-
柱花草(Stylosanthes spp.)起源于南美洲,其产量高、草质好且具有耐贫瘠、耐酸性土壤的特点,是世界热带地区重要的豆科牧草和绿肥资源[1-2]。柱花草炭疽病(Stylosanthes anthracnose)是危害柱花草生产的严重病害,可导致牧草及种子产量锐减甚至绝收,以及饲草品质大幅下降[3-4]。柱花草炭疽病主要由半知菌炭疽病菌属的胶孢炭疽菌(Colletotrichum gloeosporioides)引起,有A型和B型2种专化型[5]。目前,对于柱花草抗炭疽病的基础研究多侧重于病原菌侵染方式和致病机理。如炭疽菌致病基因CgDN3及SSD1的鉴定和致病机制研究[6-7],胶孢炭疽菌采取活体寄生和死体腐生双阶段侵染策略的发现[8],以及炭疽病的致病性和喀斯特炭疽菌的发掘等[9]。但是,对于柱花草抗炭疽菌机制尚未完全了解,仍有待深入研究。游离氨基酸(Free amino acids)约占植物体内总氮的2%,是重要的生物活性物质。近年来,游离氨基酸在植物生长发育及抵抗生物与非生物逆境胁迫过程中发挥的生物学功能受到广泛关注,相关研究取得诸多进展。例如,脯氨酸的大量积累是水稻(Oryza sativa)、小麦(Triticum aestivum)、玉米(Zea mays)等作物中普遍存在的适应低温环境的机制[10];干旱胁迫下,植物体内的脯氨酸和赖氨酸含量会上升,从而调节渗透势以缓解胁迫对植株带来的损伤[11];γ−氨基丁酸(GABA)作为信号分子,通过调节应答分子网络提高植物耐盐与耐铝毒胁迫能力[12];β−氨基丁酸(BABA)通过诱导烟草(Nicotiana tabacum)体内水杨酸、过氧化氢及病程相关蛋白(PR蛋白)的积累,有效抵抗了烟草花叶病侵染等[13]。但是,游离氨基酸在柱花草抗炭疽病方面的研究仍未见报道。本研究将分析胶孢炭疽菌侵染柱花草后,其叶片游离氨基酸的变化,并对显著增加积累的叶片游离氨基酸开展进一步抑菌活性分析,鉴定参与柱花草抗炭疽病的游离氨基酸种类,旨在为解析柱花草抗炭疽病机理及绿色防治药物的开发提供参考依据。
HTML
-
以柱花草‘热研2号’(Stylosanthes guianensis cv. ‘Reyan No.2’)为实验材料(由中国热带农业科学院热带作物品种资源研究所牧草业研究室提供)。供试胶孢炭疽菌(Colletotrichum gloeosporioides)菌株为本实验室收集保存。
-
本实验在海南大学热带作物学院科研基地的大棚中进行。将柱花草种子去种皮后,选取大小一致、饱满的种子于80 ℃水浴3 min,冷却至室温,黑暗条件下萌发2~3 d后,将幼苗转移到基质为V营养土∶V蛭石= 1∶1的育苗盒中继续培养。培养4周后,接种炭疽菌。将炭疽菌菌丝块置于PDA平板上,于28 ℃恒温箱中黑暗培养7 d。用无菌水冲洗菌丝,两层无菌纱布过滤洗脱炭疽菌孢子,将孢子悬浮液离心后用无菌水将孢子重悬,用血球计数板统计孢子密度,控制孢子密度在1×107 个·mL−1,加入φ=0.02% silweet-77。实验设置2个处理,采用全株喷洒的方式,将孢子悬浮液均匀喷洒热研2号柱花草至叶片上凝成水珠,对照用含φ=0.02% silweet-77的等量无菌水处理。接种胶孢炭疽菌96 h后收取样品,每个处理包含4个生物学重复。
-
参照文献[14]的方法分析柱花草叶片的矿质元素浓度,并略作改动。其中,磷(P)浓度用钼黄比色法;钙(Ca)浓度用EDTA络合滴定法;钾(K)、镁(Mg)、铁(Fe)和钠(Na)浓度用原子吸收分光光度法测定。
-
采用分光光度比色法[15]测定柱花草叶片抗氧化酶活性和次级代谢物浓度,包括过氧化物酶(POD)、总超氧化物歧化酶(T-SOD)和苯丙氨酸解氨酶(PAL)活性,总酚、类黄酮和原花青素浓度。参照文献[16]采用黄嘌呤氧化酶法测定铜锌−超氧化物歧化酶(CuZn-SOD)的活性。参照文献[17]的方法,并略作修改,利用铁离子还原/抗氧化能力法(FRAP法)检测总抗氧化能力(T-AOC),其中,用考马斯亮蓝法[18]测定可溶性蛋白质浓度。
-
采用高效液相色谱-串联质谱(LC-MS/MS)[19] 分析柱花草叶片氨基酸浓度。
-
对柱花草叶片中显著积累的氨基酸,采用琼脂平板法进行病原菌抑菌活性测定。将氨基酸溶解后过滤除菌,分别配制含不同氨基酸的PDA培养基,且培养基中氨基酸质量浓度均为200 mg·L−1,对照组培养基中加入等量溶剂。将配置好的培养基用移液枪加入直径9 cm培养皿中,每皿5 mL培养基。用直径4 mm的无菌打孔器打取大小一致的菌饼,接种于上述培养皿中心。每组6个重复。暗培养6 d后,通过十字交叉法测量各平皿中的菌落直径,并计算该代谢物对胶孢炭疽菌生长的抑菌率。
-
采用Microsoft Office 2016软件进行数据分析,实验结果以平均值和标准误表示,用IBM SPSS Statistics 20(SPSS Institute,美国)软件进行独立样本t检验来分析差异的显著性。
1.1. 材料
1.2. 材料的种植及接菌处理
1.3. 矿质元素浓度测定
1.4. 抗氧化相关指标的测定
1.5. 氨基酸浓度分析
1.6. 氨基酸对病原菌的抑菌活性测定
1.7. 数据处理
-
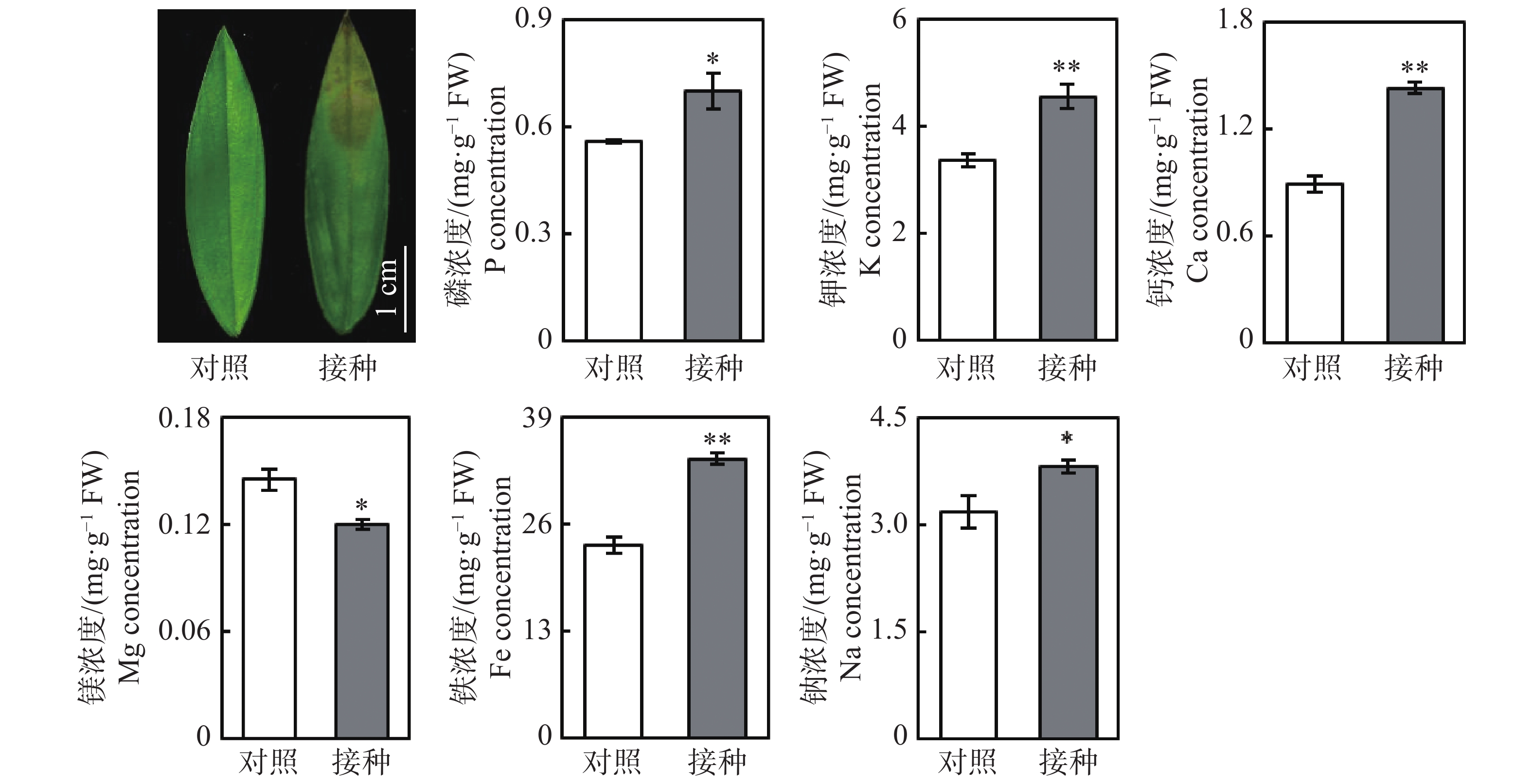
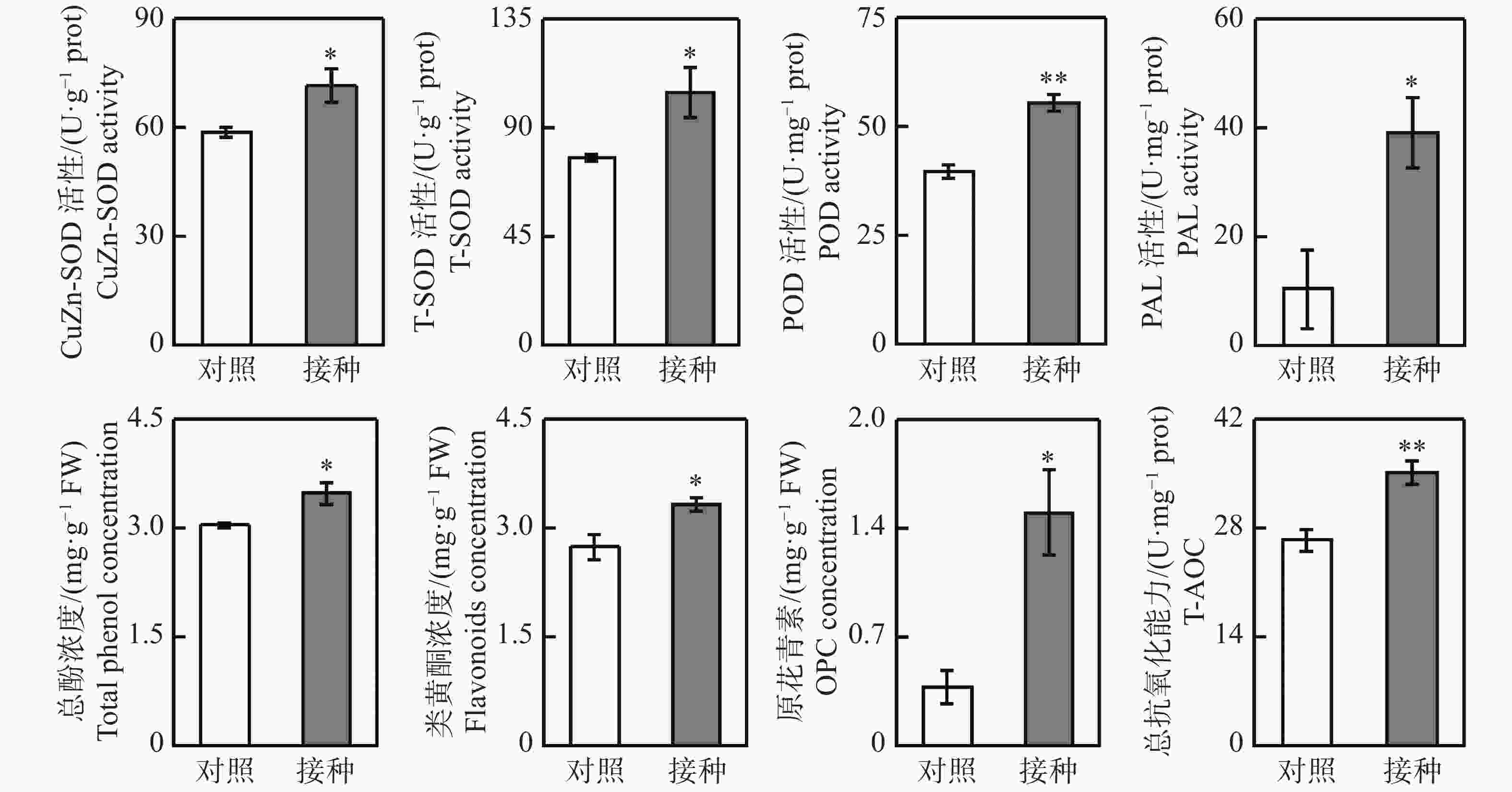
从图1可知,与对照组相比,接种96 h后,柱花草叶片呈现褐色软腐水渍状病症,磷、钾、钙、铁和钠浓度分别显著提高了25.4%、35.5%、60.2%、44.9%和19.9%,而镁离子浓度显著降低了17.4%。随后,测定了4种抗氧化酶系的活性变化,接种炭疽菌后,柱花草叶片的CuZn-SOD、T-SOD、POD和PAL活性分别显著提高了21.6%、35.0%、39.6%和274.4%(图2)。此外,总酚浓度、类黄酮浓度、原花青素浓度和总抗氧化能力在接种炭疽菌后分别显著提高了14.5%、21.4%、290.7%和33.1%(图2)。
-
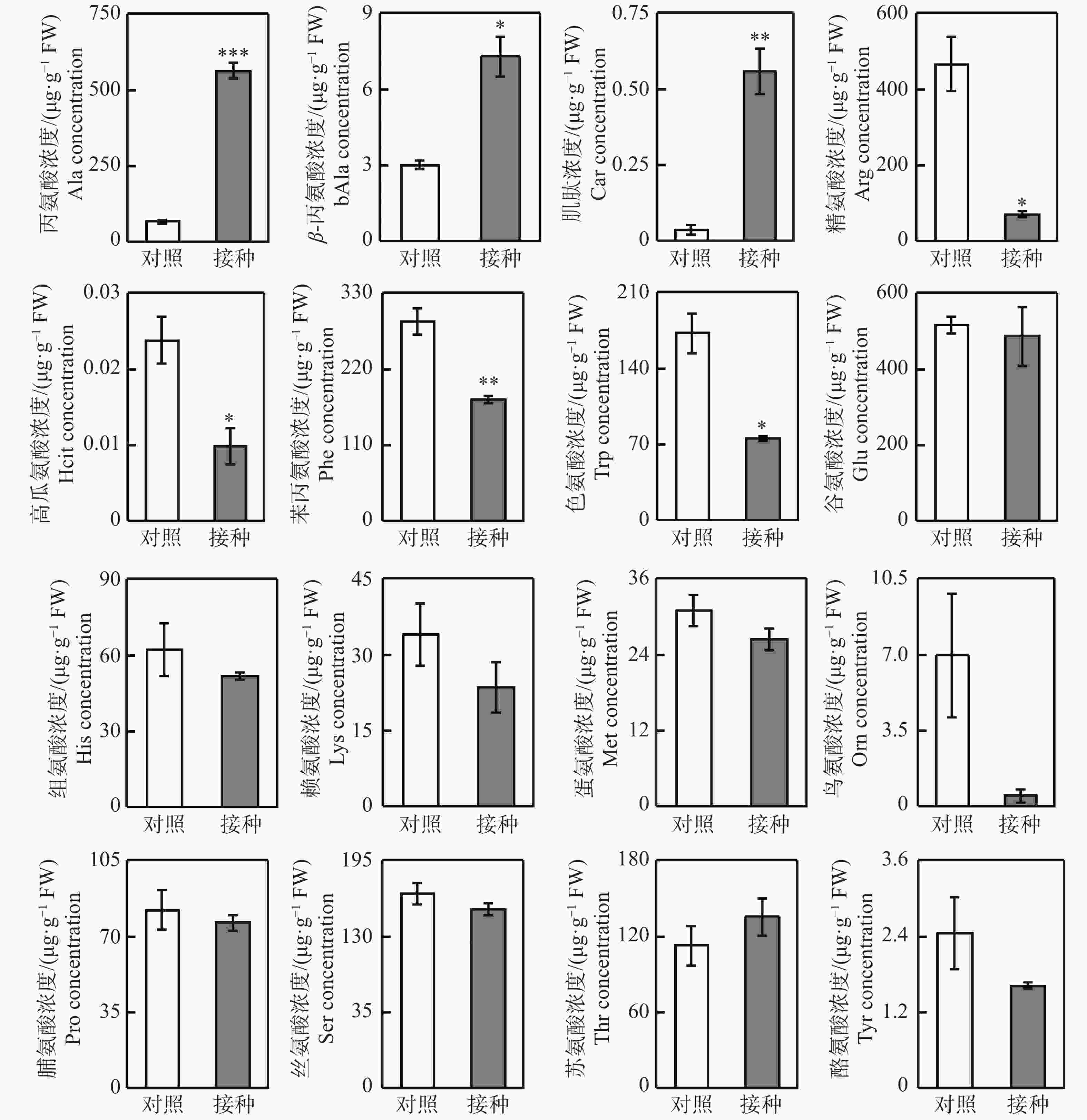
利用LC-MS/MS检测柱花草叶片16种氨基酸的浓度变化(图3),结果发现,与对照相比,接种炭疽菌使得柱花草叶片丙氨酸(Ala)、β−丙氨酸(bAla)和肌肽(Car)这3种氨基酸的浓度分别显著提高了7.61倍、1.43倍和15.09倍。与之相反,精氨酸(Arg)、高瓜氨酸(Hcit)、苯丙氨酸(Phe)和色氨酸(Trp)这4种氨基酸的浓度在炭疽菌侵染后分别显著降低了85.1%、58.7%、39.0%和56.5%。而其他9种氨基酸,即:谷氨酸(Glu)、组氨酸(His)、赖氨酸(Lys)、蛋氨酸(Met)、鸟氨酸(Orn)、脯氨酸(Pro)、丝氨酸(Ser)、苏氨酸(Thr)和酪氨酸(Tyr)浓度在对照与接种处理间无显著差异。
-
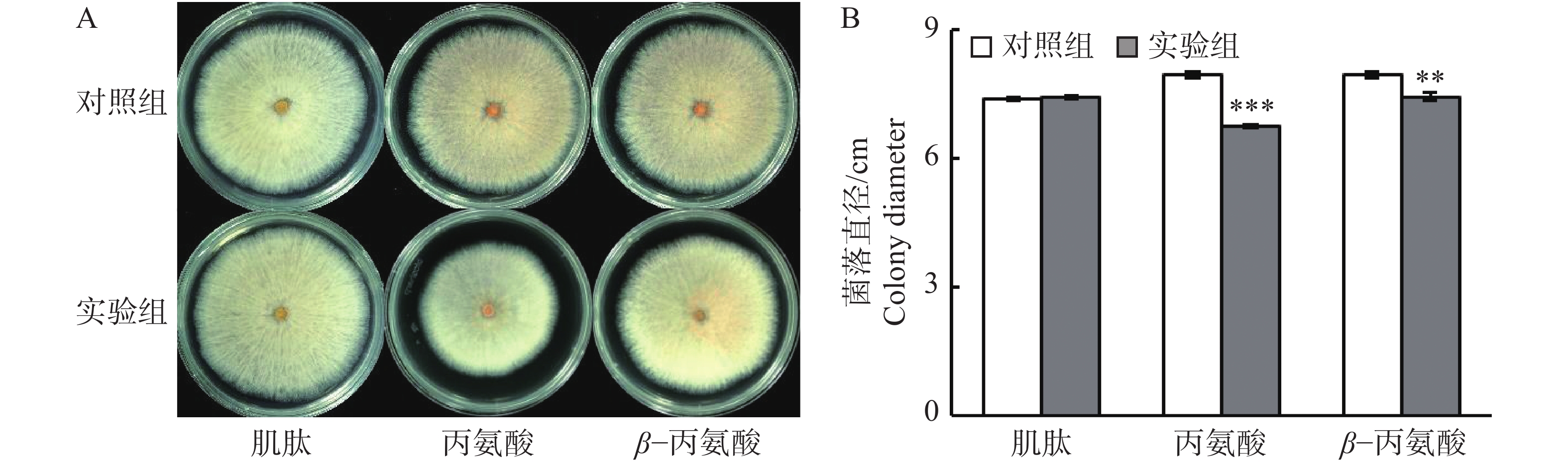
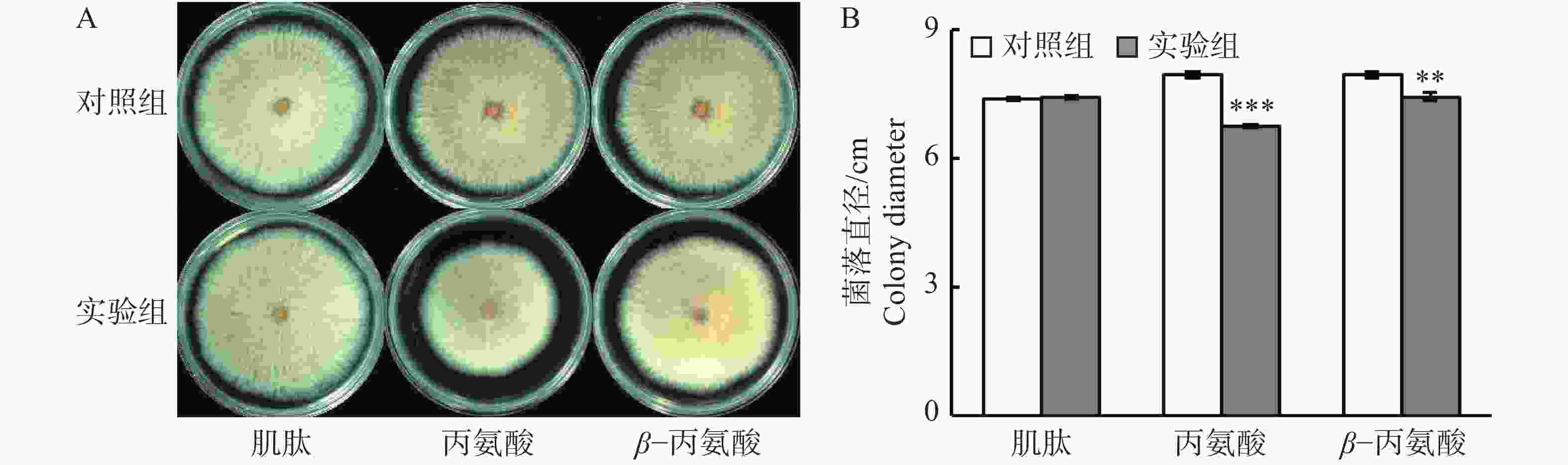
鉴于柱花草叶片在接种炭疽菌侵染后,肌肽、丙氨酸和β−丙氨酸的浓度显著提高,在PDA培养基中通过添加这3种游离氨基酸,分析它们对胶孢炭疽菌生长的体外抑制能力。结果发现(图4),添加肌肽对胶孢炭疽菌的生长无显著影响(图4-A)。但是,与对照相比,添加丙氨酸和β−丙氨酸分别使得菌落直径显著减小27.9%和12.4%(图4-B),表明丙氨酸和β−丙氨酸对胶孢炭疽菌具有抑制作用。
2.1. 炭疽菌侵染对柱花草叶片矿质元素浓度及抗氧化系统的影响
2.2. 炭疽菌侵染对柱花草叶片游离氨基酸浓度的影响
2.3. 3种游离氨基酸对胶孢炭疽菌的体外抑菌能力分析
-
柱花草炭疽病的病原菌为胶孢炭疽菌,危害我国柱花草生产的主要是B型胶孢炭疽菌[3-4]。当遭受炭疽菌侵染时,柱花草可能通过多种生理生化反应来提高抗病能力。本研究发现,接种炭疽菌后,柱花草叶片中5种矿质元素的浓度显著升高,其中钙浓度提高最多,达60.2%。钙对于生物膜的稳定性具有重要作用,研究发现,氯化钙、丙酸钙及硅酸钙对胶孢炭疽菌和尖孢炭疽菌的生长及侵染均有抑制效果[20]。柱花草可能通过提高钙离子浓度来增强对炭疽菌侵染的抵抗能力。
植物体中存在一套清除活性氧的抗氧化系统,分别是酶促抗氧化系统和非酶促抗氧化系统。酶促抗氧化系统主要包括SOD、POD等抗氧化酶;而非酶促抗氧化系统主要包括脯氨酸、多酚、类黄酮、生物碱等抗氧化代谢物产物[21]。抗氧化系统已被报道在植物抵抗生物与非生物胁迫中发挥着重要作用。本研究发现,炭疽菌侵染导致柱花草叶片中3种酶促抗氧化系统相关酶POD、CuZn-SOD、T-SOD的活性显著提高。此外,接种炭疽菌后,柱花草叶片中总酚、类黄酮和原花青素这3种非酶促抗氧化系统相关代谢物产物的浓度也显著提高。总酚、类黄酮和原花青素均属于苯丙烷代谢途径的下游代谢产物,而PAL是催化该途径第一步反应的关键酶和限速酶[22]。柱花草叶片的PAL活性在接种炭疽菌后显著提高2.7倍。以上结果说明,柱花草叶片通过提高PAL酶活性促进下游抗氧化代谢物合成与积累,协同抗氧化酶系统的增强共同提高了叶片的总抗氧化能力,以减轻炭疽菌侵染对其造成的氧化损伤。当遭受病原菌侵染后,植物细胞结构受到破坏,同时植物体内的新陈代谢活动及游离氨基酸等主要营养物质也会随之改变。游离氨基酸具有生物活性,已被报道在植物抵抗病原菌侵染中发挥着重要作用[23]。如:茶饼病菌(Exobasidium vexans)侵染茶树(Camellia sinensis)后,导致其叶片的γ−氨基丁酸和茶氨酸浓度显著增加[24];尖孢镰孢菌(Fusarium oxysporum)侵染黄瓜(Cucumis sativus)后,使其叶片的丙氨酸、瓜氨酸浓度显著提高[25];兰花胶孢炭疽菌(Colletotrichum gloeosporioides)侵染墨兰(Cymbidium sinense)后,致使其叶片的天门氨酸、胱氨酸和谷氨酸浓度显著提高[26]。笔者发现,柱花草叶片中丙氨酸、β−丙氨酸和肌肽这3种游离氨基酸的浓度在接种炭疽菌后显著提高,而随后的体外抑菌实验表明,其中只有丙氨酸和β−丙氨酸对胶孢炭疽菌生长具有显著抑制作用。游离氨基酸可作为碳源和氮源被病原菌直接吸收,而有致害作用的游离氨基酸被吸收后可对病原菌生长产生抑制作用[25,27]。生物防治是指利用有益生物或者生物代谢产物来防治植物病害的各种措施[28]。但目前尚未有柱花草炭疽病生物防治药剂研究的公开报道。本研究结果表明,丙氨酸和β−丙氨酸具有用于开发生物防治药剂的潜力,但仍需进一步研究验证。
综上所述,当遭受炭疽菌侵染时,柱花草通过抗氧化系统来抵抗病原菌侵染引起的氧化损伤,同时通过丙氨酸和β−丙氨酸的积累来抑制炭疽菌的生长。本研究结果将为解析柱花草抗炭疽病机理及绿色防治药物的开发提供参考依据。






 DownLoad:
DownLoad: