 2022-06-27
- Available Online: 2023-04-23
- Publish Date: 2023-09-25
-
Key words:
- indoxacarb /
- chiral isomers /
- Bombyx mori /
- enantioselective /
- oxidative stress
Abstract: Indoxacarb belongs to oxadiazine insecticide, which blocks the insect sodium channel. Lepidopteran pests, such as Cotton Bollworm, Plutella xylostella, and Spodoptera litura, were effectively controlled by indoxacarb. The scholars have focused on the selective toxicity of indoxacarb isomers to non-target organisms. In this study the acute toxicity of indoxacarb and its isomers, (+)-S-indoxacarb and (−)-R-indoxacarb, on the silkworm Bombyx mori was evaluated by spray method. Additionally, the toxic effects of indoxacarb and its isomers on the silkworm were evaluated by measuring the changes in the activities of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) in the silkworm. The results showed that the 96 h-LC50 of indoxacarb, (+)-S-indoxacarb and (−)-R-indoxacarb to the silkworm were 0.379, 0.041 and 0.113 mg·kg−1 mulberry leaf, respectively. The (+)-S- indoxacarb and (−)-R- indoxacarb were highly toxic to the silkworm. The 96 h-LC50 of the (+)-S-indoxacarb was 2.750 folds higher than that of the (−)-R-indoxacarb, which suggested an enantioselective toxicity to the silkworms. The mixture of the (+)-S-indoxacarb and (−)-R-indoxacarb showed an antagonism effect with the co-toxicity coefficient being 12.868. The activities of SOD and CAT in the silkworm were significantly increased in the indoxacarb treatment groups, which induced oxidative stress in the silkworm. The activities of SOD significantly increased in the (+)-S-indoxacarb and (−)-R-indoxacarb treatment groups with the concentrations of 0.730, 1.460, and 2.920 mg·kg−1 mulberry leaf. The activities of CAT significantly increased in the (+)-S-indoxacarb and (−)-R-indoxacarb treatment groups with all the concentrations except the concentration of 0.183 mg·kg−1. The activities of POD were decreased in all the indoxacarb treatment groups. The effect of indoxacarb on SOD, CAT and POD was lower than the sum of the individual effects of the (+)-S-indoxacarb and (−)-R-indoxacarb, indicating that the mixture of the (+)-S-indoxacarb and (−)-R-indoxacarb produced antagonistic effects on inducing of oxidative stress in the silkworm. The toxic effects of indoxacarb, (+)-S-indoxacarb and (−)-R-indoxacarb on silkworms were preliminarily evaluated, which provides reference for the application of indoxacarb in the field. This result suggests that the indoxacarb when sprayed in the field should be kept away from mulberry gardens to avoid harm to silkworms.
| Citation: | ZHAO Wenyu, ZHANG Jie, ZHENG Shixiang, WANG Xinyu, SU Xiaoting, FAN Yongmei. The acute toxic effects of chiral indoxacarb on Bombyx mori[J]. Journal of Tropical Biology, 2023, 14(5): 514-520. doi: 10.15886/j.cnki.rdswxb.20220035 |








 DownLoad:
DownLoad: