-
珊瑚礁是多样性极丰富和生产力极高的海洋生态系统[1], 也是无数海洋生物的产卵、育苗、繁殖和觅食地[2]。在过去的几十年里,由于全球气候变化、海岸工程建设、富营养化和过度捕捞等多种影响因素[3] ,世界各地的珊瑚礁覆盖面积急剧减少。退化的珊瑚礁通常很难在没有人类干预的情况下自然恢复[4],因此科学家们开展了大量的珊瑚礁保护工作,通过持续优化珊瑚礁管理政策和开发多种珊瑚修复技术,辅助珊瑚礁恢复以确保未来的生态系统服务功能[5]。目前,珊瑚礁保护工作取得了跨越性进展,很多珊瑚修复方法和理念被广泛应用,如珊瑚园艺理念、简易修复装置、人工辅助有性繁殖等[6-7]。当前恢复退化珊瑚礁最成功的方法是“珊瑚园艺”,即依靠珊瑚无性繁殖的能力在苗圃上生长成为成熟的群体(育苗阶段),然后将其种植在受损的珊瑚礁上(修复阶段)[8]。珊瑚苗圃是指在一个相对受保护的环境中(降低天敌侵害威胁、相对低浓度的悬浮物、更少的藻类竞争等)种植珊瑚,能提高珊瑚应对环境压力的能力,促进珊瑚幼体补充[9],同时追求最大化的珊瑚生长率和存活率[10],这将为修复阶段的珊瑚供体量提供保障,保证移植阶段的修复效果。科研工作者们利用珊瑚苗圃已在世界各地的各种珊瑚礁上培育上百种珊瑚,繁殖的珊瑚群落多达数十万,目前,研究人员已经针对珊瑚苗圃的多个方面进行了实验研究,包括苗圃类型、苗圃位置、苗圃珊瑚附着等[11-13]。研究大都聚焦于检验和评估各类型珊瑚苗圃在本土环境下的适用性[14],但苗圃内部基础材料的结构设计却很少被重视。人工基底作为移植珊瑚的附着生长基质,是珊瑚苗圃的基本应用材料,其对珊瑚生长和存活有着决定性作用[15],同时也起到为珊瑚幼体提供沉降附着的作用。目前,多种园艺式苗圃使用镂空式材料作为繁育培养珊瑚的基底,如以色列的中层水苗圃及中国涠洲岛的浮动式苗圃、铁架式苗圃均采用塑料板、塑料无结网的材料[16-17];甚至有些珊瑚修复技术也使用镂空式材料来固定珊瑚礁区的碎石,如在菲律宾保护区修复破碎化的礁盘使用了塑料筛网,并将其作为珊瑚移植基底[18];在中国蜈支洲岛北部退化区域的长期修复研究中应用了镁铝合金网格板材料作为人工礁移植基底,并取得不错的修复效果[19]。镂空式基底材料在珊瑚修复领域的应用已经屡见不鲜,但这些简单廉价、易获取基底材料的孔径尺寸大小对珊瑚生长效果的影响是未知的,且缺乏相关实验数据参考。因此,笔者在蜈支洲岛的北部退化珊瑚礁区域使用不同孔径尺寸的铝制网格板搭建珊瑚苗圃,以此来探究人工基底的孔径尺寸大小对移植珊瑚生长、存活和生理状态的影响,并对适宜珊瑚生长的网格板孔径尺寸进行筛选,旨在为未来珊瑚苗圃的设计和珊瑚修复技术的开发提供研究基础。
-
2020年11月20日,选择三亚蜈支洲岛北部观瑚亭附近(18°19.025′N, 109°45.858′E)珊瑚礁退化严重的海域作为实验珊瑚苗圃地点。实验海域水深约6 m,投放有钢筋混凝土制作的长方体中空框架礁体,礁体的长宽高尺寸为200 cm×100 cm×100 cm,礁体的长段中部由20 cm×20 cm的承重柱连接,每个礁体质量约3 t。选择4个相邻的礁体在其上方框架铺设4种不同孔径尺寸的菱形铝镁合金材料的网格板,并使用直径5 mm的尼龙绳固定四周,将其作为珊瑚移植基底。每个实验组设置单个尺寸为200 cm×100 cm的网格板,共4个实验组,孔径规格为A组:1.0 cm×2.0 cm;B组:2.5 cm×2.5 cm;C组:4.5 cm×4.5 cm;D组:6.0 cm×6.0 cm。经截线样带法评估,不同实验组的本底环境均是以碎石、砂和珊瑚断枝残骸为主的高度破碎化区域。
-
采用小叶鹿角珊瑚(Acropora microphthalma,分枝形珊瑚)为实验对象。小叶鹿角珊瑚具有生长迅速、对环境变化敏感等特征,且小叶鹿角珊瑚在蜈支洲岛海域分布较为广泛。从实验海域的成熟期珊瑚苗圃上采集120株健康的珊瑚断枝,断枝的尺寸均在5~10 cm,将120株珊瑚断枝分为4组,每组各30株重复。断枝收集后,潜水员在水下迅速使用扎带移植到已搭建好的4种网格板上,移植时尽可能减少对珊瑚断枝的机械损伤,并且保证移植的珊瑚断枝彼此间隔大于30 cm,以满足移植珊瑚的生长空间需求。珊瑚移植完成后,使用奥林巴斯TG-6相机记录珊瑚初始面积,之后定期每两个月(实验具体的时间段)对移植珊瑚进行生长监测、生理指标及环境指标监测。
-
每两个月定期监测时,对4种网格板进行取样并带回实验室,进一步识别网格板上附着藻类并使用游标卡尺随机测量10条附着的草皮海藻类细丝长度,取平均值得出每种网格板上的平均草皮海藻类长度[20]; 截取面积为10 cm2样框内的网格板(N=3)进行烘干处理,差量法称量附着藻类干质量。使用浊度仪(Aqualogger210)原位记录贴近网格板的水体浊度和温度。使用便携笔式盐度计(BK-056)、便携式 pH /电导率仪(420C-01A Orion Star,美国)原位测量海水盐度和pH;使用1 L水瓶在每种网格板上方10 cm左右原位采集海水,海水经过0.45 μm混合纤维素酯微孔滤膜抽滤,滤膜用锡纸包裹后带回实验室进行水样叶绿素的检测;再使用滤膜过滤海水,称量过滤后海水收集100 mL到塑料试剂瓶中,利用营养盐全自动分析仪(德国SEAL,AA3)检测海水中无机硅酸盐(SiO32−)、无机氮(NH4+、NO2−、NO32−)、磷酸盐(P043−)的含量。
-
每两个月对4组珊瑚进行采样,潜水员使用斜口钳随机采集每组珊瑚中的2~3株,采集后放入盛有海水的密闭保温箱中暂养暗适应后,使用叶绿素调制荧光仪MINI-PAM-Ⅱ测量珊瑚共生虫黄藻的Fv/Fm(最大光化学量子产量),测量时将PAM光纤探头固定在距离珊瑚1cm的位置,每组珊瑚测量3~5个不同位置[21]。
-
珊瑚表面积的测定利用锡纸包裹法:截取一定面积的科研锡纸,称取其质量,通过面积-质量关系式得出科研锡纸的密度;锡纸包裹实验珊瑚表面,利用锡纸质量间接计算出锡纸面积进一步估算为实验珊瑚的表面积[22]。使用美国洁碧牌冲牙器和0.45 μm滤膜过滤后的海水冲刷珊瑚表面组织,收集虫黄藻样液并记录溶液体积,量取10 mL藻液,并使用血球计数板置于显微镜下计数,重复8次取平均值,虫黄藻密度用个·cm−2表示。
收集10 mL藻液并经过0.45 μm滤膜抽滤后,锡纸包裹避光保存,将滤膜研磨加丙酮萃取后,通过Trilogy实验室荧光仪(Turner Designs,7200-000)检测叶绿素a含量,最终叶绿素含量用μg·cm−2表示。
-
通过珊瑚垂直投影面积的方式,在水下潜水使用钢尺和奥林巴斯TG-6相机,对每株珊瑚进行垂直投影拍照。收集照片数据后,利用ImageJ软件测量珊瑚投影面积,计算移植珊瑚的面积增比及生长率[23],计算公式如下:
式中,S表示珊瑚面积增长的百分比(%);S1表示珊瑚断枝增长后的面积(cm2);S0表示珊瑚断枝的初始面积(cm2);V表示珊瑚生长速度(cm2·月);SA表示统计的珊瑚群落平均面积(cm2);Δm表示珊瑚生长的月数;N表示珊瑚存活率(%);N1表示珊瑚存活数量(株);N0表示珊瑚死亡数量(株)。认定珊瑚死亡的标准为珊瑚组织全部脱离死亡,只剩下珊瑚骨骼残骸,无任何生命迹象。
-
通过Excel处理得到监测实验数据。通过IBM SPSS Statistic 23进行单因素方差分析(one-way ANOVA)检验每个阶段不同组别之间的差异性,对数据进行方差齐性检验;事后比较选用假定等方差的LSD和未假定等方差的Tamhane's T2,以P < 0.05认为显著差异;使用独立样本t检验对两个样本的数据进行数据分析。实验结果表示为均值±标准误差(mean±SE),本实验数据作图在Origin 2021b中完成。
-
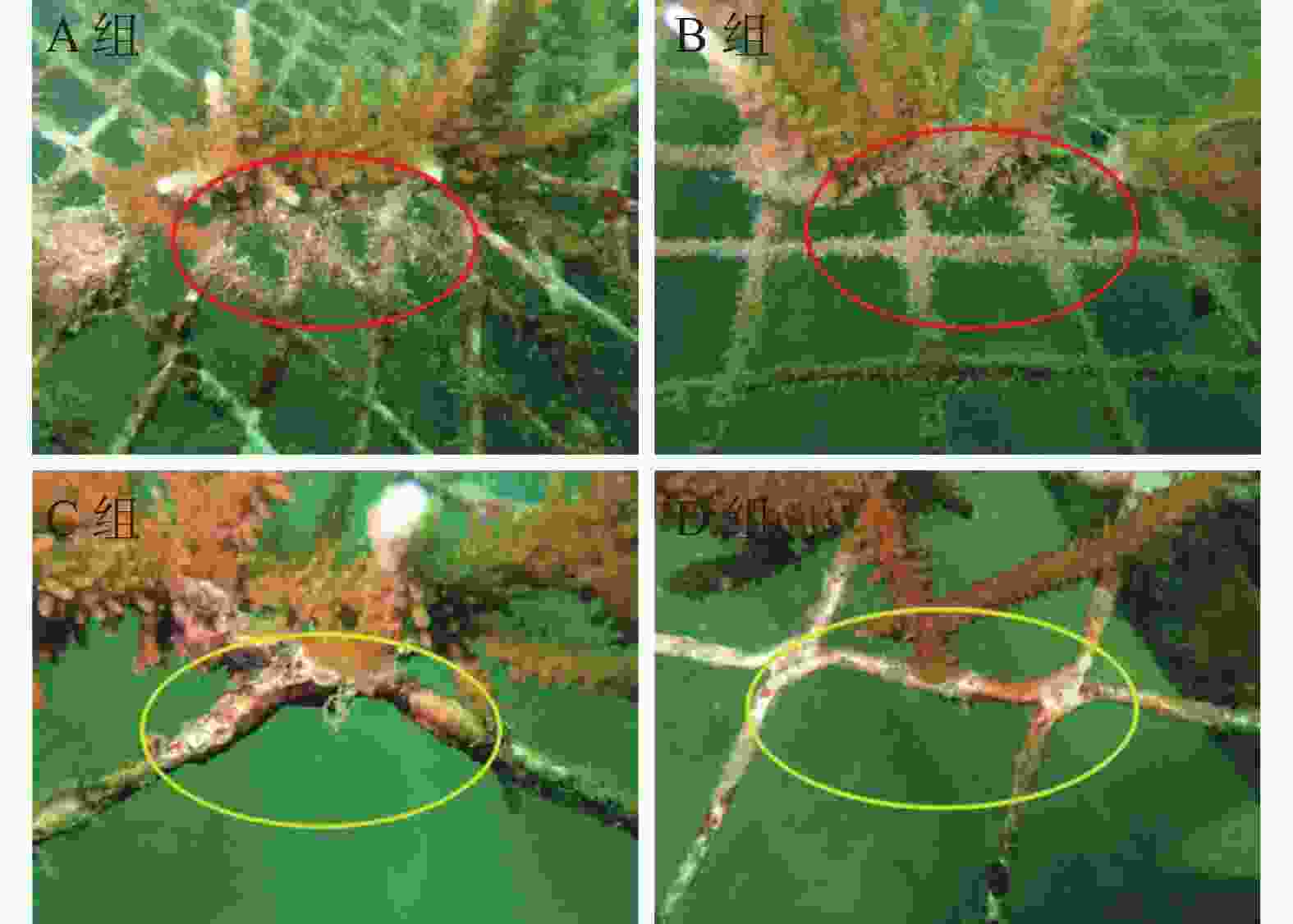
如图1所示,4组网格板的附着藻类情况有明显差异,现场观察到移植珊瑚后A组与B组的网格板生长有不同程度的丝状草皮海藻,即短而多产的草皮海藻类群落,而C组和D组多为钙化的壳状珊瑚藻,藻类分析情况如表1所示。3次监测中,随时间的推移,丝状藻长度均增长,3组的丝状藻长度均显著低于A组;同时,C组和D组的丝状藻干质量均显著低于A组。仅在C组和D组网格上附着有壳状珊瑚藻。
参数 第2月(2021年1月) 第4月(2021年3月) 第6月(2021年5月) A组 B组 C组 D组 A组 B组 C组 D组 A组 B组 C组 D组 草皮海藻
长度/mm1.81±
0.08a0.85±
0.04b0.81±
0.05b0.77±
0.07b2.10±
0.20a1.30±
0.06b1.28±
0.07b1.26±
0.09b6.12±
0.04a4.00±
0.04b2.99±
0.04c3.05±
0.04c草皮海藻
干质量/(g·cm−2)0.144±
0.004a0.027±
0.002b0.012±
0.001c0.009±
0.001c0.182±
0.006a0.042±
0.001b0.015±
0.001c0.011±
0.001c0.218±
0.003a0.057±
0.002b0.017±
0.001c0.012±
0.002c壳状珊瑚藻
干质量/(g·cm−2)— — 0.089±
0.006a0.055±
0.006b— — 0.235±
0.002a0.187±
0.008b— — 0.299±
0.019a0.192±
0.008b注:所有环境参数均使用标准差±标准误差的表示,不同字母表示不同时期下各组别在P<0.05水平上的显著;“—”代表网格板上未发现此藻类。 部分环境参数如表2所示,在实验期监测中,海水温度随时间变化逐渐升高,3次监测的日间海水温度依次为22.5、26.7、30.7℃,盐度范围维持在33.2~34.0之间,pH范围在8.20~8.32之间,温度、盐度和pH指标在同期监测时,组间均表现为无显著差异。在移植后第2月监测时,各组之间的浊度无显著差异,移植后第4月时A组浊度显著高于C组和D组,移植6月监测发现A组浊度显著高于其他3组。不同组间的水质环境变化不大,营养盐水平均在低浓度范围内。根据国家海水水质标准(GB 3097—1997)划分,实验海域的溶解无机氮(DIN)和磷酸盐(DIP)平均浓度均属于第一类海水。
环境参数 第2月 第4月 第6月 A组 B组 C组 D组 A组 B组 C组 D组 A组 B组 C组 D组 浊度/FTU 0.88±
0.05a0.82±
0.02a0.84±
0.06a0.82±
0.03a0.35±
0.01a0.22±
0.01b0.20±
0.02b0.19±
0.01b0.79±
0.01a0.40±
0.03b0.51±
0.06b0.34±
0.01b硅酸盐/(μmol·L−1) 2.45±
0.01a2.41±
0.02a2.08±
0.01b1.70±
0.01c1.06±
0.07a0.82±
0.03b0.68±
0.01b0.84±
0.03b1.29±
0.03a1.19±
0.0b1.24±
0.0ab1.25±
0.0ab无机氮/(μmol·L−1 ) 4.82±
0.01c5.46±
0.04a4.67±
0.04d5.84±
0.03b6.65±
0.24a6.47±
0.41a4.68±
0.26b6.28±
0.36ab6.88±
0.07并5.27±
0.08d7.26±
0.08a6.07±
0.02c磷酸盐/(μmol·L−1) 0.04±
0.01b0.04±
0.01b0.08±
0.01a0.02±
0.01b0.08±
0.0a0.04±
0.0b0.02±
0.0c0.02±
0.0c0.0±
0.0a0.0±
0.0a0.0±
0.0a0.0±
0.0a注:所有环境参数均使用标准差±标准误差的表示,不同字母表示不同时期下各组别在P<0.05水平上的显著。 -
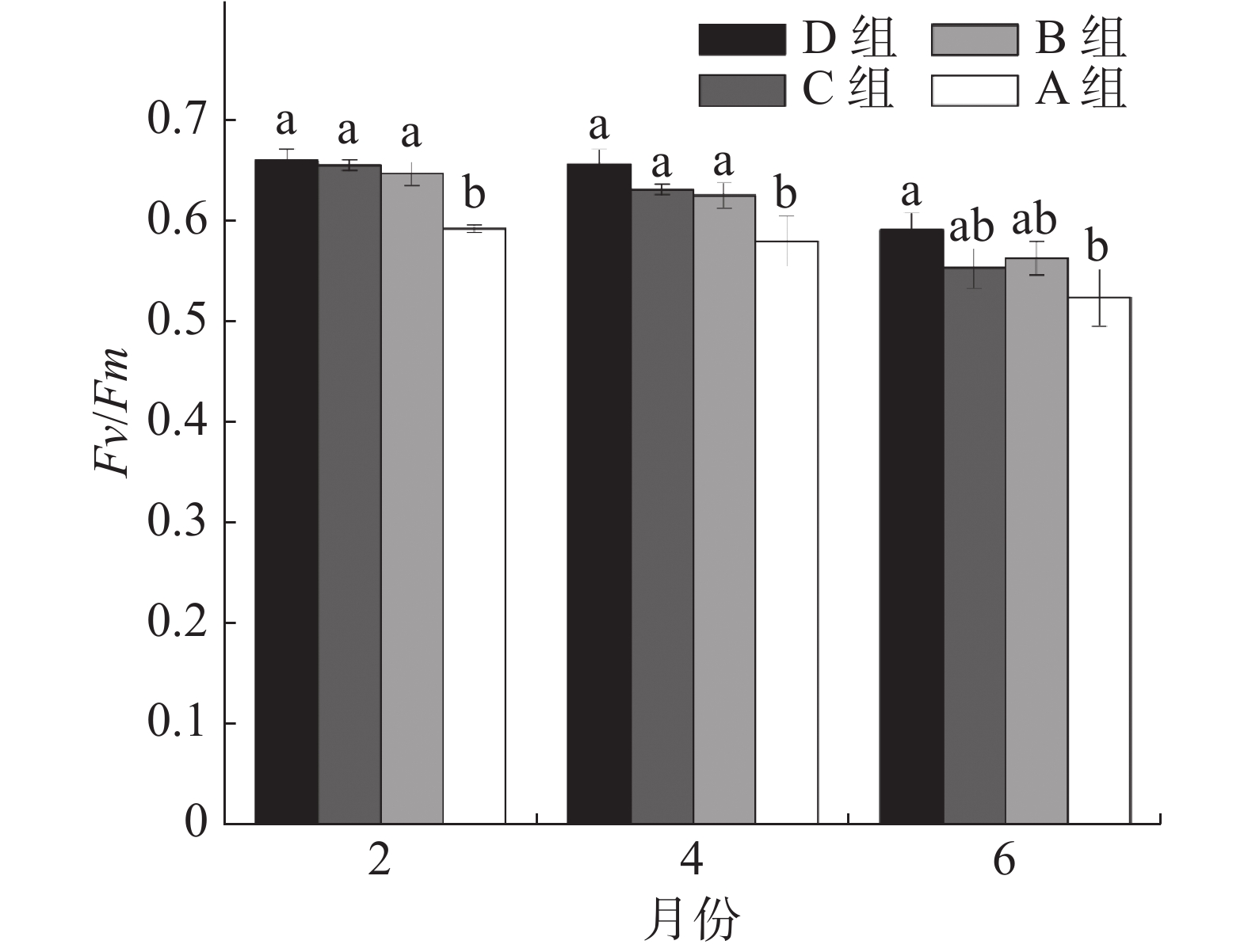
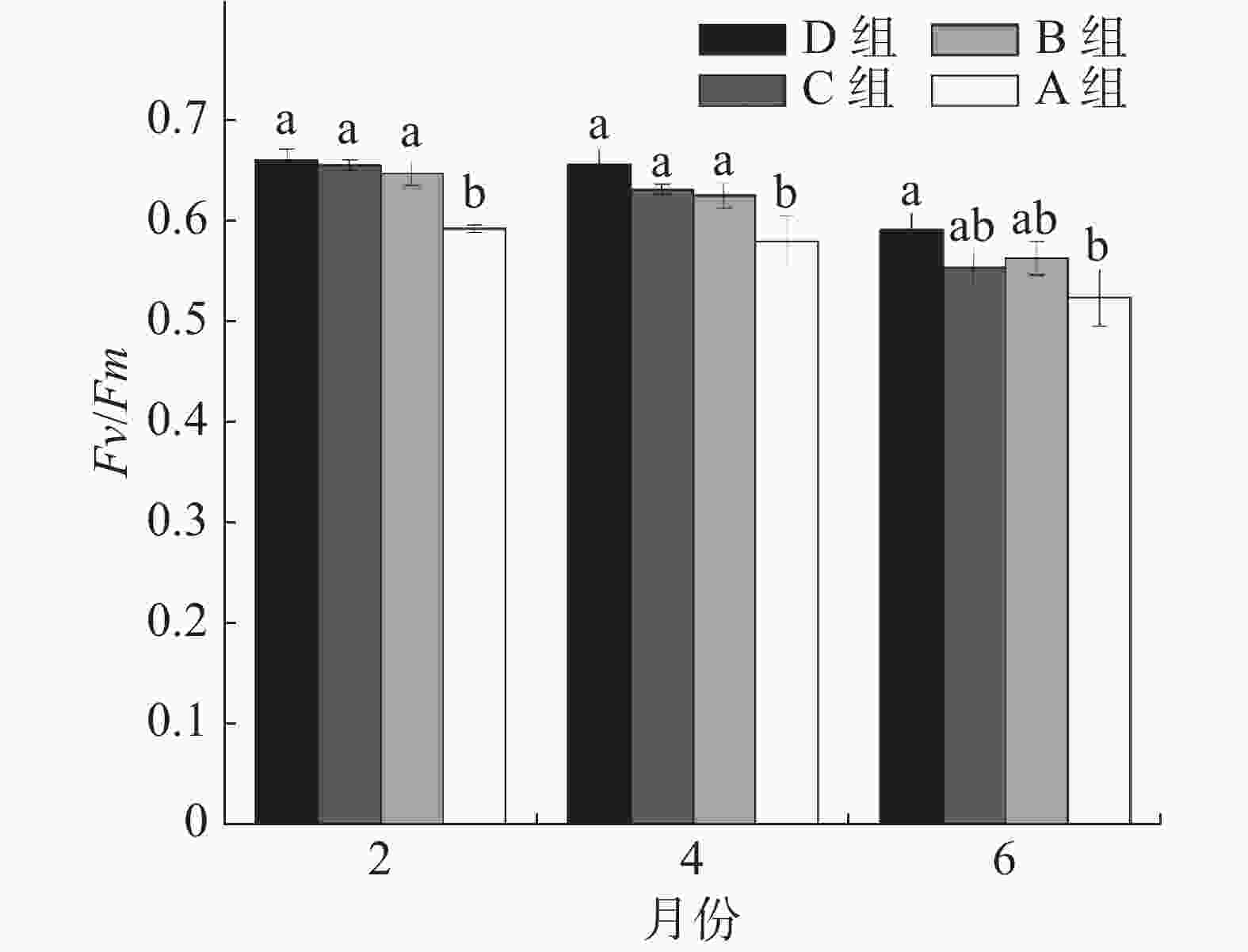
如图2所示,移植后第2月,A组珊瑚的Fv/Fm均值仅为0.615,B、C、D组分别比A组增加8%、10%、10%,且B、C、D 组与A组均存在显著差异。移植后第4月, B、C、D组珊瑚仍显著高于A组,分别增加7%、8%和12%。移植6个月后, D组显著高于A组(P<0.05),比A组高12%。
-
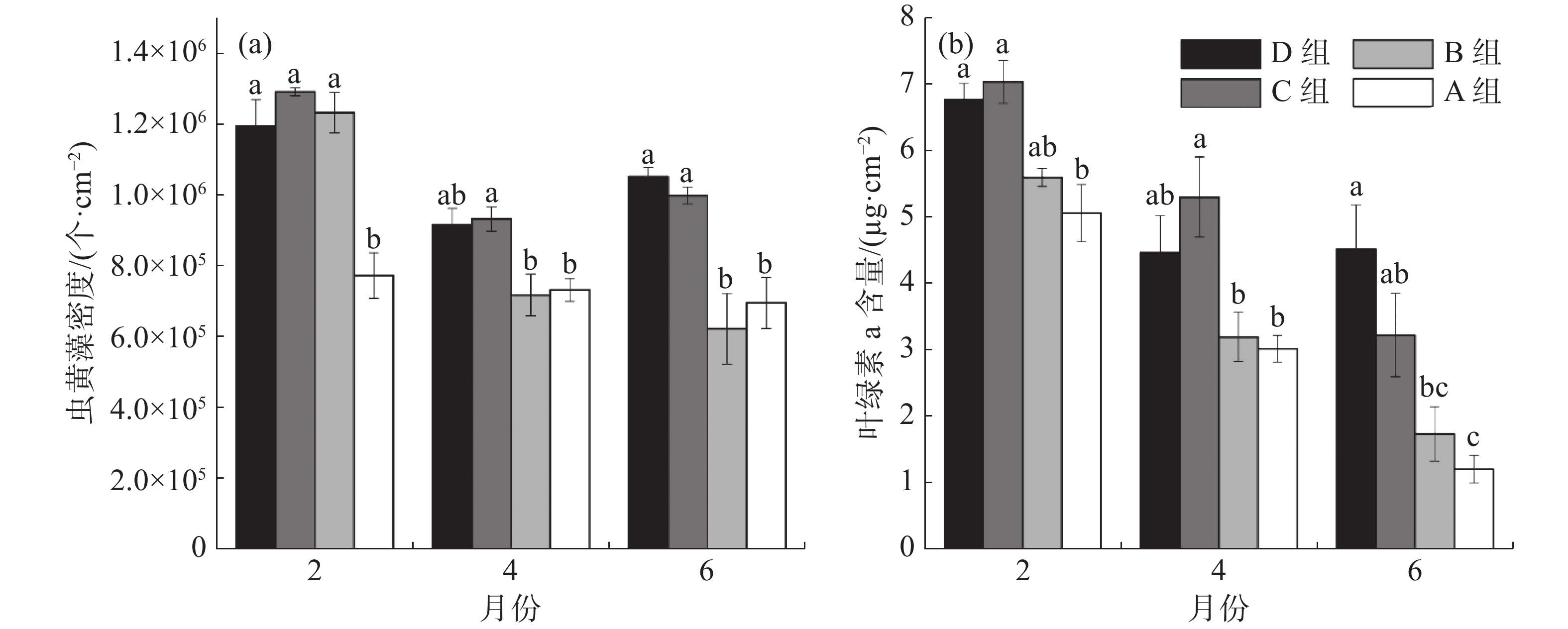
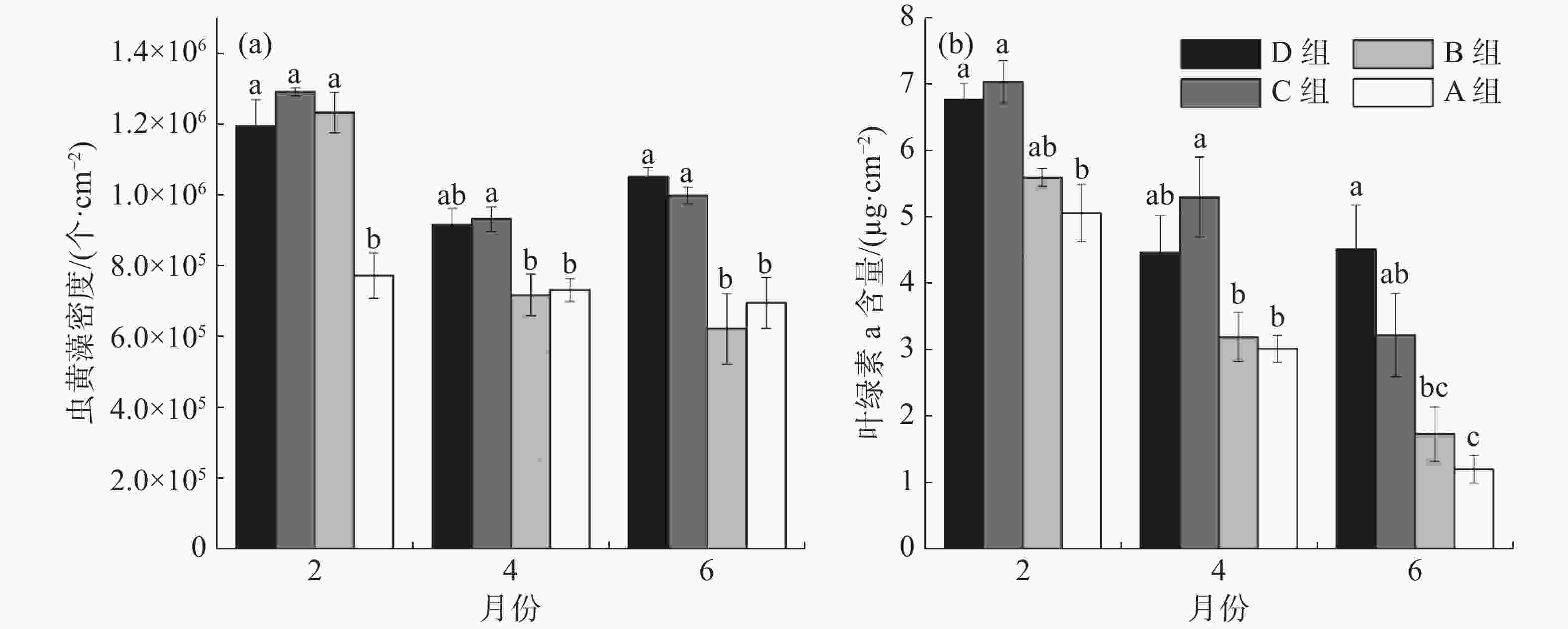
如图3-a所示,在移植后第2个月, B、C、D组的虫黄藻密度显著高于A组,分别增加了37%、40%和35%(P<0.05)。移植后第4个月, C组虫黄藻密度比A组增加了21%,比B组增加了23%,P<0.05。移植后第6个月,C、D组的虫黄藻密度均显著高于A组和B组,与A组相比分别增加了34%和30%,与B组相比分别增加了37%和41% 。从图3-b可知,移植后第2月, C、D组的珊瑚叶绿素a含量显著高于A组,分别增加了28%和25%(P<0.05); C组的叶绿素a含量相比B组增加了21%。移植后第4月, C组的叶绿素a含量仍显著高于A组与B组,分别增加43%和39%(P<0.05)。移植后第6月,D组叶绿素a含量最高, C、D组的叶绿素含量比A组分别增加了63%和73%,P<0,05; D组叶绿素a含量比B组增加61%(P<0.05)。
-
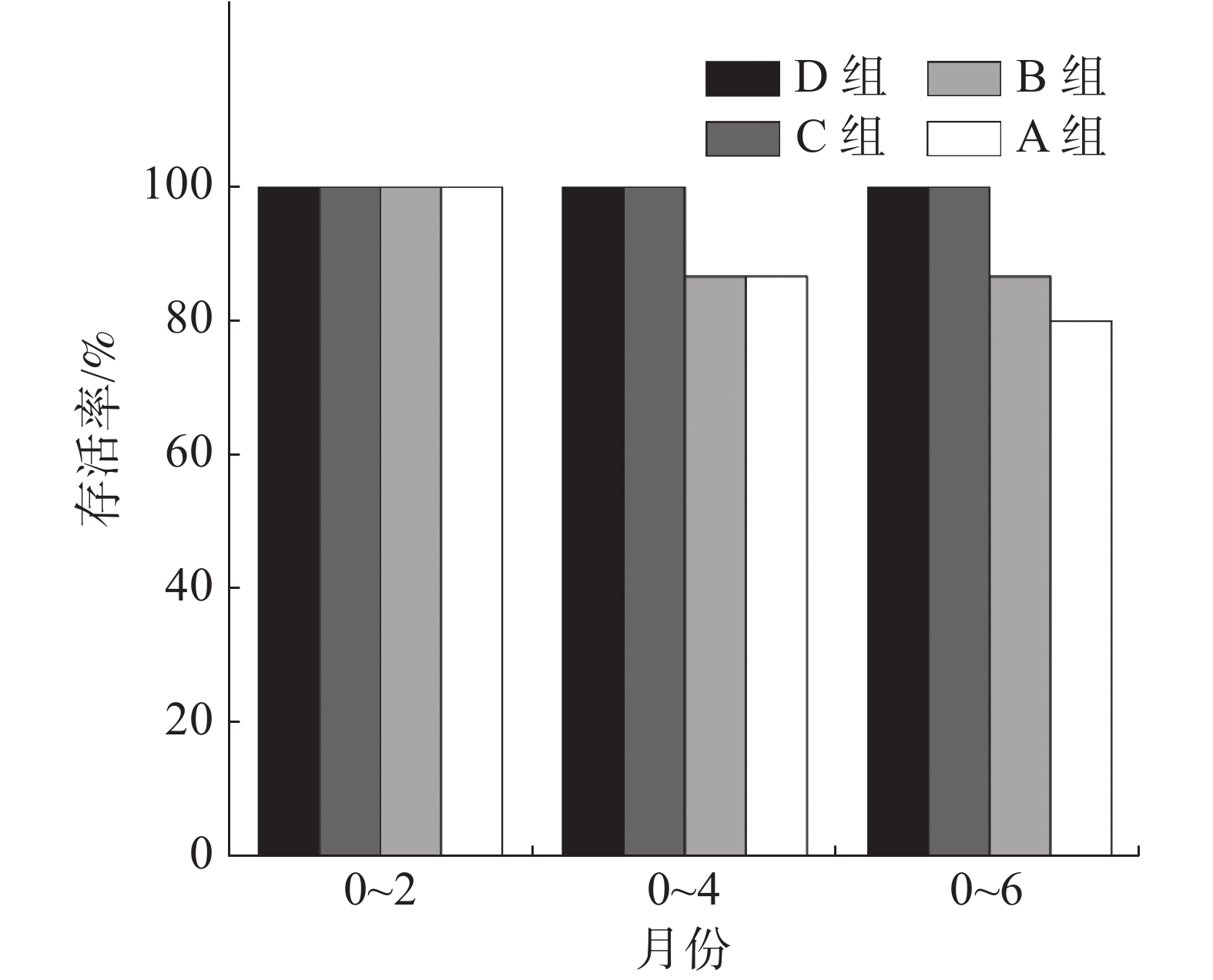
通过现场观测到珊瑚的残骸完整,且实验苗圃区域未暴发长棘海星和核果螺等敌害生物,排除了鱼类啃噬和敌害侵蚀的死亡原因。结果如图4所示,在整个实验期内移植的4组共120株珊瑚中,C组和D组珊瑚无移植个体死亡,存活率为100%;移植2个月时,4组珊瑚无死亡植株;移植4个月时,A组和B组珊瑚死亡4株(N=30),存活率约为87%;移植6个月时,A组在移植6个月时仍有死亡个体增加,存活率降为80%,B组仍为87%。
-
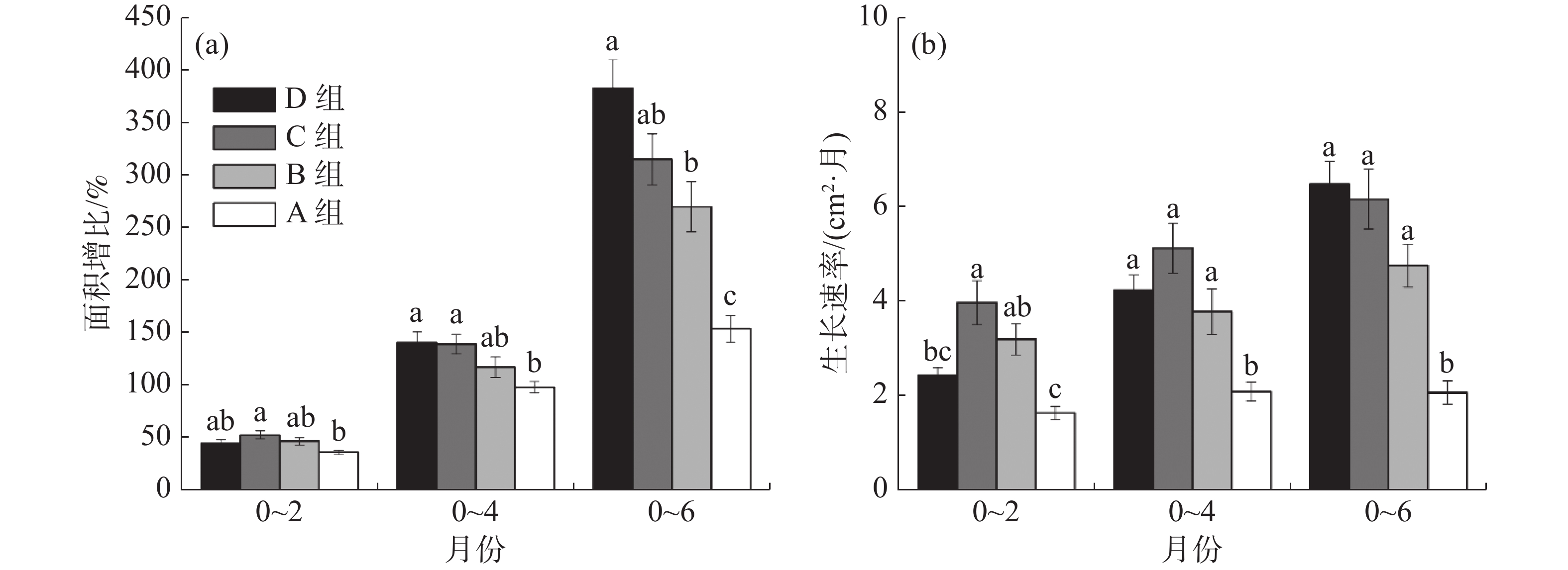
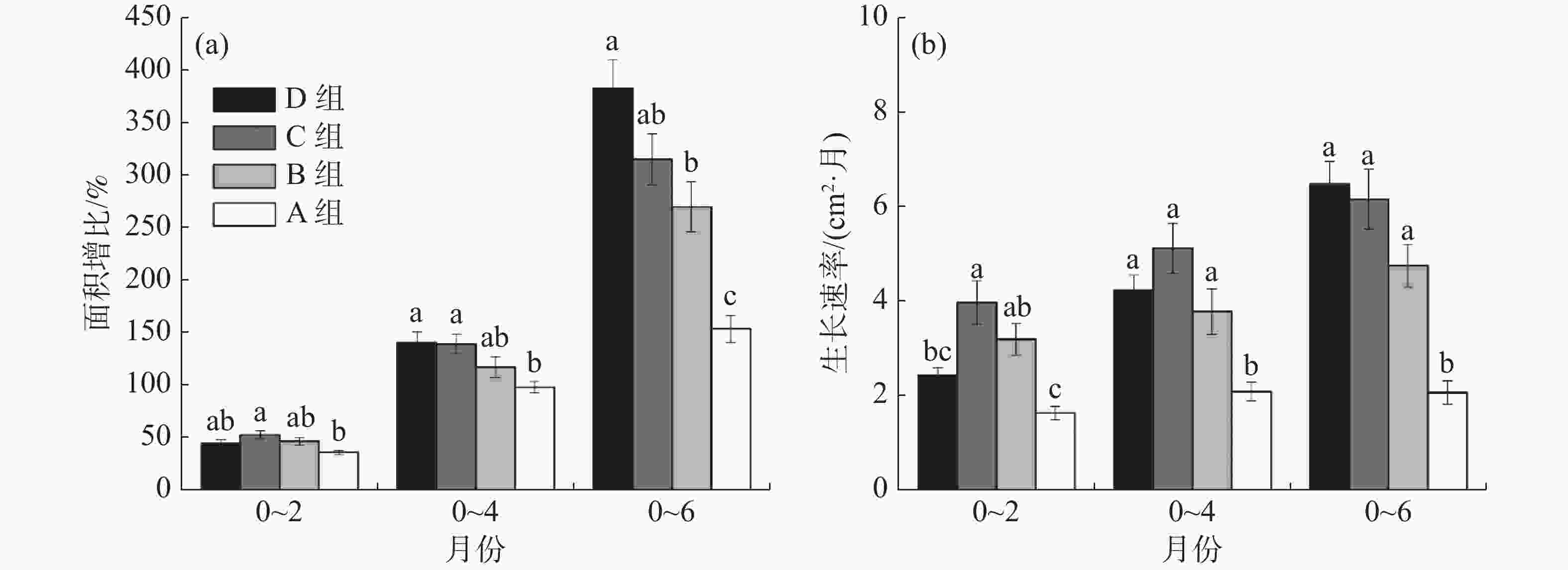
在6个月的实验期内,各孔径网格板上移植珊瑚的生长速率如图5所示。从图5-a面积增比的累积效应来比较,移植2个月后,C组的面积增比均值最高,为52.14%;B组和D组移植珊瑚的面积增比均值分别为45.58%和44.28%;A组最低,仅为35.02%。移植后4个月,C组和D组珊瑚面积增比均值为138.81%和140.12%,B组的珊瑚面积增比均值为111.88%,D组已经明显高于B组,A组仍最低,为98.65%,且C组、D组与A组具有显著差异。移植6个月后,D组珊瑚面积增比均值为382.82%,B组和C组珊瑚面积增比均值分别为269.86%和315.04%,D组已大幅度高于C组,与B组差异显著,A组珊瑚面积增比仍为最低,仅153.36%,且A组与其余3组均有显著差异。
由图5-b可知,A组珊瑚生长速率始终最低。移植2个月后, C组珊瑚生长速率比A组珊瑚增加60%(P<0.05),D组比C组降低38%(P<0.05),B、C、D组之间均无显著差异;移植4个月后, C组比A组增加了52%(P<0.05), C组比D组增加了16%;移植6个月后,D组生长速率达到最高,D组比C组增加了5%, D组比A组增加了68%(P<0.05)。
-
珊瑚苗圃能为野外移植提供丰富的珊瑚断枝资源,因此最大限度地提高珊瑚存活率和生长率成为珊瑚苗圃的主要目标之一[24],也是决定最终修复效果的关键[25]。本研究中,笔者基于现场监测和垂直拍照投影法获得珊瑚存活率和生长率结果: C组和D组的移植珊瑚全部存活;而A组和B组的珊瑚有死亡植株(A组死亡6株,B组死亡4株)。C组和D组苗圃的珊瑚的存活率(100%)均高于Putchim等[26] 在中层水苗圃上使用小孔径塑料网移植的鹿角珊瑚存活率(94.8%),而A组和B组的存活率(A组80%,B组87%)均低于上述研究中存活率;同时,C、D 2组珊瑚的生长率高于Mbije等 [27]在中层水苗圃绳网上移植的鹿角珊瑚生长率。通过实地研究及与上述研究的比较,C组和D组具有较高的生长率和存活率,因此,可以推测大孔径的网格板(≥4.5 cm×4.5 cm)更有利于珊瑚的生长和存活,作为珊瑚苗圃的移植基底可能更具优势。
-
现场环境监测分析发现,不同孔径尺寸的网格板上附着藻类有明显差异:随时间的推移,A组基底的海水浑浊度逐渐高于其他3组,第3次监测时,A组的浊度比D组高约2.32倍,虽然笔者监测时浊度平均水平低于1 FTU(0.13 mg·L−1),明显低于胁迫珊瑚的沉积物阈值[28],但值得考虑的是,沉积物随时间变化产生的累积效应对珊瑚的影响可能会很强烈。草皮海藻是珊瑚的主要藻类竞争者,珊瑚与壳状珊瑚藻接触的相互作用产生的有害影响要远小于草皮海藻[29]。因此,推测C、D 2组附着较多的壳状珊瑚藻与珊瑚之间无竞争作用,甚至有助于珊瑚的生长。国外学者利用高光谱图像技术观察珊瑚与皮壳状珊瑚藻之间的相互作用,发现壳状珊瑚藻边缘附近的珊瑚组织没有受到破坏或明显压力,并揭示壳状珊瑚藻不会刺激或改变与珊瑚组织相关的微生物群落,也不会通过释放化感物质直接损害或杀死珊瑚组织[30];反而有助于巩固珊瑚骨架和抑制其他藻类的生长[31-32]。而低密度和较短的草皮海藻并未到达过度生长的范围,不足以对C组和D组的珊瑚形成严重损伤,研究表明只有珊瑚完全被草皮海藻包围,且珊瑚活组织减少一定面积时,才会对珊瑚产生致死威胁[33]。
A组和B组附着的草皮海藻完全覆盖整个基底,通过物理摩擦和化感作用侵蚀珊瑚的底部组织,与移植珊瑚竞争附着空间[34]。草皮海藻还可能释放有害细菌,对A、B 2组珊瑚造成压力[35],但这需要进一步从微生物层面进行研究证明。基底的孔径较小可能会引起悬浮物浓度的升高,A组附近环境的浑浊度升高可能由于基底附着的草皮海藻会捕获珊瑚的粘液并吸附海水中的悬浮物[36],形成草皮海藻沉积物,并且小孔径引起的低流速会增加沉积物的堆积速率[37],在海水运动的作用下导致沉积物沉积和再悬浮不断反复。相比之下,A组的沉积物堆积比B组更明显,因为A组的基底截面面积大,所承载的悬浮物高于B组,并且草皮海藻密度和长度要高于B组。沉积物的累积效应会导致A组草皮海藻获得更高的营养水平[38],生长繁殖速度加快,草皮海藻的藻丝长度显著增加[39]。过度生长的草皮海藻和沉积物的叠加作用可能会增加草皮海藻在珊瑚-藻类作用中的竞争力,一方面通过窒息和组织埋藏对珊瑚边缘组织产生胁迫和致死后果[40- 41],直接减少珊瑚的活组织面积并占据死亡骨骼,进一步与活珊瑚组织竞争直至珊瑚完全死亡。另一方面,A组和B组珊瑚为应对草皮海藻过度生长的竞争压力可能会将用于组织生长的能量转移到抵御藻类胁迫 [42] ,导致珊瑚生长能量显著低于正常水平,生长率显著低于C、D 2组。
-
Fv/Fm能指示造礁石珊瑚共生虫黄藻的潜在光合能力和生理状态[43-44]。在进行的3次监测中,C组和D组均保持较高的Fv/Fm水平,而A组的Fv/Fm均低于其他3组。A组的最大量子产率降低,推测是由于过度生长的草皮海藻不断对珊瑚的摩擦作用引起。虫黄藻的光合效率降低,光合产物减少,降低珊瑚的钙化生长[45]。结果表明,C、D 2组的虫黄藻密度超过A、B 2组30%。这表明C、D 2组比A、B 2组可能具有更高的光合产物,促进其稳定钙化生长。研究结果表明,虫黄藻光合作用过程中产生的大部分有机碳化合物和氧气被输送到宿主体内,满足日常代谢需求和维持珊瑚的有氧条件,造礁石珊瑚的钙化生长的能量主要来自虫黄藻光合作用[46]。A、B 2组虫黄藻密度的降低可能是因为珊瑚遭受草皮海草的压力,从而从体内排出过量的虫黄藻,珊瑚-虫黄藻共生关系破裂[47],间接引起生长率下降。虫黄藻密度降低会引起光合产物的减少,珊瑚虫可能会通过捕食浮游动物的异养方式进行弥补,但相关研究结果表明,珊瑚异养对石珊瑚骨骼生长的影响很弱,难以促进珊瑚的钙化生长[48-49]。叶绿素a作为主要的光合色素,是体现珊瑚自养性的代表指数之一。国外学者的研究结果也证明与被丝状草皮海藻包围的珊瑚相比,被壳状珊瑚藻包围的珊瑚显示出较小的压力[46],其自养能力更高,这可能是C、D 2组具有更高叶绿素a值,而A、B 2组珊瑚叶绿素a含量下降的原因。 将共生藻类和光合能力的数据进行比较,结果表明,C、D 2组珊瑚的共生藻类健康状态和光合速率要大于A、B 2组,更有利于珊瑚的生长和存活。
-
本研究通过借助工程化礁体搭建珊瑚苗圃,评估了不同孔径尺寸基底移植珊瑚的生长率和死亡率差异,结合光合生理指标和环境因子阐述了引起差异的可能因素,并筛选了适宜应用于苗圃基底的孔径尺寸。结果表明,大孔径网格板(≥4.5 cm×4.5 cm)上珊瑚的生长率和存活率均高于小孔径网格板(≤ 2.5 cm×2.5 cm),大孔径的网格板能比小孔径的网格板附着更高密度的壳状珊瑚藻,有利于珊瑚的生长存活;而附着的草皮海藻长度和密度较低,对珊瑚的威胁可能较弱,因而保障了移植珊瑚有较高的生长速率和存活率。结合长期移植效果考虑,建议工程化修复和珊瑚苗圃等技术手段选用珊瑚移植基底的网格板孔径应选择≥ 4.5 cm×4.5 cm的尺寸,尽可能保证移植珊瑚的较高存活率和生长速率,减缓苗圃阶段的繁育期,在修复工作中获取更大的效益。
Evaluation of the transplantation effect of artificial substrates with different apertures on Acropora microphthalma
DOI: 10.15886/j.cnki.rdswxb.20220007
- Received Date: 2022-03-14
- Accepted Date: 2023-05-12
- Rev Recd Date: 2022-06-06
- Available Online: 2023-07-11
- Publish Date: 2023-09-25
-
Key words:
- coral restoration /
- artificial substrate /
- coral growth /
- turf algae
Abstract: Coral reef degradation trends are becoming more and more pronounced, and restoration methods such as coral nurseries and engineered reef bodies that are currently used require laying materials such as grid plates as artificial substrates to facilitate the immobilization of transplanted corals, but there are few studies on the effects of the pore size of the skeletonized substrate on corals that are widely used. An attempt was made to established coral nurseries to explore the growth effects of four metal mesh plates of different pore sizes (A, B, C, D, aperture sizes of 1 cm×2 cm, 2.5 cm× 2.5 cm, 4.5 cm×4.5 cm, 6 cm×6 cm) on the growth of Acropora microphthalma. The results showed that the corals in groups C and D maintained 100% survival at the end of the experimental period, while the coral survival rates in groups A and B dropped to 80% and 87%. The main algae attached to the substrates in groups C and D were crustose coralline algae, and the dry weight of algae attached to the turf algae was significantly lower in groups C and D than in groups A and B. The average length of turf algae attached to the substrates was significantly lower in groups C and D than in groups A and B. The turf algae attached to the substrates of small aperture could obtain nutrients from the suspended matter in the water and lead to excessive growth, thus inhibiting coral growth and even death. Based on symbiotic algae density and photosynthetic physiology, corals in groups C and D had higher photosynthetic capacity, which ensured coral calcification growth. Therefore, the grid plate with large aperture (≥4.5 cm×4.5 cm) is more favorable for the growth of transplanted coral, and more suitable as the transplanted substrate of engineered reef.
| Citation: | LIU Xiangbo, ZHU Wentao, XIA Jingquan, ZHU Ming, REN Yuxiao, CHEN Rouwen, WANG Aimin, LI Xiubao. Evaluation of the transplantation effect of artificial substrates with different apertures on Acropora microphthalma[J]. Journal of Tropical Biology, 2023, 14(5): 536-544. doi: 10.15886/j.cnki.rdswxb.20220007 |








 DownLoad:
DownLoad: