-
鲍曼不动杆菌(Acinetobacter baumannii)是一种糖不发酵的革兰氏阴性菌[1-3]。在电镜下,鲍曼不动杆菌大小为(600 ~ 1 000 nm)×(1000 ~ 1 600 nm),正常外形呈球杆状,两端钝圆,但是在培养基中常常是以杆状形态存在。鲍曼不动杆菌对温度的敏感度较低,可在19 ℃ ~ 43 ℃大量繁殖,最适生存温度为37 ℃,气温过高时会异常滋长[4-5]。鲍曼不动杆菌菌体常以单独或成对形式存在于大自然和医院环境中,在水、土壤、皮肤、肠道和生殖道中均能生存。该细菌属于机会性致病菌,不仅感染动物,还可通过接触传播感染人(如饲养员,兽医等),引起严重的医源性感染,如伤口感染、尿道感染、脑膜炎、败血症等,易在重症监护病房流行[6]。目前科研人员从医院、宠物所等地发现不少人和动物感染鲍曼不动杆菌的案例,其中尤以多重耐药鲍曼不动杆菌最突出,药物治疗方面较棘手,一旦感染将对畜牧业带来一定的经济损失,同时威胁人类的健康安全。国外研究人员通过近几十年的追踪报道发现该细菌的感染主要分布在天气炎热、气候湿润的地区。我国对该细菌的研究起步较晚,缺少对该病菌的耐药性、毒力、疫苗及流行病学有关研究[7-8],对于牛感染鲍曼不动杆菌研究很少,仅见王孝武等[9]报道了鲍曼不动杆菌引起牛子宫内膜炎,葛东红等[10]在生牛乳中发现了鲍曼不动杆菌。2019年10月海南省某牛场有3头牛死亡,发病早期气喘严重,中期伴随着发热,暮气沉沉,后期无食欲,直至死亡,从病死牛的肺脏内分离出1株病原菌,并对其进行生化试验和细菌16S rDNA 鉴定,结果证实该分离菌为鲍曼不动杆菌。本试验从病死牛肺脏中分离得到鲍曼不动杆菌菌株通过16S rDNA及生化鉴定等方法确定致病菌遗传背景,同时测定该致病菌对小鼠的半数致死量及其药物敏感性,旨在为预防感染鲍曼不动杆菌和感染鲍曼不动杆菌的治疗提供理论依据[11]。
-
海南省东方市某牛场病死牛的肺脏。
-
6周龄昆明(KM)小鼠,购自湖南斯莱克景达实验动物有限公司。
-
胰蛋白胨大豆肉汤(tryptic soy broth, TSB)培养基和胰胨蛋白胨酵母膏葡萄糖琼脂培养基(tryptone-peptone-yeast extract-glucose agar,TPYG agar),购自青岛海博生物技术有限公司;麦氏培养基(MacConkey medium),购自北京北纳创联生物技术研究院;药敏试纸,购自杭州微生物试剂有限公司;生化鉴定管,购自广东环凯微生物科技有限公司。
-
在无菌环境下采集病死牛肺脏组织样,涂布于胰胨蛋白胨酵母膏葡萄糖琼脂培养基(含5%绵羊脱纤血)血平板和麦氏培养基上。对菌群进行染色观察并从中挑选单一菌落,37 ℃下在胰蛋白胨大豆肉汤培养基(TSB)中培养12 h,然后划线于胰胨蛋白胨酵母膏葡萄糖琼脂培养基(含5%绵羊脱纤血)血平板进行分离培养,每隔一段时间观察菌落生长情况。取单克隆菌落进行革兰氏染色。
-
参照文献[12]中细菌16S rDNA 通用引物序列,将本实验分离菌株送公司合成,通过PCR扩增及产物电泳试验,对扩增产物进行测序。在基因库(gene bank)中对该菌株16S rDNA序列进行同源性分析,鉴定细菌的种属。按照试剂盒说明书提取细菌DNA。
-
参考伯杰手册中细菌生理生化鉴定方法[13],将分离的菌株置于尿素酶、氧化酶、靛基质、甘露醇、接触酶、麦芽糖、乳糖及葡萄糖等生化鉴定管内,反应24 h之后观察并记录试验结果。
-
将分离培养后的鲍曼不动杆菌接种于胰蛋白胨大豆肉汤培养基中,恒温振荡培养9 h,然后在胰胨蛋白胨酵母膏葡萄糖琼脂培养基上划线培养。用无菌磷酸盐缓冲盐溶液(PBS)调整含菌量为2.5×107、5×107、1×108、2×108、4×108 CFU·mL−1。36只试验小鼠随机分成6组,每组6只,第1组为空白组,第2 ~ 6组为试验接种组。接种方式为腹腔内注射,试验组分别注射5个不同剂量(2.5×107、5×107、1×108、2×108、4×108 CFU·mL−1)的菌液各200 μL,空白组注射等量无菌PBS,每天接种1次,连续接种7 d。每天定时观察并记录试验小鼠的生长状态、发病状况及死亡数量。对试验中死亡的小鼠进行剖检。根据公式计算LD50[14-15]。
式中,Xm:最大剂量的对数值; i:相邻两剂量组对数值的差; ∑p:各剂量组死亡率的总和。
-
采用纸片扩散法。将鲍曼不动杆菌作为指示菌培养于含有5% 胎牛血清的胰蛋白胨大豆肉汤培养基中,将头孢氨苄、氧氟沙星、环丙沙星等常用抗生素的药敏纸片置于含指示菌的胰蛋白胨大豆肉汤培养基中37 ℃恒温培养24 h,测量抑菌环直径(mm)。药敏片距离≥25 mm。遵照国际检测准则记录试验结果[16]。
-
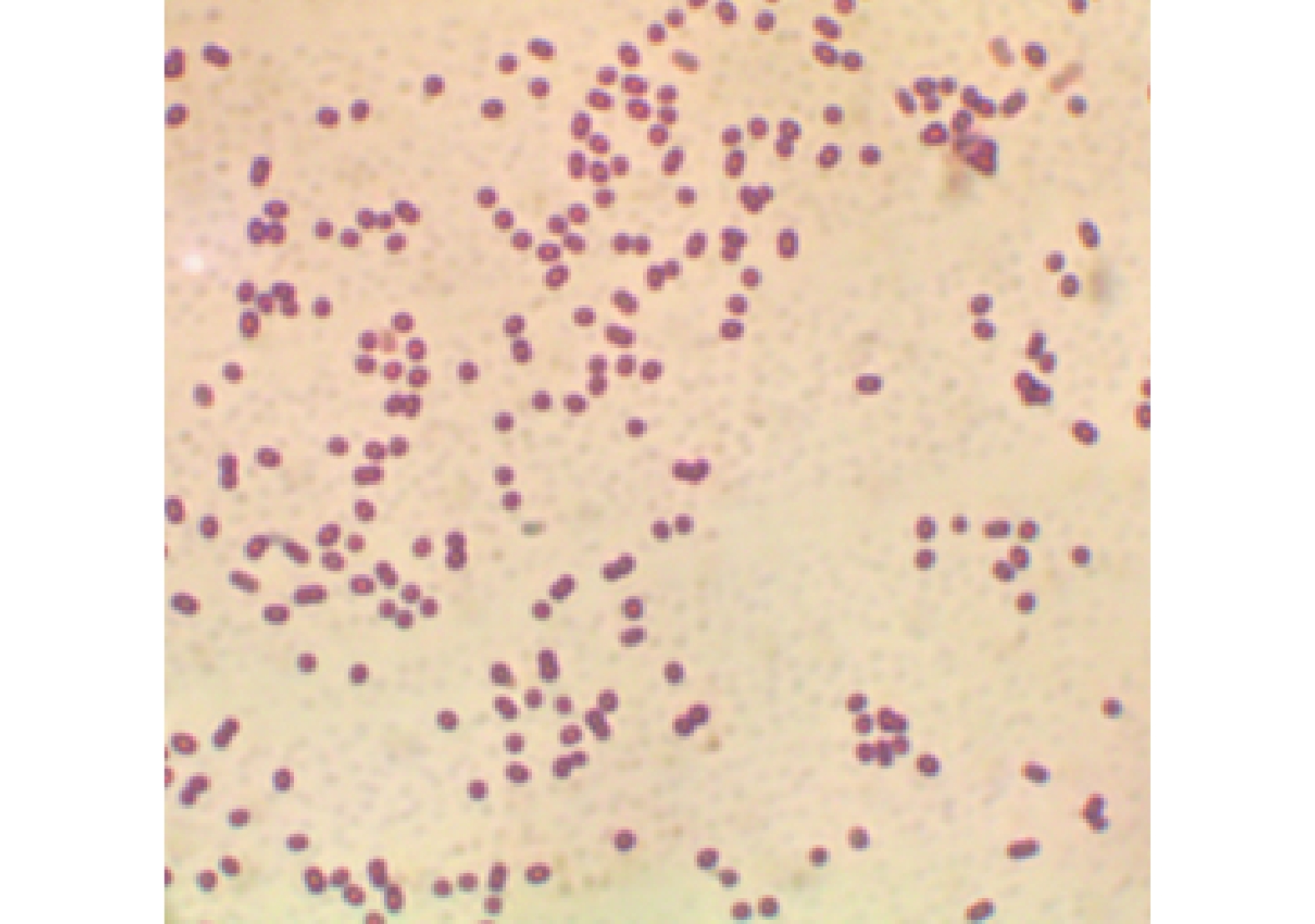
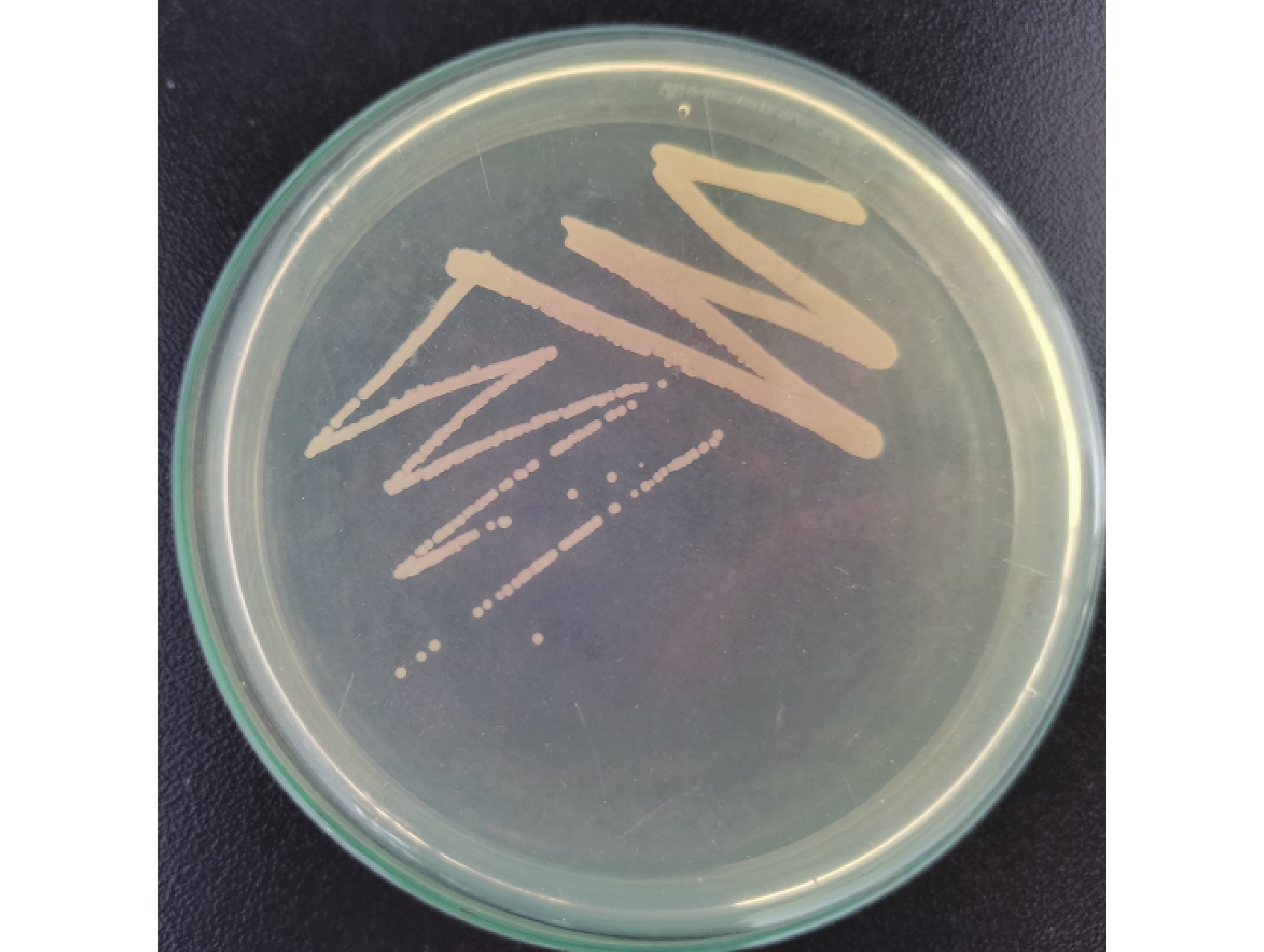
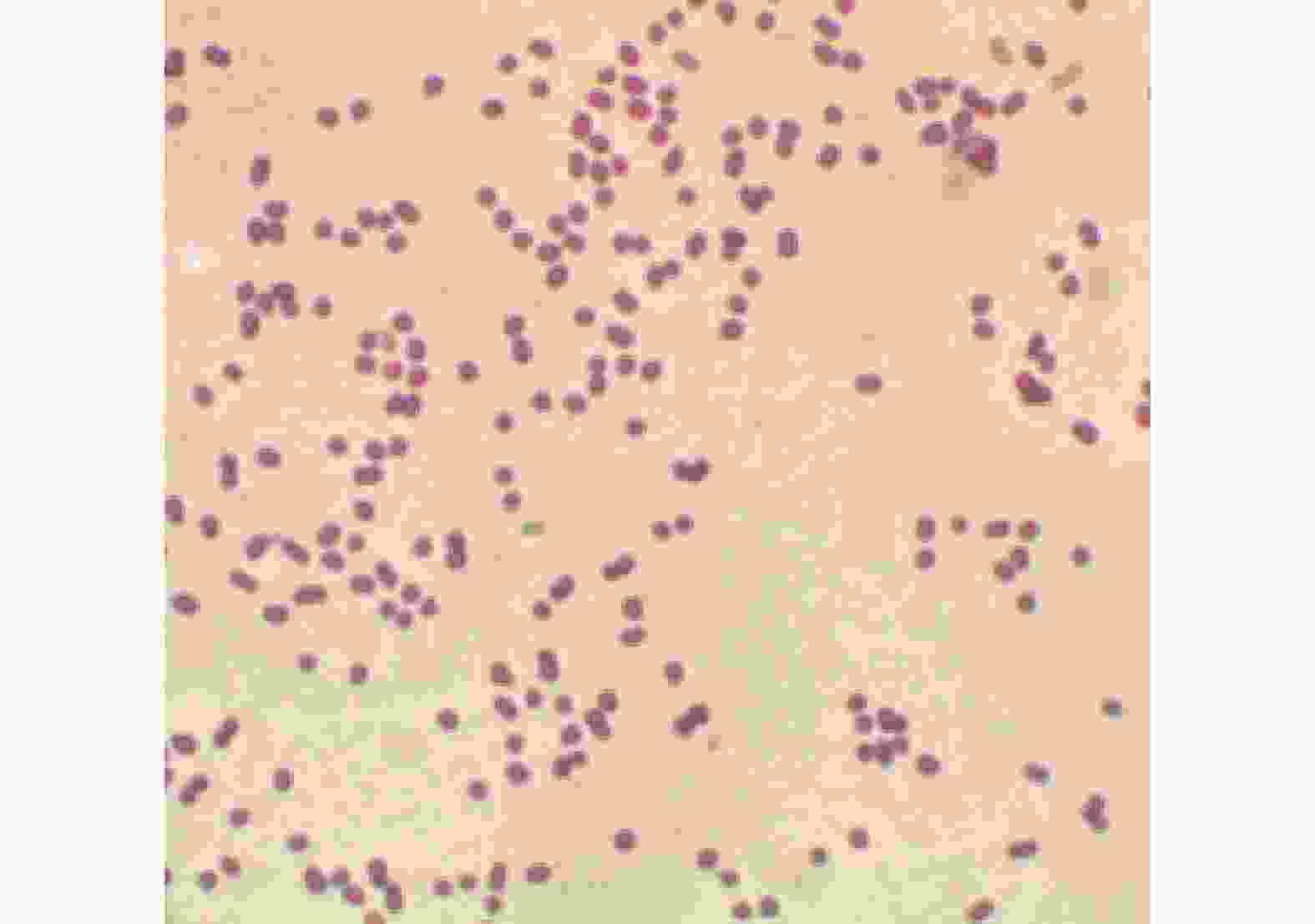
从病死牛体内分离出的病原菌放在胰胨蛋白胨酵母膏葡萄糖琼脂培养基上培养的结果见图1。从图1可见,菌落呈淡白色,生长状态良好,多以单个或成对菌落存在,有些菌落凸起,透明度很低,不溶血。由病死牛肺脏制成的切片,在显微镜下可见多数菌体呈短杆状,无鞭毛。病原菌革兰氏染色呈阴性(图2)与不动杆菌特性相符,初步证实该病原菌分离株为牛源鲍曼不动杆菌。
-
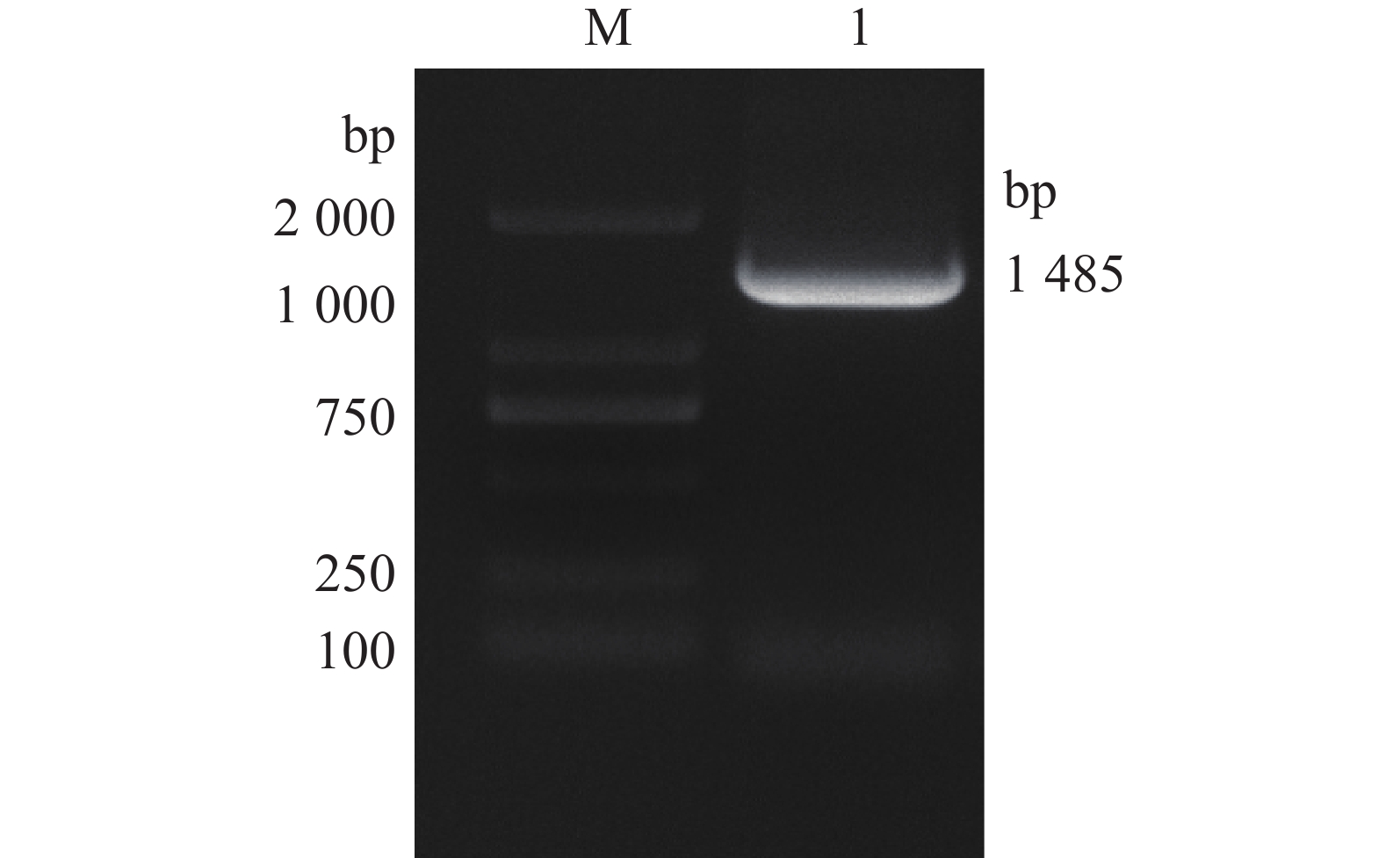
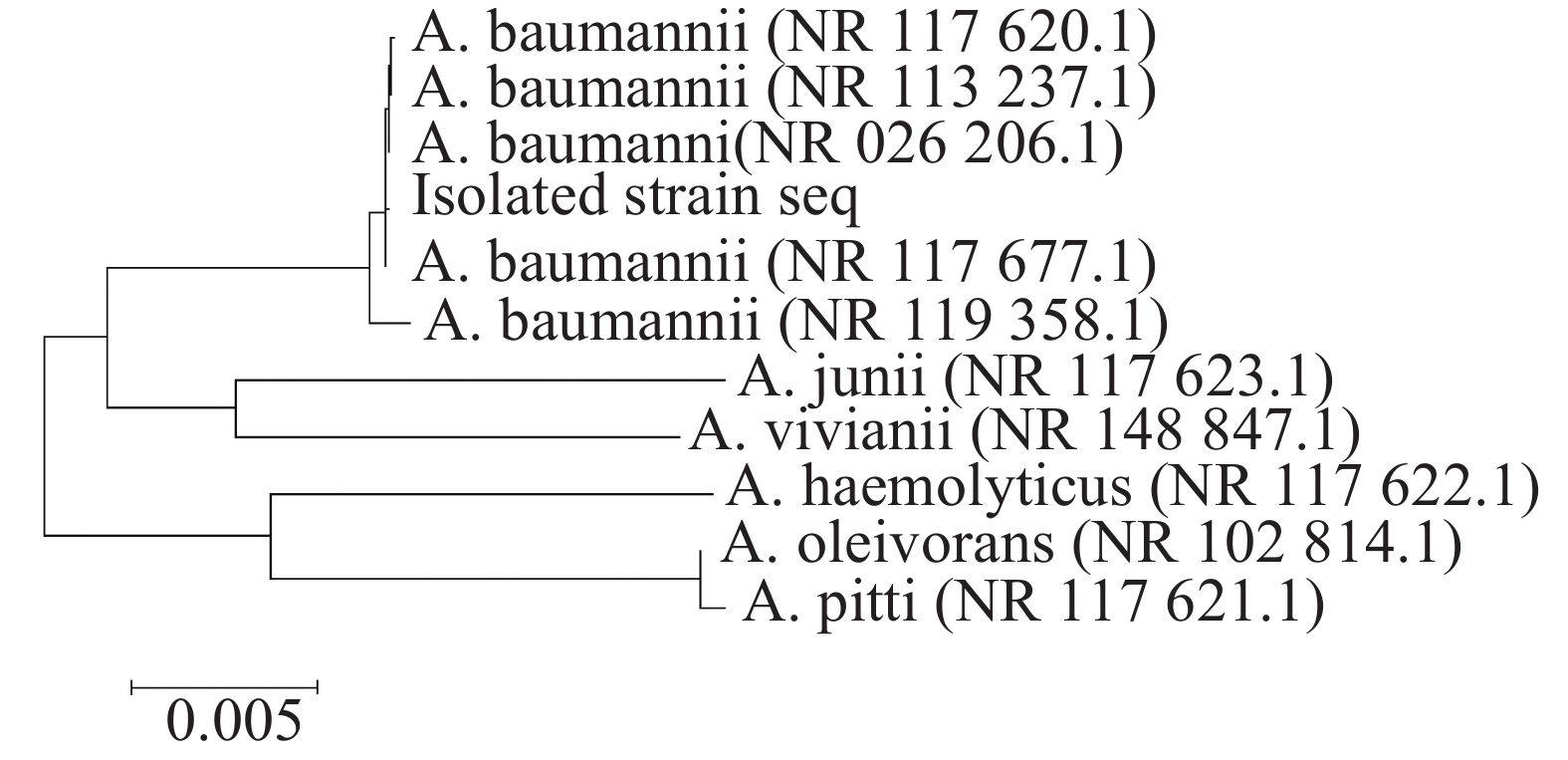
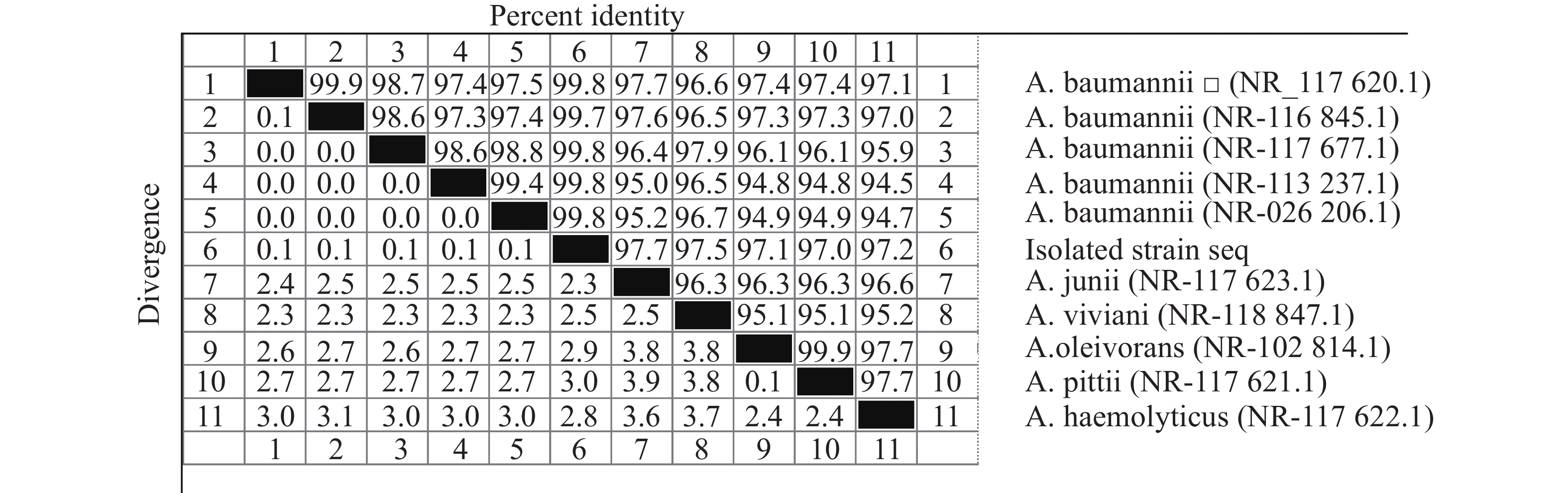
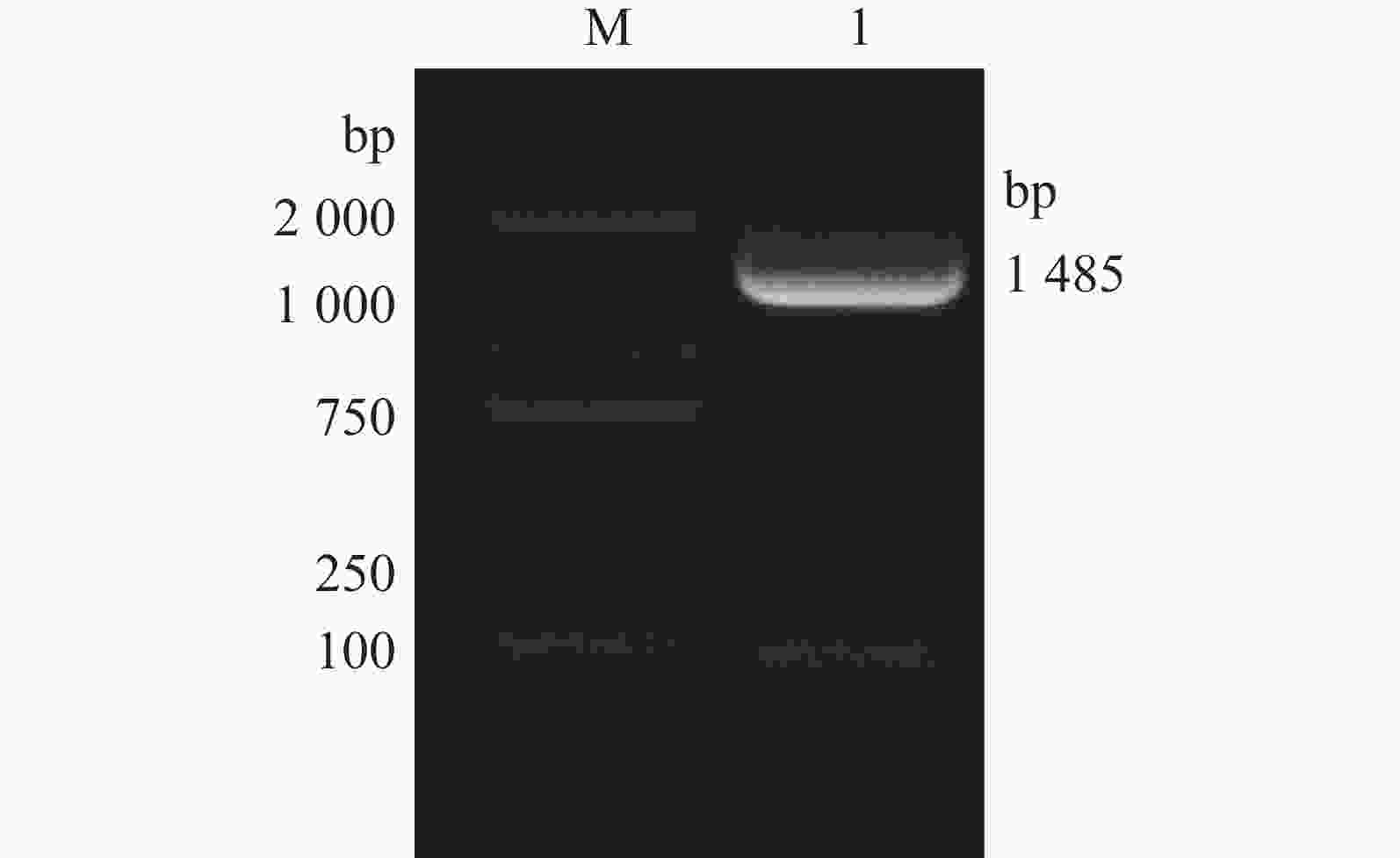
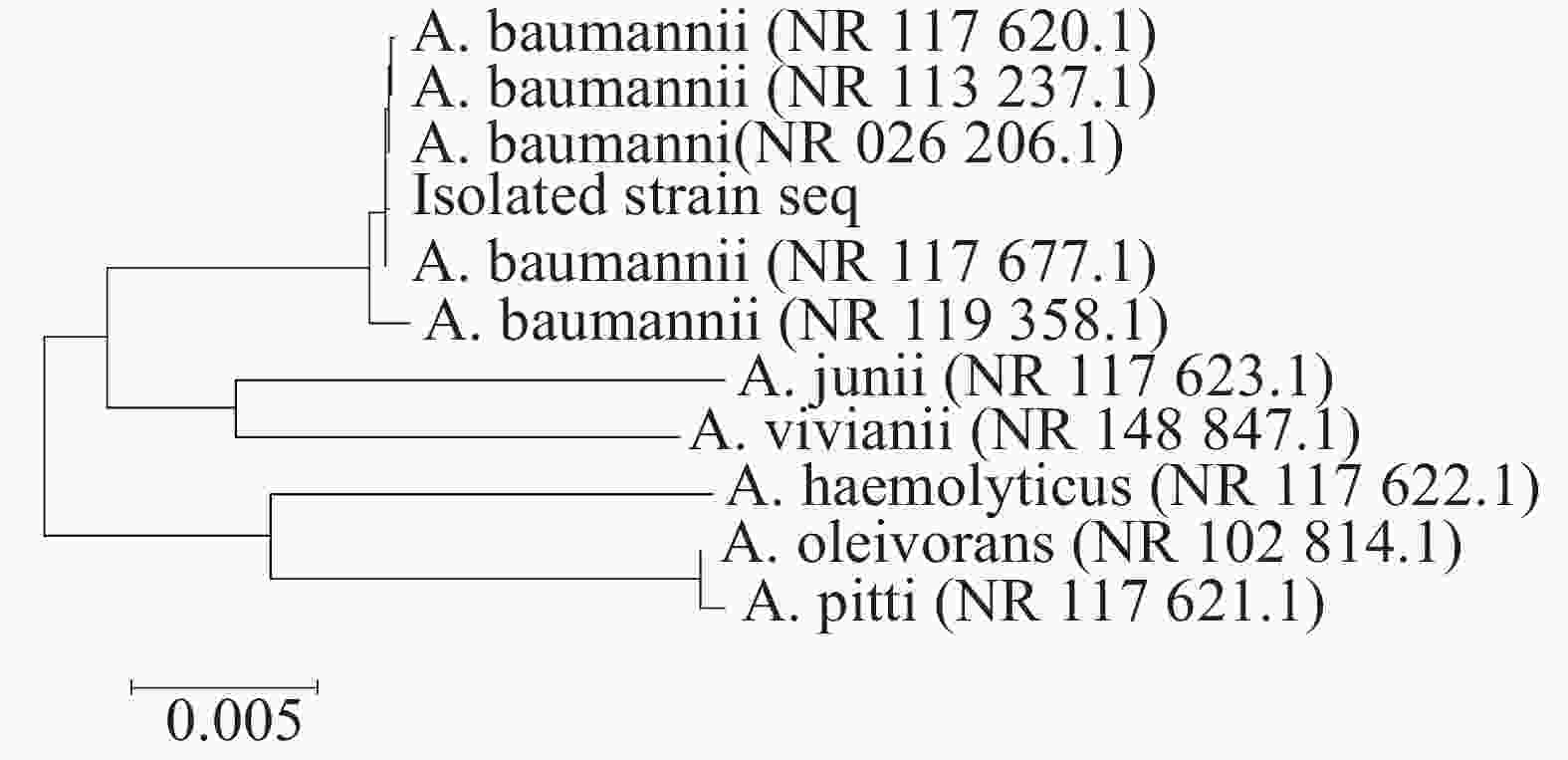
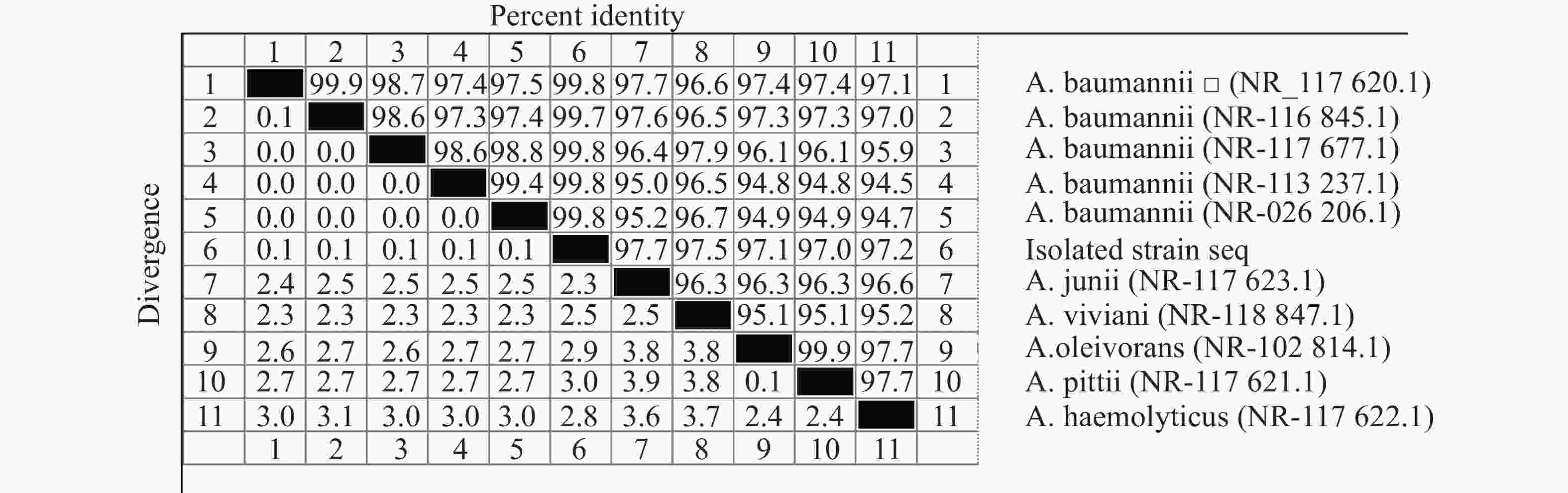
采用16S rDNA引物对分离的单一菌落进行聚合酶链反应(PCR)扩增,扩增产物琼脂糖凝胶电泳可见一条约1485 bp条带(如图3所示)。电泳产物回收后送公司测序,上传序列至基因库中,登录号为(NR_117620.1)并与基因库中鲍曼不动杆菌序列进行比对,结果显示与鲍曼不动杆菌同源率高达99.8%,系统发育进化树结果表明,本实验分离的菌株与鲍曼不动杆菌处于同一亚枝,亲缘关系较近(图4、图5),进一步证明该分离菌株为鲍曼不动杆菌。
-
遵循生化反应试剂盒中的说明书对病原菌进行生化鉴定。结果(表1)表明,分离株的氧化酶阴性,接触酶阳性;分离株利用葡萄糖做碳源;麦芽糖、乳糖、MR及VP均呈阴性,鸟氨酸、尿素酶、半乳糖、枸橼酸盐和葡萄糖等呈阳性,与伯杰手册中的报道基本相符。结合16S rDNA鉴定结果可以证实本实验分离株为鲍曼不动杆菌。
生化鉴定 结果 生化鉴定 结果 蕈糖 − 蔗糖 − 鸟氨酸 + 靛基质 − 尿素酶 − 半乳糖 + 甘露醇 − 枸橼酸盐 + 麦芽糖 − 硫化氢 − 乳糖 − 氧化酶 − 葡萄糖 + Voges Proskauer VP − 接触酶 CAT + 甲基红 MR − 注:“+”表示阳性,“−”表示阴性。
Note:“+”: Positive; “−”: NegativeTable 1. Biochemical identification of Acinetobacter baumannii isolates
-
空白对照组小鼠状态良好,接种细菌组小鼠食欲减退,活动减缓,精神萎靡,后期出现呼吸急促、气喘,身体蜷缩,直至死亡。取发病小鼠的肺组织,于TSA培养基上划线培养8 h,生理生化及细菌生化鉴定试验结果与分离株各试验指标基本一致,鉴定本实验分离的病原菌为鲍曼不动杆菌[17]。
-
分离株感染试验组小鼠结果为通过对小鼠腹腔内注射细菌剂量为5×107 CFU·mL−1组小鼠在48 h 死亡2只;1×108 CFU·mL−1 组小鼠在48 h 死亡3只;2×108 CFU·mL−1 组小鼠在48 h 死亡5只;4×108 CFU·mL−1 组小鼠在48 h内全部死亡。2.5×107 CFU·mL−1 组小鼠在48 h内无死亡。试验组小鼠腹腔注射LD50为8.89×107 CFU·mL−1。
-
药敏性鉴定结果见表2。根据药敏判定标准,该株鲍曼不动杆菌分离株对青霉素、头孢他啶、苯唑青霉素、氨苄青霉素、头孢氨苄、头孢唑林、头孢拉定、麦迪霉素耐药,对羧苄青霉素、氧氟沙星、环丙沙星敏感。这表明此分离株的耐药能力高,在上述药物鉴定试验中该牛源鲍曼不动杆菌没有高敏药物,仅有3种敏感药物可用于治疗。
药物
Drug抑菌圈直径标准/mm
Inhibition zone diameter standard抑菌圈直径/mm
Inhibition zone diameter/mm敏感性
SensitivityR I S 青霉素(Penicillin) ≤10 11-19 <20 0 R 苯唑青霉素(Oxacillin) ≤10 11-20 <21 7 R 氨苄青霉素(Ampicillin) ≤11 12-19 <20 9 R 头孢氨苄(Cephalexin) ≤12 13-20 <21 8 R 头孢唑林(Cefazolin) ≤10 11-19 <20 7 R 头孢拉定(Cefradine) ≤10 11-20 <21 5 R 头孢他啶(Ceftazidime) ≤10 11-21 <22 6 R 麦迪霉素(Medicin) ≤11 12-21 <22 7 R 羧苄青霉素(Carbenicillin) ≤11 12-19 <20 20 S 氧氟沙星(Ofloxacin) ≤10 11-19 <20 20 S 环丙沙星(Ciprofloxacin) ≤13 14-21 <22 22 S 注:R. 抗性;I. 中介;S. 敏感
Note: R: Resistance; I: Intermediary; S: SensitiveTable 2. Results of drug sensitivity test
-
国内外研究结果表明,鲍曼不动杆菌对多数抗生素药物不敏感,仅有部分抗生素药物可用于治疗,但是效果不甚满意,耐药形势十分严峻[18]。其中刘勃兴等[19]人从某鸡场分离出的1株鲍曼不动杆菌仅对诺氟沙星表现中敏,对其他抗生素均不敏感,但是诺氟沙星是兽用禁药,该菌株面临着无药可用的局面,应引起重视。
近些年来,多重耐药性鲍曼不动杆菌在临床细菌分离率占据前三,已经变为一种日益重要的病原体,对该病菌的研究具有一定的公共卫生学意义[20]。国内外对鲍曼不动杆菌的耐药性能力研究较多,主要与细菌内含有的氨基修饰酶、β内酰胺酶、耐药基因岛和整合子等密切相关[21]。而对于鲍曼不动杆菌的致病机制研究较少,目前该细菌的致病机理尚不清楚,对已知的毒力因子外膜蛋白A的研究最为透彻[22]。研究报道,多粘菌素E作为鲍曼不动杆菌有效的体外活性菌剂,可与多种抗生素搭配使用,效果显著[23]。
本研究从病死牛肺脏内分离出鲍曼不动杆菌,在动物回归实验中,4×108 CFU·mL−1组试验小鼠在48 h 内全部死亡,证明该菌具有一定的致病力。药物敏感试验结果显示该株鲍曼不动杆菌对多数抗生素表现耐药,只对羧苄青霉素、氧氟沙星、环丙沙星敏感。目前,国内外对牛源鲍曼不动杆菌的报道尤其对其致病性的研究报道较少。随着我国牛养殖规模的不断增长,鲍曼不动杆菌感染将会成为养牛业的又一难题。建议加强养殖户对鲍曼不动杆菌的感染预防意识,防止牛感染该菌,同时保障饲养人员的健康安全。
Isolation and Identification of Acinetobacter Baumannii from Cattle
doi: 10.15886/j.cnki.rdswxb.2021.04.005
- Received Date: 2021-05-18
- Rev Recd Date: 2021-10-09
- Available Online: 2021-12-28
- Publish Date: 2021-12-25
Abstract: In order to identify the pathogenic bacteria of dead cattle in a cattle farm in Dongfang City, Hainan Province, and to explore the specific causes of cattle deaths in the cattle farm, the lung tissues of the diseased cattle were collected, and the pathogenic strains were isolated. purified and determined by 16S rDNA and biochemical identification methods to identify the genetic background of the pathogenic bacteria, and the 50% lethal dose of the pathogenic bacteria to mice and its drug sensitivity were determined at the same time. The experimental results showed that the homology between the pathogenic strain and Acinetobacter baumannii was as high as 99.8%. The pathogenic strain was highly pathogenic to experimental mice, and its half lethal dose in mice was 8.89 × 107 CFU·mL−1. Drug sensitivity tests showed that the pathogenic strain is sensitive to carbenicillin, ofloxacin and ciprofloxacin.
| Citation: | YANG Zidong, CHENG Yiwen, ZHANG Zhenxing, WU Haotian, WANG Chengqiang, CHEN Qiaoling, CHEN Si, MANCHU Riga. Isolation and Identification of Acinetobacter Baumannii from Cattle[J]. Journal of Tropical Biology, 2021, 12(4): 435-440. doi: 10.15886/j.cnki.rdswxb.2021.04.005 |






 DownLoad:
DownLoad: