-
火龙果 (Hylocereus polyrhizus) 属于仙人掌科的热带植物,原产于中美洲和南美洲[1]。火龙果果实颜色亮丽并含有多酚类化合物[2],红肉火龙果含有甜菜红素[3-4]等丰富的生物活性成分,具有抗氧化、清肠通便等多种生理功能,有很高的营养价值和经济价值[5-7]。近年来,火龙果在我国种植面积增长十分迅速,截至2020年,我国火龙果种植面积已经超过6.67万 hm2。随着种植面积的扩大,火龙果的病害问题日趋严重,其中危害最严重的是火龙果溃疡病[8-9]。2012年,火龙果溃疡病在中国台湾首次被报道[10],随后在马来西亚、以色列、墨西哥、美国、尼加拉瓜和中国的广东、广西、云南、贵州、海南等火龙果种植区均有发现[9,11-12],火龙果溃疡病严重危害火龙果的产量和品质,在夏季,火龙果茎部溃疡病病株发病率高达60%,该病具有发病快、传播广、危害大等特点。经研究表明,引起该病的病原菌为新暗色柱节孢菌 (Neoscytalidium dimidiatum)[13],该病菌主要损害火龙果树的茎和果实,导致果实和茎条先出现白色病斑,随着时间的推移转变为棕色,最后表现为茎腐病、果实开裂[14-15]、内部褐腐病和黑腐病[11,14],此病害在高温和潮湿的环境中更具侵袭性[16],对火龙果的产量、质量产生有害影响,进而影响其商业价值[17]。目前,已经分离和鉴定了火龙果溃疡病的致病菌,但关于其遗传转化体系的研究仍为空白,而有效的遗传转化体系对于新暗色柱节孢菌致病机理及相关致病基因的研究将起到至关重要的作用。真菌遗传转化主要分为原生质体转化和农杆菌介导转化2种途径[18]。原生质体法操作简单,但转化效率低、变异率高,而且PEG有一定的毒性,可能会对原生质体的生长有一定影响,制备原生质体程序繁琐,因此,在丝状真菌的转化应用中受限[4,19-21]。2001年MULLINS 等[22]通过构建二元载体,通过农杆菌的介导实现了对镰刀菌的有效遗传转化,到目前为止已有大量的农杆菌介导转化真菌的报道[23],但是因为真菌材料的性质差异,所用农杆菌菌株和真菌分离株及共培养条件的不同,对不同的真菌物种进行优化是一项艰巨的任务。迄今为止,国内外还没有关于农杆菌介导新暗色柱节孢菌转化的相关报道,其在新暗色柱节孢菌中的有效性尚待进一步研究。笔者利用农杆菌介导转化法,将含有潮霉素抗性基因(hyg)和绿色荧光蛋白基因(mGFP)的双元表达载体转化到新暗色柱节孢菌中,并在阳性转化子中检测绿色荧光蛋白的表达,旨在为进一步研究新暗色柱节孢菌的菌丝生长过程、形态特征、致病菌与火龙果之间的互作致病机制等提供技术支撑。
-
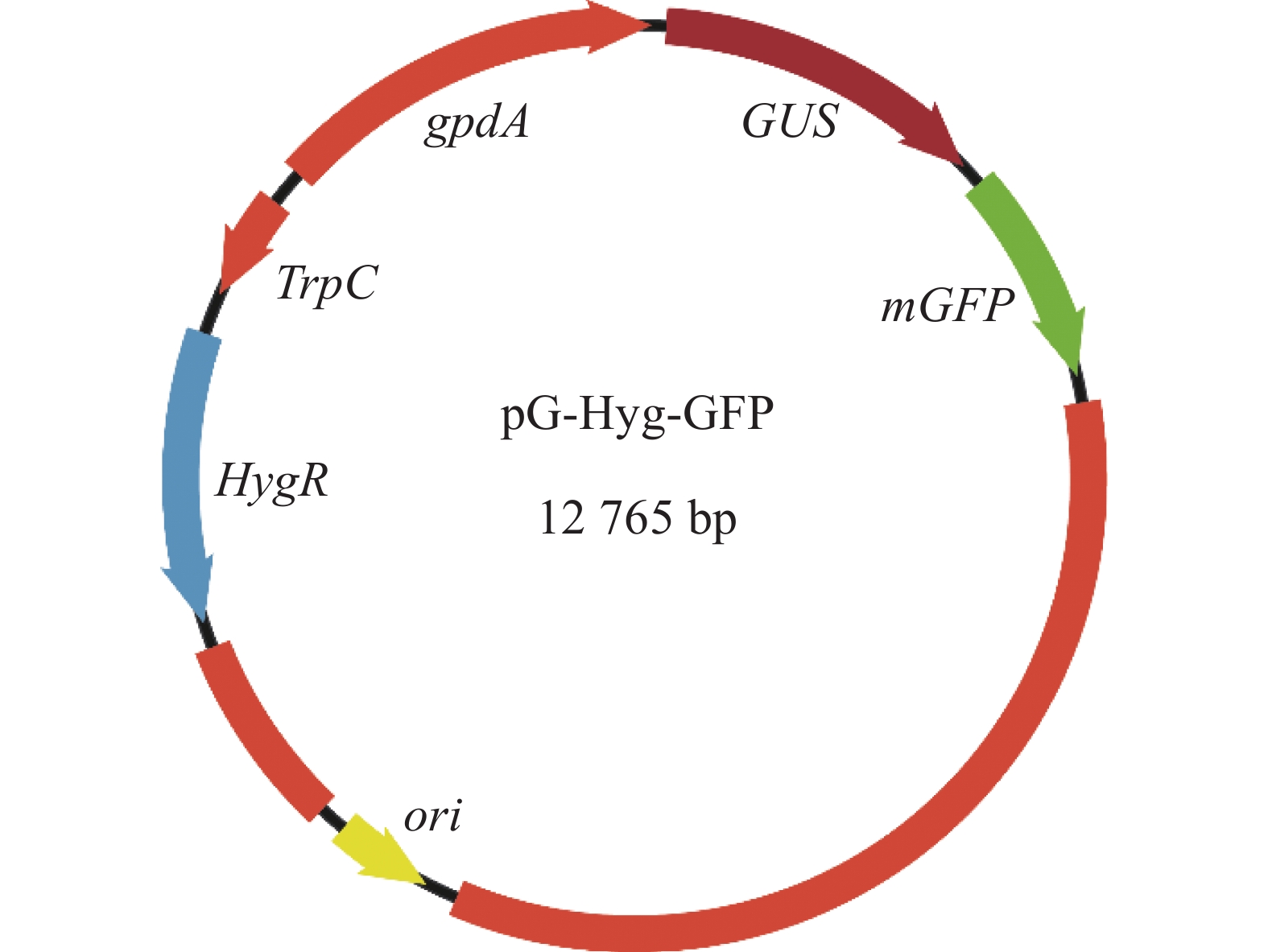
新暗色柱节孢菌是本实验室从红皮红肉火龙果茎条分离鉴定并保存的。双元表达载体pG-Hyg-GFP是以pCAMBIA1300为骨架,插入trpC启动子、潮霉素抗性基因(hyg)和绿色荧光蛋白基因(mGFP),方法参照MULLINS 等[22]。其中潮霉素抗性基因来源于构巢曲霉Aspergillus nidulans的trpC启动子控制其转录,绿色荧光蛋白基因来源于3−磷酸甘油脱氢酶基因(gpdA)启动子控制其转录。利用化学转化法转入根癌农杆菌AGL-1中,获得农杆菌pG-Hyg-GFP(图1)。
-
马铃薯葡萄糖琼脂(PDA)培养基:称取PDA培养基粉62.5 g,加水定容至1 000 mL,灭菌后4 ℃保存。马铃薯葡萄糖液体(PDB)培养基:称取PDB培养基粉26.1 g,加水定容至1 000 mL,调pH至7.0后灭菌,4 ℃保存。基本培养基(minimal medium, MM): KH2PO4 3.625 g,K2HPO4 5.125 g,NaCl 0.375 g,MgSO47H2O 1.250 g,CaCL22H2O 0.165 g,FeSO4·7H2O 0.0062 g,(NH4)SO4 1.250 g,定容至1 000 mL,室温储存。AIM培养基:MM培养基400 mL,0.5%(V/V)甘油5 mL,MES 8.5 g,1 mol·L−1过滤除菌的葡萄糖5 mL,将pH调至5.3,定容至1 000 mL,固体加入15 g的琼脂粉,室温储存。LB培养基:酵母提取物5 g,胰蛋白胨10 g,NaCl 10 g,将pH调至7.0,4 ℃储存。噻孢霉素(Cefotaxime):称取1 g Cef溶于1 mL灭菌水中,配置成100 g·L−1,过滤分装,−20 ℃储存。乙酰丁香酮(Acetosyringone):称取 196.2 mg AS,溶于 10 mL二甲基亚砜(Dimethyl sulfoxide),配置成100 mmol·L−1,−20 ℃储存。卡那霉素(Kanamycin):称取50 mg Kan,溶于1 mL的灭菌水中,配置成50 g·L−1,−20 ℃储存。利福平(Rifampicin):称取0.25 g Rif,溶于10 mL无菌水中,过滤分装,−20 ℃储存。
-
将野生的新暗色柱节孢菌丝接到PDA培养基平板上培养3~5 d,先用5~7 mL灭菌水洗涤菌丝,再用枪头把菌丝悬浮液吸至含有50 mL的PDB培养基中,在摇床中28 ℃培养3 d后,用3层擦镜纸过滤获得孢子悬浮液,并稀释成1×107个·mL−1,吸取20 µL孢子悬浮液点接到分别含有0、10、20、30、40、50、60、70 µg·mL−1潮霉素B的PDA平板上,置于28 ℃恒温培养箱中培养7 d,每天观察其生长状况并记录。
-
农杆菌转化参照文献[22]的方法培养。首先将−80 ℃保存的含有重组质粒的农杆菌AGL-1菌液接到液体LB培养基中(含有100 µg·mL−1的Kan和25 µg·mL−1的Rif),28 ℃,180 r·min−1摇床培养至浑浊;然后取培养好的农杆菌50 µL接种到MM培养基中(含有100 µg·mL−1的Kan和25 µg·mL−1的Rif),28℃,180 r·min−1摇床培养2 d,室温,5 000 r·min−1离心10 min,弃上清,加入20 mL的AIM液体培养基(含有200 µmol·L−1的AS和40 µmol·L−1的MES)重悬,5 000 r·min−1室温离心10 min,弃上清;再用IM液体培养基(含有200 µmol·L−1的AS和40 µmol·L−1的MES)调农杆菌A600=0.15,28 ℃,180 r·min−1摇床培养12 h至A600=0.6,备用。
-
挑取适量的菌丝于PDB培养基中(含100 µg·mL−1的Kan),28 ℃暗培养3 d,经3层擦镜纸过滤,收集孢子悬浮液,调整孢子悬浮液的密度为1×107个·mL−1,备用。
-
将A600=0.6的根癌农杆菌与新暗色柱节孢的分生孢子悬浮液(1×107个·mL−1)按体积比为1:1的比例混合,取200 µL混合液,用涂布器均匀的涂布于铺有硝酸纤维素滤膜IM(含200 µmol·L−1的AS和40 µmol·L−1的MES)固体培养基上,25 ℃培养48 h。
-
将完成共培养48 h的硝酸纤维素薄膜转移至PDA培养基上(含200 µg·mL−1 Cef、50 µg·mL−1的潮霉素B),25 ℃暗培养7 d后,用接种环挑取转化子于新的PDA培养基上(含200 µg·mL−1 Cef、50 µg·mL−1的潮霉素B)培养7 d,进行检测。
-
在无菌条件下,将转化子接种到选择培养基上,25 ℃培养7 d,滴一滴dH2O于载玻片中央,用接种针挑取嫩的菌丝于水中,将菌丝打散,盖上盖玻片,在黑暗条件下,将载玻片放到带有绿色激发滤光460~550 nm的OLMPUS IX71荧光显微镜下,观察绿色荧光蛋白表达情况。先用自然光找到菌丝体,再关闭自然光改为蓝光,分别记录在自然光和绿光下的曝光情况,并用OLMPUS相机拍照。
-
将转化子用接种环接种在选择培养基上培养7 d,待长满培养皿时,用接种环将刮取菌丝并利用真菌微量DNA提取试剂盒提取DNA,用于PCR检测。具体操作如下:以新暗色柱节孢菌全基因组DNA为模板,根据双元表达载体(pG-Hyg-GFP)上的潮霉素磷酸转移酶基因设计上下游引物,分别为HPH-F:5′-AATTCGGTCGAAAAAAGAAAAGG-3′和HPH-R:5′-CTATTTCTTTGCCCTCGGACG-3′,PCR扩增体系为:TaKaRa Ex Taq 0.25 µL,10×buffer 5 µL,dNTP mixture 4 µL,模板2 µL,上下游引物各1 µL,dH2O补充至50 µL。PCR扩增条件为:95 ℃预变性5 min,95 ℃变性30 s,59 ℃退火30 s,72 ℃延伸100 s,30次循环,72 ℃延伸5 min。PCR产物片段大小为1 604 bp。
-
从获得的转化子中随机挑选出12个转化子接种至不含有潮霉素抗性的PDA培养基上培养,3 d后从边缘挑取菌丝接种至另一个不含有潮霉素抗性的PDA培养基上培养,连续转接5代,再将菌丝转接至含有潮霉素抗性PDA的平板上培养,观察转化子的情况。
-
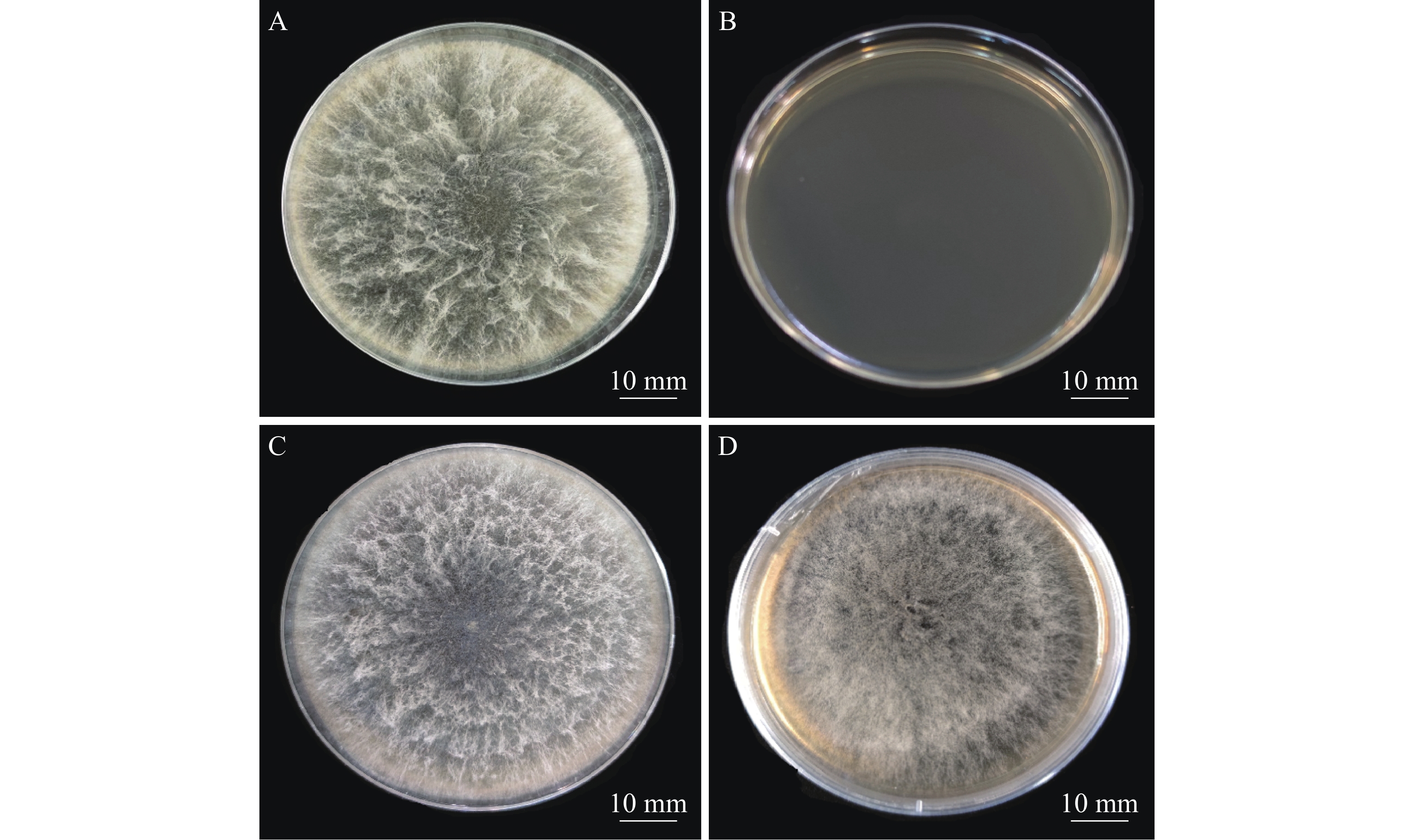
将野生型的新暗色柱节孢菌株接在含有不同浓度的潮霉素B的PDA平板上,其在含有20 µg·mL−1潮霉素B的PDA培养基平板上受到明显的抑制,在40 µg·mL−1潮霉素B的PDA培养基平板上能够完全抑制其生长,为了防止假阳性,选择培养基选用50 µg·mL−1潮霉素的浓度。
-
将农杆菌和新暗色柱节孢菌在IM培养基上共培养48 h后,将硝酸纤维素薄膜转移至含有50 µg·mL−1的潮霉素的PDA培养基上培养7 d,培养基上会长出新的菌丝。将其再挑到新的含50 µg·mL−1潮霉素B和200 µg·mL−1 Cef (抑制农杆菌生长)的PDA培养基上培养。结果表明,新暗色柱节孢菌转化子可以在含有50 µg·mL−1潮霉素B的PDA平板上生长(图2),而野生型菌株不生长,说明新生新暗色柱节孢菌转化子具有潮霉素抗性。
-
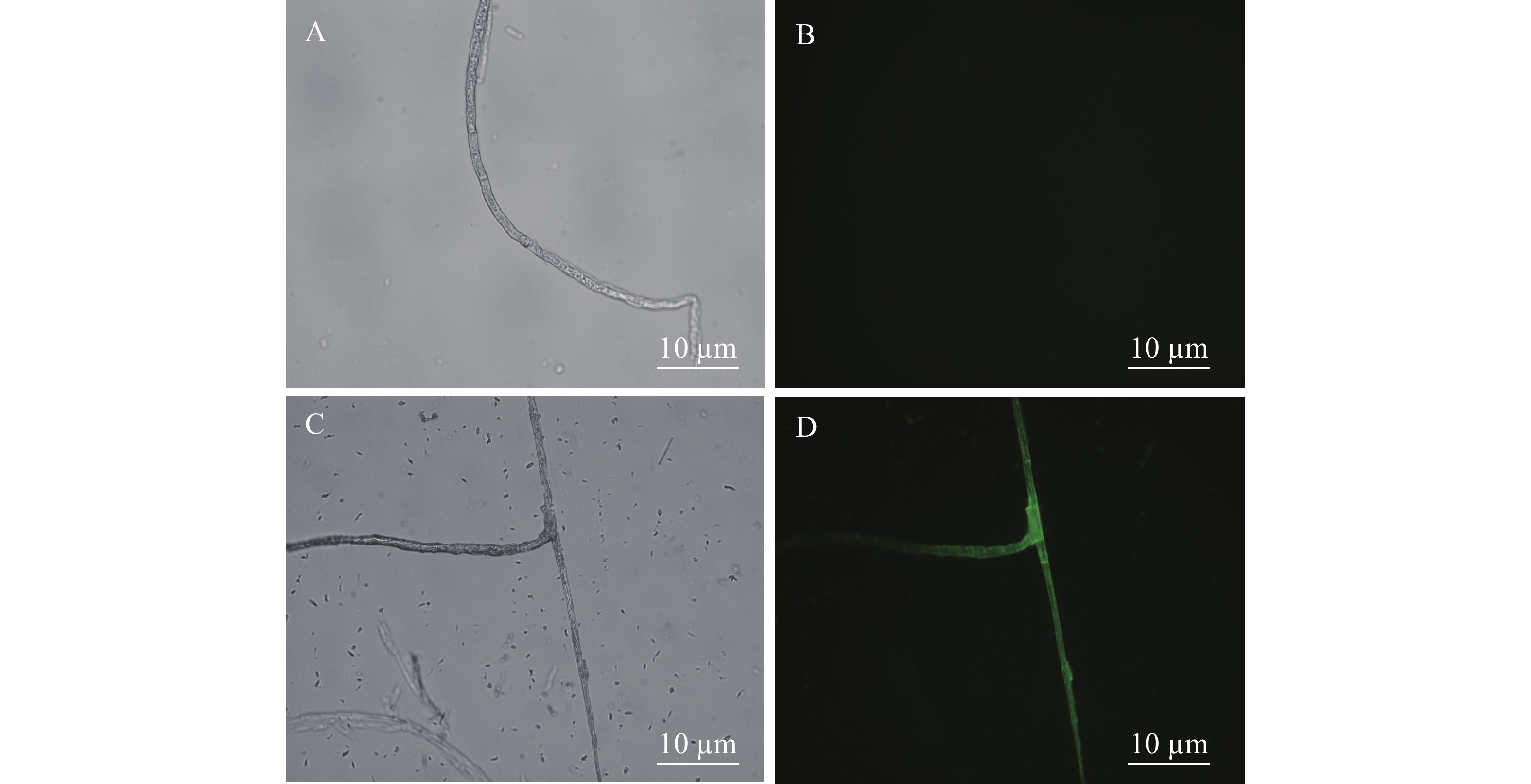
在荧光显微镜的蓝光(488 nm)激发下,观察和检测mGFP的表达情况。图3-A、3-C分别为野生型新暗色柱节孢菌和转化子在荧光显微镜自然光中的形态;图3-B、3-D分别为野生型新暗色柱节孢菌和转化子在荧光显微镜荧光场中的形态。在荧光场中,新暗色柱节孢菌的野生型菌丝观察不到荧光,而新暗色柱节孢菌的转化子的菌丝上有明显的绿色荧光。说明在转化子中,GFP基因已成功转到新暗色柱节孢菌中并得以表达。
-
随机挑取3个新暗色柱节孢菌转化子在新的PDA培养基上(含有50 µg·mL−1潮霉素B)培养,待菌丝长满平板。分别提取新暗色柱节孢菌转化子和野生型的DNA,以HPH-F和HPH-R作为上下游引物进行PCR检测,结果(图4)表明,野生型对照没有扩增出条带,转化子能够扩增出1 604 bp的潮霉素基因片段,说明T-DNA已插入新暗色柱节孢菌中。
-
将挑取的12个转化子接种至无潮霉素抗性的PDA培养基上连续培养5代,再转接至含有潮霉素抗性的PDA培养基上培养,发现所有的转化子均可以正常生长,说明转化子获得了抗潮霉素抗性,可以稳定遗传。
-
通过构建双元表达载体,利用构巢曲霉(Aspergillus nidulans)的trpC启动子控制潮霉素磷酸转移酶的转录;利用构巢曲霉(Aspergillus nidulans)的3-磷酸甘油脱氢酶基因(gpdA)启动子控制绿色荧光蛋白(mGFP)的转录。在农杆菌介导下,成功将含有潮霉素抗性基因和含有绿色荧光蛋白基因(mGFP)导入火龙果溃疡病致病菌—新暗色柱节孢菌中,并通过PCR扩增检测到潮霉素基因片段,通过荧光显微镜检测到绿色荧光蛋白的表达,说明外源基因能够整合到新暗色柱节孢菌基因组上并成功表达;将转化子菌株转接5代仍然可以在含有潮霉素B(50 µg·mL−1)的PDA培养基上生长,说明其具有遗传稳定性。
目前,国内外对于新暗色柱节孢菌的研究较少,相关的文献报道基本都处于新暗色柱节孢菌的分离和鉴定阶段[1,17,24],也有在不同物种上发现了新暗色柱节孢菌的报道,如在加利福尼亚的无花果[25]、马来西亚的菠萝[26]、中国广西的剑麻[27]等上面,均发现了新暗色柱节孢菌,导致植物发生溃疡病。近几年有关于火龙果对新暗色柱节孢菌侵染的响应研究报道,如2019年,XU 等[28]从海南红皮红肉火龙果中分离得到新暗色柱节孢菌,使用Illumina RNA-Seq 技术研究了红肉火龙果 (H. polyrhizus) 对Neoscytalidium dimidiatum的宿主反应;2020年,XU 等[9]全面概述了红肉火龙果在转录水平上响应Neoscytalidium dimidiatum感染的HpLRR家族基因;2021年,PAN 等[29]采用定量蛋白组学分析法研究了火龙果对Neoscytalidium dimidiatum感染的免疫反应,但新暗色柱节孢菌对火龙果的致病性机理的研究还未见报道。
建立成功的转化系统是研究基因功能的前提,目前已经有利用该方法获得转化子并开展基因功能的研究报道[30-31]。因此,建立有效的新暗色柱节孢菌遗传转化体系,对于研究新暗色柱节孢菌的致病基因及其基因功能极其重要。虽然已有大量农杆菌介导的真菌遗传转化体系被建立,但未见新暗色柱节孢菌遗传转化相关的报道。本研究成功获得稳定表达的转化子,将为后续开展新暗色柱节孢菌侵染火龙果的发育发展进程和探究新暗色柱节孢菌侵染火龙果的致病机理研究提供材料;还可以为后续突变体库的建立提供技术基础,通过筛选表型异常的突变体开展基因功能的研究。
Agrobacterium tumefaciens-mediated Transformation of Neoscytalidium dimidiatumcausing Stem Canker on Pitaya
doi: 10.15886/j.cnki.rdswxb.2021.04.002
- Received Date: 2021-03-04
- Accepted Date: 2021-12-10
- Rev Recd Date: 2021-06-30
- Available Online: 2021-12-28
- Publish Date: 2021-12-25
-
Key words:
- pitaya canker disease /
- Neoscytalidium dimidiatum /
- hygromycin resistant gene /
- green fluorescent protein /
- Agrobacterium tumefaciens‐mediated transformation
Abstract: In recent years, pitaya (Hylocereus spp.) has gradually become a newly-emerging tropical fruit. With the continuous increase of planting area, diseases infecting pitaya have become more serious. Among them stem canker is the most important disease, which is caused by Neoscytalidium dimidiatum. To explore the genetic variation and gene function of N. dimidiatum, an effective genetic transformation system must be established. However, there have been no reports documented at home or abroad in this aspect. A binary expression vector containing gpdA promoter, green fluorescent protein (mGFP) and hygromycin resistance gene as screening markers was constructed to establish a genetic transformation system for N. dimidiatum through Agrobacterium tumefacien-mediation, and positive transformants from the spores of N. dimidiatumwere successfully generated. Fluorescence microscope observations showed that the positive transformant hyphae could produce green fluorescence, while the wild-type hyphae could not produce green fluorescence. The PCR test confirmed the integration of hygromycin resistance gene in the transformant genome. Therefore, this Agrobacterium tumefaciens mediated transformation system produced stable genetic expression of mGFP gene in N. dimidiatum , which lays a technical foundation for the further study of the pathogenicity mechanism of dragon fruit canker disease.
| Citation: | WANG Meng, WANG Zhouwen, DING Yi, XU Min, GUO Panyang, LUI Chengli, LI Jiaxue, LI Tao, WEI Shuangshuang, TANG Hua. Agrobacterium tumefaciens-mediated Transformation of Neoscytalidium dimidiatumcausing Stem Canker on Pitaya[J]. Journal of Tropical Biology, 2021, 12(4): 412-418. doi: 10.15886/j.cnki.rdswxb.2021.04.002 |










 DownLoad:
DownLoad: