-
中南鱼藤(Derris fordii Oliv.),又名毒鱼藤、白药根、雷公藤蹄,是豆科鱼藤属中的一种攀援灌木[1],广泛分布于我国长江以南地区,通常生长于浙江、湖北、湖南、海南、贵州、云南等省市的灌木林或疏林中[2]。中南鱼藤在医学方面常用于治疗游走性关节炎、湿癞、老年阴道炎等。农业方面,中南鱼藤大多应用于3个方面:(1)控释肥料。多用于水稻[3]、辣椒、马铃薯[4]、南瓜、番茄、白萝卜[5]、天冬、火龙果[6]、荔枝、香蕉、百香果[7-9]、山楂[10]、茶树[11]等植物。(2)抗菌杀虫处理剂。中南鱼藤与其他材料制成的处理剂有较好的杀虫效果,用于防治根结线虫、蛴螬、蚜虫等病虫害[12],多在水稻、瓜果类蔬菜[13]、果树(贡柑)、板蓝根等上使用,能提高产品的产量及品质。此外,这些处理剂针对园林植物种子进行防霉变处理,能显著提高园林植物成活率[14]。(3)菇类培养基。中南鱼藤被制成香菇[15]、杏鲍菇[16]等菇类的培养基,能促进菇类的生长,提高产量且品质更优。此外,中南鱼藤还可作为一种天然成分的甲醛去除剂[17]以及其与根瘤菌共生体可能在生态修复和农业生产中具有应用潜力。在海南,中南鱼藤有着悠久的使用历史,其为三大传统杀虫植物之一[18],从中南鱼藤根皮甲醇提取物中分离出了鱼藤酮、12ɑ-羟基鱼藤酮、6-甲氧基黄酮3种化合物,其中鱼藤酮、12ɑ-羟基鱼藤酮表现出良好的杀虫活性[19]。鱼藤酮是从鱼藤属植物中分离出来的一种化合物,具有较好的杀虫活性,用于蚜虫、菜粉蝶幼虫、小菜蛾、孑孓等害虫的防治[20-21]。目前,对中南鱼藤的研究多在杀虫活性方面,在抑菌方面还没有系统的研究。本研究利用中南鱼藤枝叶提取物,以9种植物病原真菌为靶标进行抑菌活性研究,以期发现中南鱼藤新用途,为中南鱼藤资源的充分利用及植物病原真菌的防治提供理论依据,为中南鱼藤在杀菌活性方面的进一步深入研究奠定基础。
-
中南鱼藤采自海南省昌江黎族自治县霸王岭,并经海南大学热带作物学院王华锋教授鉴定;实验所用有机溶剂(甲醇、石油醚、乙酸乙酯、正丁醇、DMF(N,N-二甲基甲酰胺)、吐温−80)均为分析纯,购于海南海道森科技有限公司;嘧菌酯(浓度为98%,上海源叶生物)和丙环唑(浓度为97%,Aladdin)原药均购自北京伊诺凯科技有限公司;豇豆种子(Vigna unguiculata(Linn.) Walp.,摘不败(广东省兴宁市庆丰盈科种子有限公司)。
-
实验供试菌种见表1。
序号 植物病原真菌 用于实验 保存地点 1 水稻纹枯病菌(Rhizoctonia solani) 抑菌活性测定 海南大学植物保护学院 2 苹果轮纹病菌(Botryosphaeria dothidea) 抑菌活性测定 海南大学植物保护学院 3 小麦赤霉病菌(Fusarium graminearum) 抑菌活性测定 海南大学植物保护学院 4 香蕉炭疽病菌(Colletotrichum musae) 抑菌活性测定 海南大学植物保护学院 5 火龙果溃疡病菌(Neoscytalidium dimidiatum) 抑菌活性测定 海南大学植物保护学院 6 茄链格孢菌(Alternaria solani) 抑菌活性测定 海南大学植物保护学院 7 辣椒疫霉病菌(Phytophthora capsici) 抑菌活性测定 海南大学植物保护学院 8 稻瘟病病菌(Magnaporthe oryzae) 抑菌活性测定 海南大学植物保护学院 9 豇豆白粉病菌(Podosphaera xanthii) 盆栽试验 海南大学植物保护学院 -
将中南鱼藤枝叶剪成10 cm左右的小段,于50 ℃下烘干并粉碎。称取500.00 g粉末,用6.00 L甲醇浸泡3 d,旋转蒸发仪减压除去溶剂,重复3次,得到中南鱼藤甲醇提取物浸膏54.05 g,于4 ℃件下保存备用。将一定量的中南鱼藤甲醇提取物用水溶解后,依次用石油醚、乙酸乙酯、正丁醇萃取,将各相萃取液浓缩后分别得到石油醚相萃取物、乙酸乙酯相萃取物、正丁醇相萃取物、水相萃取物。将各相萃取物置于4 ℃环境中保存备用。
-
利用菌丝生长速率法测定中南鱼藤甲醇提取物及各相萃取物对供试真菌的抑制活性。称取一定量的中南鱼藤甲醇提取物浸膏及各相萃取物,用DMF(N,N-二甲基甲酰胺)充分溶解后,配制成供试药液,将药液与马铃薯葡萄糖琼脂培养基(PDA培养基)混匀,制成提取物浓度为2.00 g·L−1、各相萃取物浓度为1.00 g·L−1的带药培养基,以相同含量DMF的PDA培养基为对照。病原真菌用直径为0.5 cm的打孔器制成菌饼,接入带药培养基中间,设置3个重复。转接好的病原菌置于28 ℃的恒温培养箱中培养,待对照组菌落直径达到5.0 cm以上时,采用十字交叉法测量菌落的直径。菌丝生长抑制率按下式计算:
式中:I表示抑制率,C表示对照组病原菌在培养上的直径,T表示处理组病原菌在培养基上的直径。
-
称取定量提取物浸膏,用DMF充分溶解配置成供试药液,加入PDA培养基中,配置成一定浓度梯度的带药培养基,其他操作同1.3.2一样。计算中南鱼藤甲醇提取物对病原真菌的EC50值。
-
称取定量的中南鱼藤甲醇提取物浸膏及各相萃取物,溶于DMF中,配置成供试药液,加入PDA培养基中充分混匀,制成一定浓度的带药培养基。吸取0.50 mL带毒培养基置于载玻片上,待凝固后,在培养基表面均匀涂抹香蕉炭疽病菌孢子悬浮液(1×107 CFU· mL−1),以含同等比例DMF的无菌水作为对照,每个处理设置3个重复,实验重复3次。处理后的载玻片置于28 ℃恒温培养箱中培养,对照组孢子萌发率超95.00%后于显微镜下观察各处理组孢子萌发情况,每个重复观察3个视野,且记录不少于300个分生孢子的萌发情况,并计算萌发率与相对抑制率。公式如下:
-
将称取的中南鱼藤甲醇提取物溶于DMF中,用0.1%吐温-80水溶液稀释成3.20、1.60 、1.00 、0.50 、0.10 g·L−1溶液。选取成熟度、大小一致、完好无损的香蕉,清洗香蕉自然晾干。将香蕉置于配置好的药液中浸泡10 min,以嘧菌酯为阳性对照,含有同等比例DMF的0.5%吐温-80水溶液为空白对照,自然晾干,24 h后,喷洒浓度为1×107 CFU·mL−1香蕉炭疽病菌分生孢子悬浮液,将处理过的香蕉置于28 ℃条件下培养,对照发病后,观察各处理发病情况并分级,试验进行3次,每次试验设置7个重复。其分级标准如下:
0级:无病;
1级:病斑面积占果实面积的<5%;
3级:病斑面积占果实面积的≥5%~<10%;
5级:病斑面积占果实面积的≥10%~<25%;
7级:病斑面积占果实面积的≥25%~<50%;
9级:病斑面积占果实面积的≥50%;
-
播种豇豆种子,待豇豆发芽后于温室下正常培养15 d,选取二叶期长势一致的豇豆苗作为供试植株。称取中南鱼藤甲醇提取物及各相萃取物,用少量DMF溶解后,加入0.1%吐温-80水溶液配置成一定浓度的供试药液备用。用毛笔收集植株上的白粉孢子,配成浓度为1×107 CFU· mL−1孢子悬浮液备用。将12 mL供试药液用喷雾机均匀喷洒于5株豇豆叶片上,喷药后等药液自然晾干后,将豇豆置于温室下正常培养,24 h后喷洒豇豆白粉病孢子悬浮液;喷洒药液及豇豆白粉病孢子悬浮液时喷雾压力均为1.5 kg·cm−2。处理后的植株置于温室(25 ℃,相对湿度80%)培养。每个处理设置5个重复。以丙环唑为阳性对照,设置等量DMF的吐温-80水溶液作为空白对照。培养14 d后调查发病情况以及防治效果。实验重复3次。豇豆白粉病分级标准如下:
0级:无病;
1级:病斑面积占叶片面积的<5%;
3级:病斑面积占叶片面积的≥5%~<10%;
5级:病斑面积占叶片面积的≥11%~<20%;
7级:病斑面积占叶片面积的≥21%~<40%;
9级:病斑面积占叶片面积的≥40%;
-
所有相关数据采用Excel 2019和SPSS 9.0软件进行统计和计算,利用Origin 8.5 软件绘制图形。
-
中南鱼藤甲醇提取物对8种植物真菌离体抑菌活性测定结果(表2)表明,在浓度为2.00 g·L−1时,中南鱼藤甲醇提取物对其中7种植物病原菌抑制效果均大于60.00%,其中对香蕉炭疽病菌效果最为突出,其抑制率可达到82.15%。中南鱼藤各相萃取物对8种植物病原真菌离体抑菌活性测定结果(表3)表明,各相萃取物浓度为1.00 g·L−1时,乙酸乙酯相萃取物表现出较好的抑制活性,其对8种真菌的抑制率为53.09%~80.50%。此外,石油醚相萃取物对8种真菌的抑制率在44.44%~64.95%;正丁醇相萃取物对火龙果溃疡病菌抑制效果最好(抑制率:54.55%),其对其余真菌抑制率均小于50%;水相萃取物对8种真菌抑制活性较差,抑制率均小于30%。
序号 植物病原真菌 菌丝生长抑制率/% 1 水稻纹枯病菌 76.00±1.02 2 苹果轮纹病菌 71.88±0.60 3 小麦赤霉病菌 48.72±0.73 4 香蕉炭疽病菌 82.15±0.60 5 火龙果溃疡病菌 64.35±0.75 6 茄链格孢菌 63.82±0.37 7 辣椒疫霉病菌 71.70±0.69 8 稻瘟病菌 75.00±1.03 -
中南鱼藤甲醇提取物对8种植物病原真菌EC50测定结果如表4所示,其对8种植物病原真菌的EC50为0.24~1.33 g·L−1,其中中南鱼藤甲醇提取物对香蕉炭疽病菌EC50最低(0.24 g·L−1),说明中南鱼藤甲醇提取物对香蕉炭疽病菌的生长抑制活性最好。
序号 植物病原真菌 EC50/(g·L−1) 1 水稻纹枯病菌 0.57 2 苹果轮纹病菌 0.45 3 小麦赤霉病菌 1.33 4 香蕉炭疽病菌 0.24 5 火龙果溃疡病菌 0.87 6 茄链格孢菌 0.85 7 辣椒疫霉病菌 1.08 8 稻瘟病菌 0.73 -
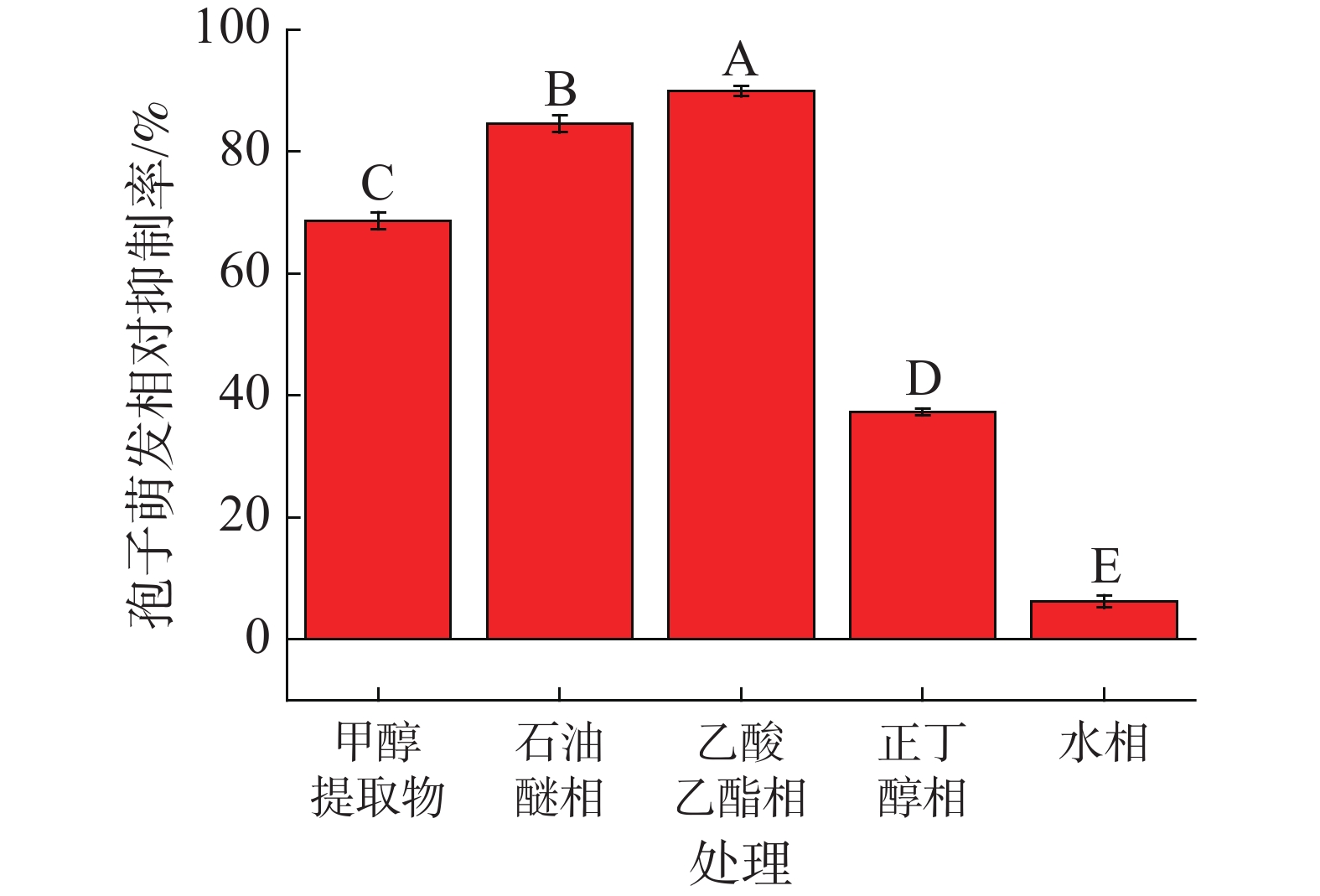
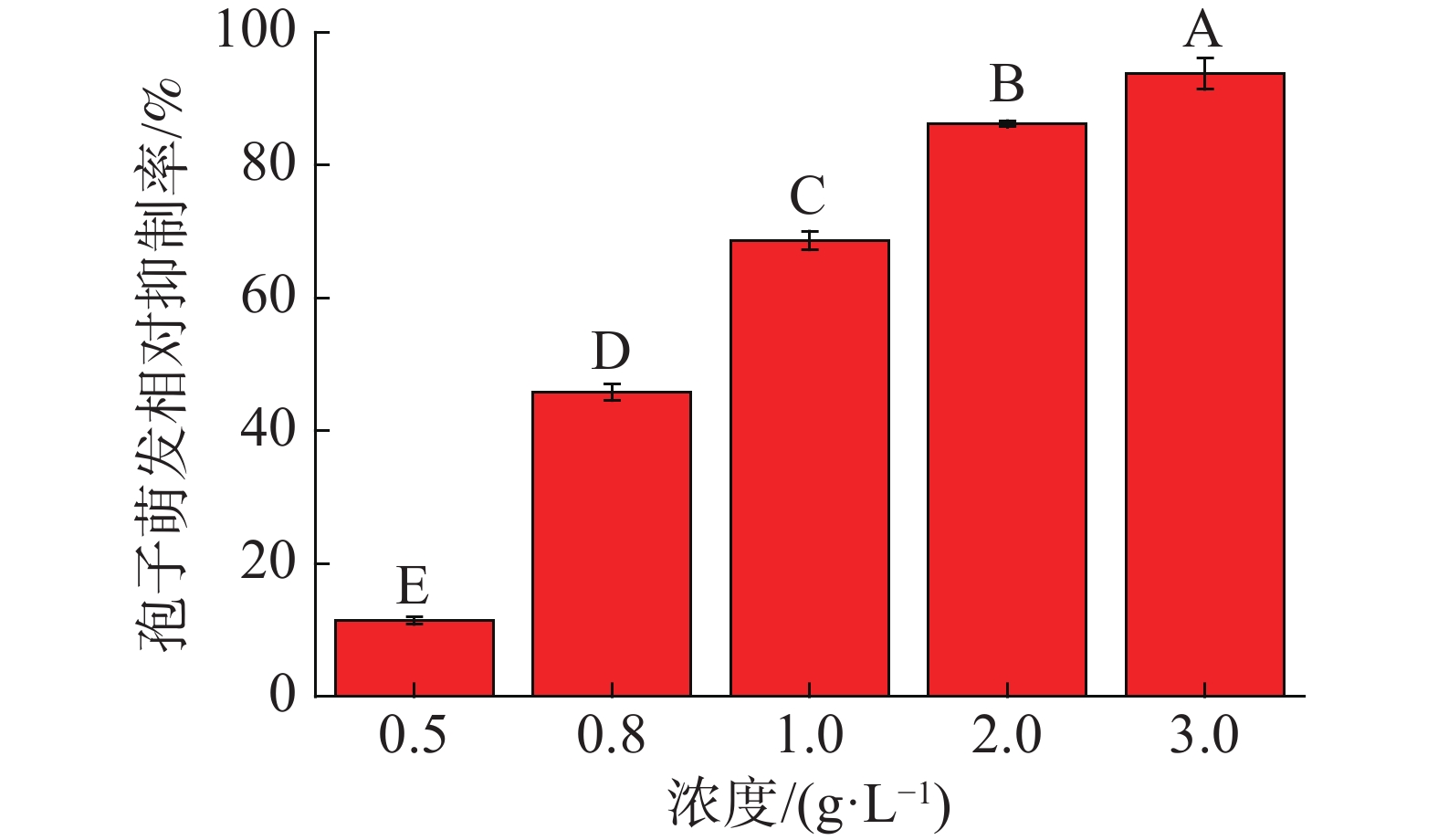
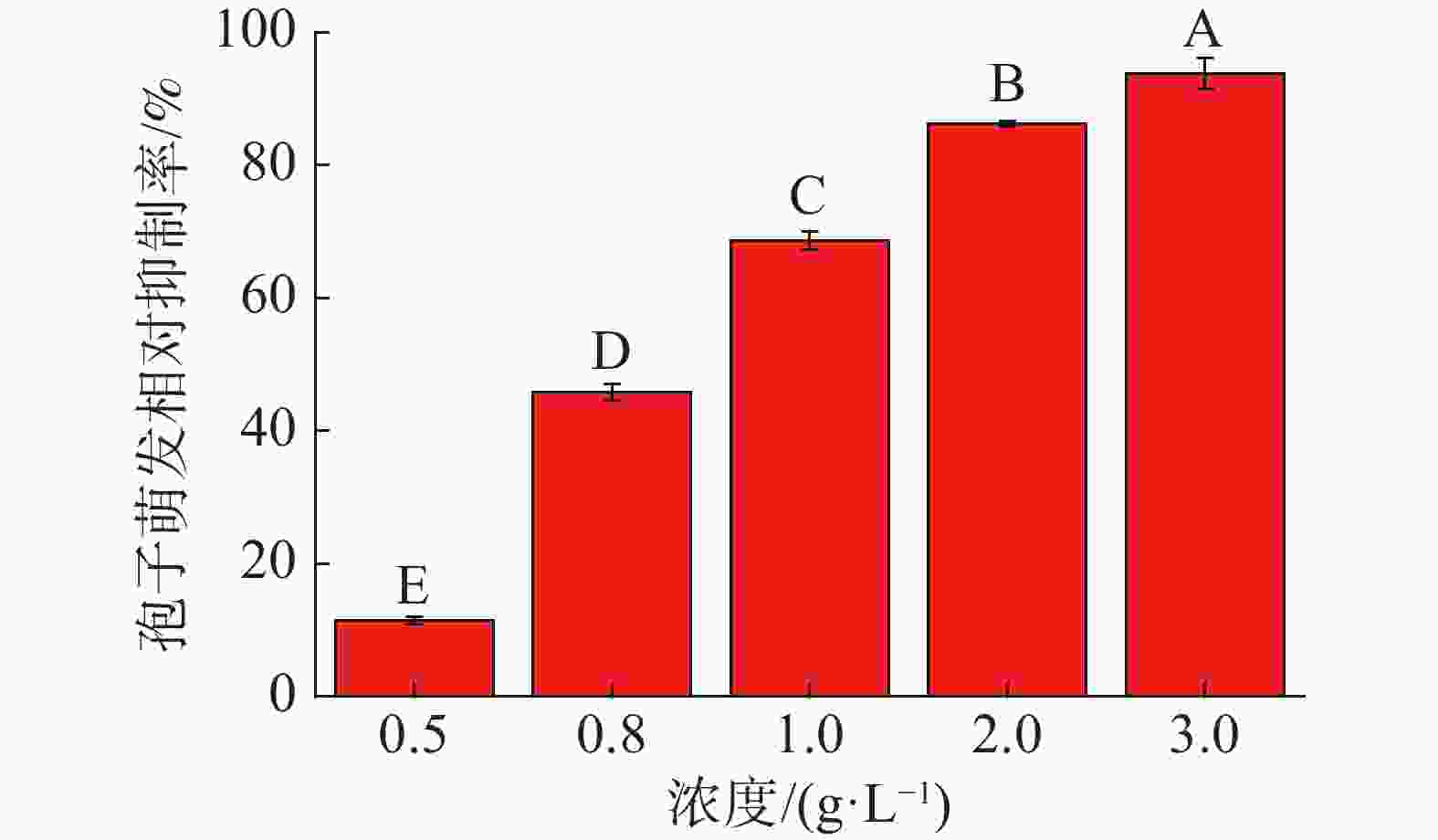
中南鱼藤甲醇提取物对香蕉炭疽病菌分生孢子萌发相对抑制率如图1所示。结果表明,随着中南鱼藤甲醇提取物浓度的增加,其对香蕉炭疽病菌分生孢子萌发抑制作用越来越明显。当中南鱼藤甲醇提取物浓度为0.50 g·L−1、0.80 g·L−1时,其相对抑制率均小于50.00%;当浓度大于1.00 g·L−1时,其相对抑制率均高于60.00%,其中当浓度为3.00 g·L−1时,抑制率可到93.76%。另外,当甲醇提取物及各相萃取物浓度均为1.00 g·L−1时(图2),相较于甲醇提取物,石油醚相萃取物与乙酸乙酯相萃取物表现出较好活性(相对抑制率分别为84.56%、89.92%)。甲醇提取物与石油醚相萃取物、乙酸乙酯相萃取物相对抑制率分别为68.68%、37.32%、6.20%。
处理 R.S B.D F.G C.M N.D A.S P.C M.O 石油醚相 61.58±1.97B 49.00±0.00B 51.43±1.04B 55.00±0.00B 40.64±1.31B 30.56±0.49B 44.44±0.98B 64.95±0.73A 乙酸乙酯相 73.16±1.29A 80.50±1.22A 60.00±1.39A 76.88±0.88A 56.68±1.31A 58.33±0.85A 71.53±0.89A 53.09±0.73B 正丁醇相 16.32±1.29D 1.00±1.25C 22.29±0.80C 27.50±1.77C 54.55±0.75A 33.33±0.00B 24.53±0.00C 10.77±0.36C 水相 29.47±0.74C 2.83±0.92C −1.14±0.40D 0.63±0.00D 10.70±0.37C 5.54±0.84C −3.14±0.89D 0.00±0.89D 注:R.S. 水稻纹枯病菌;B.D. 苹果轮纹病菌;F.G. 小麦赤霉病菌;C.M. 香蕉炭疽病菌;N.D. 火龙果溃疡病菌;A.S. 茄链格孢菌;P.C. 辣椒疫霉病菌;M.O. 稻瘟病菌。表中不同字母代表1.0 g·L−1浓度下中南鱼藤不同相萃取物对同一种病菌菌丝生长抑制率差异显著[17](P<0.01)。 -
中南鱼藤甲醇提取物对香蕉果实炭疽病防治效果(表5)表明,随着提取物浓度的增加,其对香蕉果实炭疽病的防治效果越来越好。提取物浓度为0.10 g·L−1、0.50 g·L−1、1.00 g·L−1时防治效果均小于50.00%;当提取物浓度大于1.60 g·L−1时防效均大于60.00%;当浓度为3.20 g·L−1时,防效为71.11%,与0.50 g·L−1嘧菌酯防效相当。
处理 浓度/(g·L−1) 病情指数 防治效果/% 中南鱼藤 0.10 86.67±0.70A 13.33±0.16A 0.50 73.33± 1.91B 26.67±1.63B 1.00 51.11±2.21C 48.09±0.82C 1.60 33.33±0.19D 66.67±2.94D 3.20 28.89±0.22E 71.11±0.83E 嘧菌酯 0.50 15.56±0.42F 84.44±0.25F 注:表中不同字母代表不同浓度下中南鱼藤甲醇提取物对香蕉果实炭疽病防治效果差异显著(P<0.01)。 -
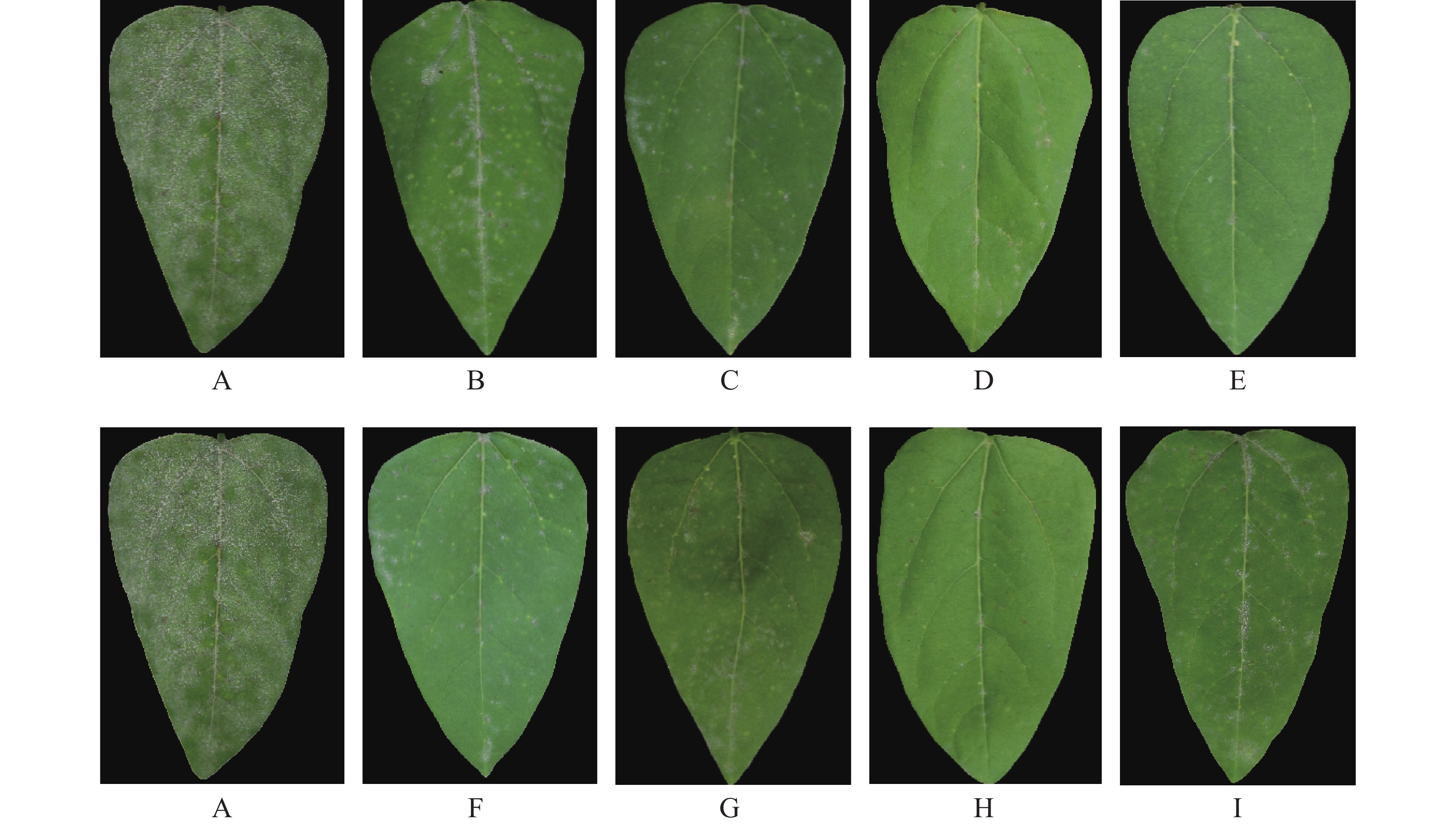
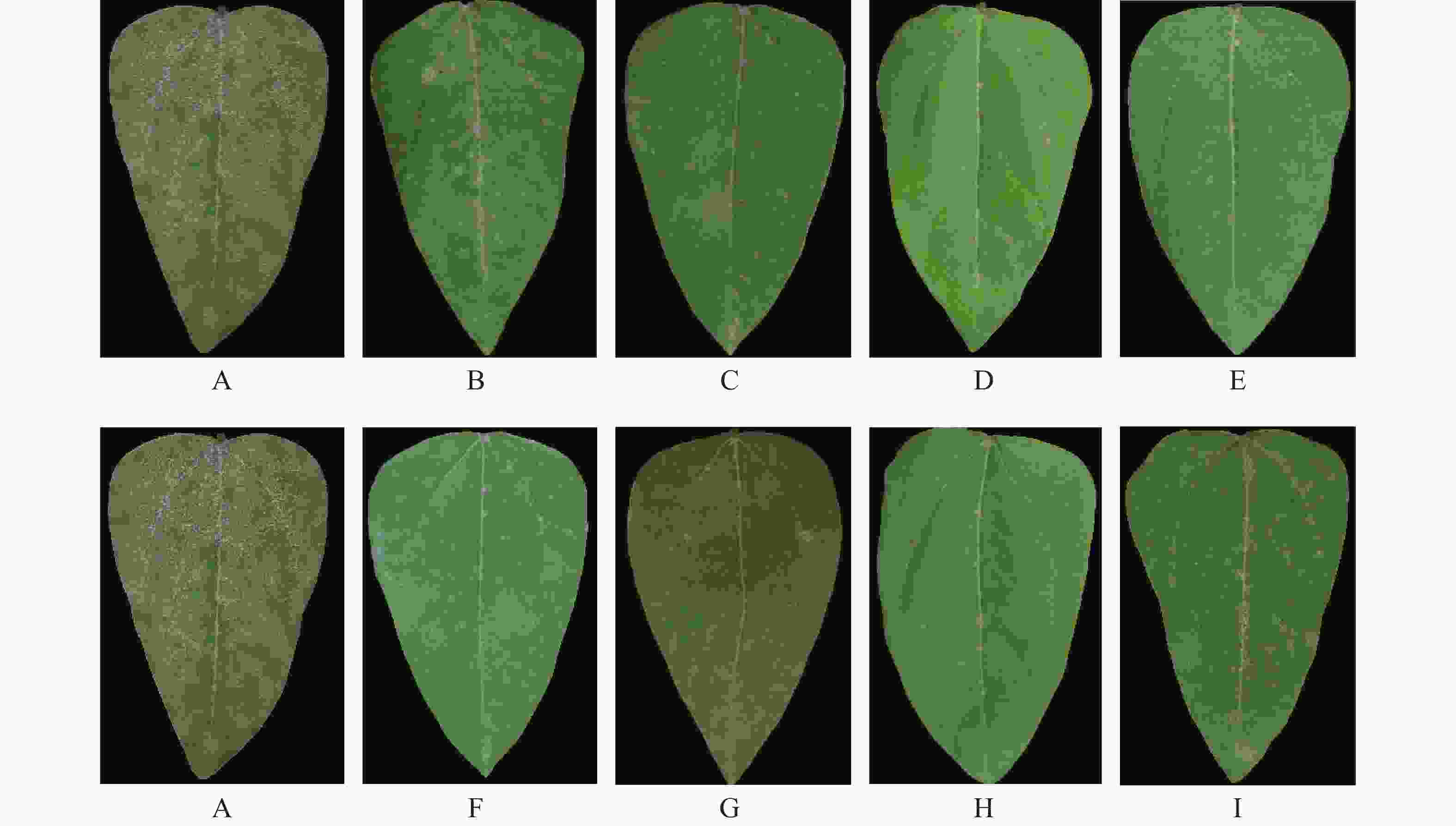
中南鱼藤甲醇提取物及各相萃取物对豇豆白粉病的室内盆栽防效实验结果(图3,表6)表明,中南鱼藤甲醇提取物对豇豆白粉病有较好的防治效果,防治效果随着中南鱼藤浓度的增加越来越好,当浓度为0.50 g·L−1防治效果可超过50.00%;当浓度为1.00 g·L−1时,防效可达91.67%。中南鱼藤甲醇提取物分相后,浓度为1.00 g·L−1时,石油醚相萃取物、乙酸乙酯相萃取物、水相萃取物防效均低于60.00%,正丁醇相萃取物防治效果最好,防效为94.07%,效果优于浓度为0.025 g·L−1的商品化农药丙环唑。
处理 病情指数 防治效果/% 0.025 mg·mL−1 0.10 mg·mL−1 0.50 mg·mL−1 1.00 mg·mL−1 0.025 mg·mL−1 0.10 mg·mL−1 0.50 mg·mL−1 1.00 mg·mL−1 提取物 - 72.22±1.89 49.50±1.09 8.33±1.54D - 27.78±0.78 50.50±1.09 91.67±0.42B 石油醚 相 - - - 40.12±0.95C - - - 59.88±0.48C 乙酸乙酯相 - - - 64.30±1.65 - - - 35.70±1.79D 正丁醇 相 - - - 5.93±0.75Ee - - - 94.07±0.51A 水相 - - - 89.97±1.48A - - - 10.03±0.56E 丙环唑 18.05±0.67 - - - 81.95±2.01 - - - 注:表中不同字母代表同一浓度下中南鱼藤甲醇提取物及不同相萃取物对豇豆白粉病防治效果差异显著(P<0.01)。“-”代表未测试数据。 -
在农业方面,鱼藤属植物是很多杀虫活性物质的主要来源, LI [22]从中南鱼藤根皮中成功分离出鱼藤酮并测定了杀虫活性。 HYMAVATHI A [23]从攀缘鱼藤(Derris scandens)中分离出9种化合物,其对绿豆象(Callosobruchus chinensis L.)、米象(Sitophilus oryzae L.)、谷蠹(Rhyzopertha dominica L.)、赤拟谷盗(Tribolium castaneum H.)四种仓贮害虫表现出不同程度的杀虫活性。WU X [24]等从毛鱼藤(Derris elliptica)中分离出5个化合物对白纹伊蚊(Aedes albopictus)幼虫表现出较好杀虫活性。鱼藤属中多种植物提取物对细菌也表现出良好抑菌活性,其中,鱼藤(Derris trifoliata)对黄色葡萄球菌(Staphylococcus aureus )和表皮葡萄球菌(Staphylococcus epidermides)表现出高活性[25];马来鱼藤(Derris malaccensis)对幽门螺杆菌 (Helicobacter pylori) 具有较好抑菌活性[26];攀缘鱼藤对枯草芽孢杆菌、芽孢杆菌、金黄色葡萄球菌和铜绿假单胞菌表现出显著的抑菌活性[27]。鱼藤属植物中关于防治植物病原真菌的相关报道较少,目前在对中南鱼藤的研究中未见关于其对植物病原真菌抑菌活性的报道。本研究结果表明,中南鱼藤枝叶提取物对8种植物病原真菌均有较好的抑制作用,中南鱼藤甲醇提取物对8种真菌的EC50均在1.35 g·L−1以下。香蕉炭疽病菌分生孢子萌发抑制活性测定结果表明,中南鱼藤甲醇提取物和乙酸乙酯相萃取物都表现出较好抑制活性,孢子萌发实验结果正好与香蕉炭疽病菌菌丝生长速率法测定结果相一致,均是乙酸乙酯相萃取物的活性较好。香蕉果实炭疽病的防治效果表明,中南鱼藤甲醇提取物对香蕉果实炭疽病有较好的防治作用,其中中南鱼藤甲醇提取物浓度为3.20 g·L−1时,防效可达71.11%。在豇豆白粉病病菌防治实验中,中南鱼藤提取物表现出较好的防治效果,其中,中南鱼藤甲醇提取物与正丁醇相萃取物浓度为1.00 g·L−1时,防效均超过90.00%。张慧等[28]的研究结果表明,毛枝鱼藤(Derris scabricanlis)叶片甲醇提取物浓度为100 g·L−1时,对荔枝霜疫霉的抑制率为88.89 %;ZAHARI R [29]测定了毛鱼藤叶部提取物对3种根腐病病原菌(Ganoderma philippii、 Phellinus noxius、Rigidoporus microporus)的抑菌活性,结果表明只有丙酮萃取物和二氯甲烷萃取物对Rigidoporus microporus表现出显著抑菌活性。KHAN M R [30]测定了鱼藤、毛鱼藤和印度鱼藤(现正名为水黄皮Pongamia pinnata (L.) Pierre)对Aspergillus niger, Aspergillus rubrum, Aspergillus versicolor等9种真菌的抑菌活性,发现3种植物对9种真菌均无抑菌活性。综上所述,笔者认为中南鱼藤在防治植物病害方面,特别是防治香蕉炭疽病和豇豆白粉病更具有潜在研究价值。
中南鱼藤乙酸乙酯相萃取物对多种植物病原真菌具有较突出的抑制作用,正丁醇相萃取物在豇豆白粉病防治中有较突出的效果,因此可进一步对中南鱼藤乙酸乙酯相萃取物及正丁醇相进行分离提纯,以期分离出对香蕉炭疽病菌及豇豆白粉病菌有高活性的化合物。此外,由于中南鱼藤提取物来源于植物,在使用其防治病害过程中具有对环境友好、不易让病原菌产生抗药等特点[31]。这些优点从侧面说明对中南鱼藤进行进一步研究的意义所在。本研究只对中南鱼藤抑菌活性进行了初步探究,未来可在中南鱼藤抑菌活性化合物分离鉴定、抑菌机制以及在田间试验中对农作物病害的防治效果等方面进行更深入的探讨。
-
中南鱼藤枝叶提取物对多种植物病原真菌具有较好的抑菌活性,可以拓展其在农作物病害防治上的新用途,特别是在助力乡村振兴和在发展有机果蔬生产上提供绿色、无公害农药的更多选择。
Fungistatic activity of the extracts from the twigs and leaves of Derris fordii against plant pathogenic fungi
doi: 10.15886/j.cnki.rdswxb.2022.03.004
- Received Date: 2021-08-07
- Accepted Date: 2022-02-25
- Rev Recd Date: 2022-01-21
- Available Online: 2022-03-21
- Publish Date: 2022-05-23
-
Key words:
- Derris fordii /
- plant pathogenic fungi /
- fungistatic activity /
- botanical pesticide
Abstract: The twigs and leaves of Derris fordii were extracted with methanol and then partitioned to factions in petroleum ether, ethyl acetate, n-butanol and water to assess the fungistatic activity of the methanol extracts and their fractions against plant pathogenic fungi. Eight plant pathogenic fungi were selected for treatment with the methanol extracts and their fractions of D. fordii to determine the mycelial growth inhibition activities by using the mycelium growth rate method, and the inhibition activities against the spore germination of Colletotrichum musae by using the spore germination method. The control effects of the methanol extracts of D. fordii against C. musae infecting banana fruit were determined by inoculation method, and the control effects of the methanol extracts of D. fordii and their solvent-partitioned fractions against Podosphaera xanthii in vivo were determined by pot culture method. The results showed that the methanol extracts and their factions of Derris fordii had different degrees of mycelial growth inhibition effects on the eight plant pathogenic fungi with the EC50 being 0.24~1.33 g·L−1, of which the methanol extract at the concentration of 2.00 g·L−1 had an inhibition rate of 82.15% against C. musae, and the ethyl acetate fraction at 1.00 g·L−1 had a relative inhibition rate of 89.92% against the spore germination of C. musae. The in vivo experiments indicated that the methanol extract of D. fordii at 3.20 g·L−1 had a control effect of 71.11% against C. musae in the inoculation experiment and that the methanol extract and the n-butanol fraction of Derris fordii when both at the concentration of 1.00 g·L−1 gave control effects of 91.67% and 94.07% against P. xanthii, respectively. Therefore, the methanol extracts and the petroleum ether, ethyl acetate, n-butanol, and water fractions of D. fordii are potentially valuable in the prevention and control of plant pathogenic fungal diseases.
| Citation: | LIU Qifeng, ZHANG Beijing, YIN Fengman, ZHANG Xi, HU Zhan, XIE Jia, SUN Ranfeng. Fungistatic activity of the extracts from the twigs and leaves of Derris fordii against plant pathogenic fungi[J]. Journal of Tropical Biology, 2022, 13(3): 227-234. doi: 10.15886/j.cnki.rdswxb.2022.03.004 |







 DownLoad:
DownLoad: