-
香蕉(Musa spp.)是我国南亚热带地区重要的经济作物。香蕉灰纹病(又称香蕉暗双孢霉叶斑病)已成为我国香蕉产区常见且危害严重的病害之一,严重威胁着香蕉产业健康持续发展[1-6]。该病主要危害叶片,且常和Sigatoka叶斑病混合发生,造成多种病原的复合侵染[7-9]。目前国外已报道的引起香蕉灰纹病的病原真菌有Neocordana musae(Cordana musae)、N. johnstonii(C. johnstonii)、N. musicola、N. musarum、N. musigena及N. malayensis等[10-15],国内已报道的有C. musae[4, 16-18],但国内相关报道只是进行形态与培养性状观察,未进行分子生物学的进一步验证。为了进一步明确海南香蕉灰纹病病原菌的种类及其生物学特性,本研究从海南香蕉主产区采集多份病叶进行组织分离纯化和致病性测定,采用传统形态学特征观察、rDNA-ITS测序及其系统发育树分析等方法对该病害病原进行鉴定,并在此基础上测定了该菌在不同培养基、温度、pH、碳源、氮源、光照、通气等条件下的生物学特性,以期为深入研究海南香蕉灰纹病的发生流行及防控提供理论依据。
HTML
-
从海南香蕉产区采集典型灰纹病症状的香蕉叶片,用无菌水冲洗干净并晾干,采用组织分离法[19]进行病原菌分离。在病健交界处取3~5 mm的组织块,先用0.1 %升汞消毒1 min,再用75 %乙醇漂洗30 s后,用无菌水漂洗3次,再用滤纸吸干水分,移至PDA培养基上培养。待菌落长出后,挑取菌落边缘菌丝块,置于PDA平板上进一步纯化培养。分离纯化后的菌种转移到PDA斜面培养基上保存备用。
-
选用PDA平板上培养7 d的菌丝块对健康香蕉植株叶片进行刺伤接种。挑选生长良好、生育期相近、植株大小比较一致的巴西蕉植株6株,先用清水冲洗巴西蕉叶片,再用无菌水清洗巴西蕉叶片,后用75%酒精冲洗灭菌进行表面消毒,无菌水冲洗3次,晾干后备用。每株选取1片叶片,用灭菌针在叶脉左右相对位置进行刺伤,取直径6 mm菌丝块接种于经灭菌针刺伤的叶片(菌丝面紧贴叶片伤口处),每片叶接种2块菌丝块,以未接菌PDA琼脂块为对照,每个处理3次重复。用脱脂棉覆盖菌丝块,期间喷无菌水保湿48 h,3 d后移去菌丝块,观察叶片发病情况。待接种叶片发病后,根据柯赫氏法则,从病斑处分离病原菌,并观察与原接种病原菌形态特征是否一致。
-
将分离纯化得到的病原菌转接至PDA平板上,于28 ℃培养箱中黑暗条件下培养7 d,期间观察并记录菌落颜色、形态、气生菌丝疏密程度等形态特征。在显微镜下观察病原菌的分生孢子和分生孢子梗形态。
-
将供试菌株接种到PDA培养基上,28 ℃培养7 d,用载玻片刮取培养基表面的菌丝备用。采用CTAB法[20]提取菌株基因组DNA,用真菌核糖体基因内转录间隔区通用引物ITS1与ITS4[21]对其rDNA-ITS区进行PCR扩增,PCR产物送英潍捷基(上海)贸易有限公司进行测序。利用EMBL数据库中Clustal Omega程序、GenBank数据库中的Blastn程序和MEGA 6软件进行各序列间的同源性和进化距离的分析与比较,并构建NJ系统进化树。
-
制备马铃薯葡萄糖琼脂培养基(PDA)、马铃薯蔗糖琼脂培养基(PSA)、燕麦片琼脂培养基(OA)、胡萝卜琼脂培养基(CA)、玉米粉琼脂培养基(CMA)、查氏培养基(CDA)及香蕉叶片煎汁琼脂培养基(LEDA)。将病原菌接种于PDA培养基上,28 ℃培养7 d,用灭菌的6 mm打孔器在菌落边缘上打取菌丝块,接种至各种培养基平板上。各处理重复5皿,在28 ℃下培养,并采用十字交叉法每隔2 d测量菌落直径,按菌丝生长速率=菌落平均直径(cm)/培养时间(d),计算菌丝在各供试培养基上3、5、7、9 d的生长速率(cm·d−1)和满皿时间(d)。
-
将接有6 mm病原菌菌丝块的PDA平板分别置于5、10、15、20、25、30、35、40 ℃恒温培养,各处理重复5皿,7 d后观察菌落生长情况,十字交叉法测量菌落直径。
-
用磷酸缓冲液(0.2 mol·L−1磷酸二氢钠、0.2 mol·L−1磷酸氢二钠)配制PDA培养基,再用1 mol·L−1磷酸、1 mol·L−1 NaOH调节pH值至4、5、6、7、8、9、10(7个梯度)。将6 mm病原菌菌丝块分别转接不同pH的PDA平板上,28 ℃恒温培养,各处理重复5皿,7 d后观察菌落生长情况,十字交叉法测量菌落直径。
-
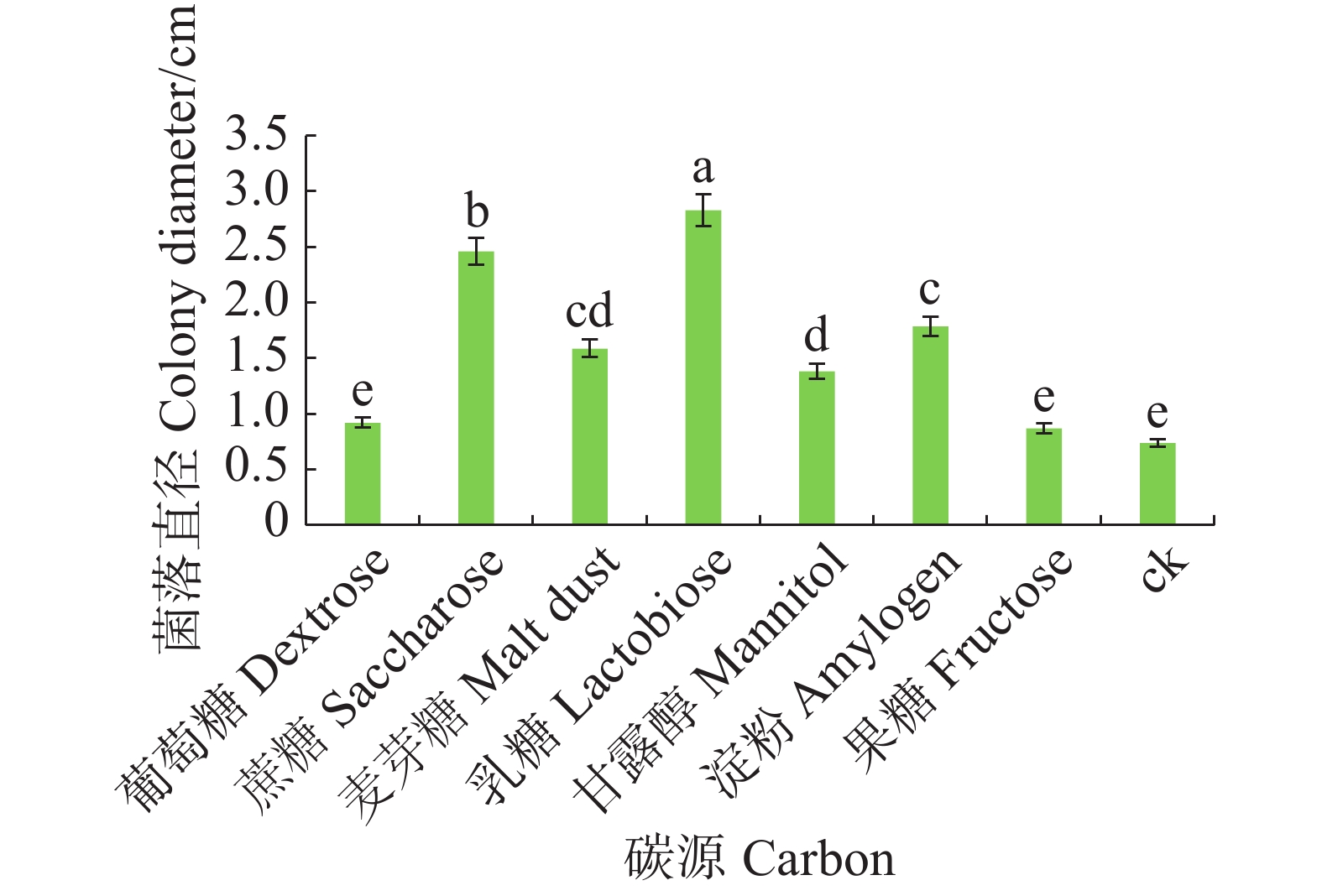
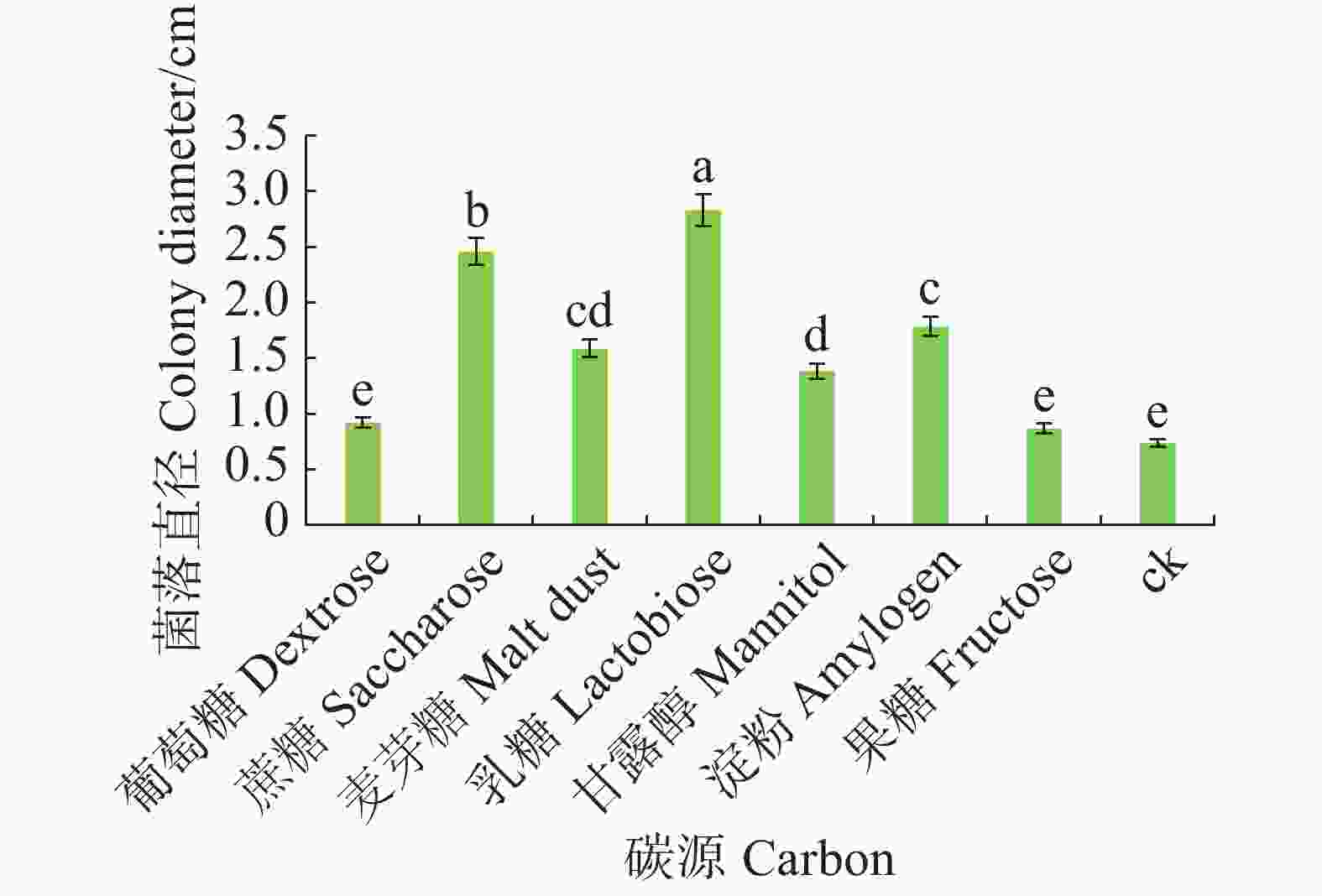
将6 mm病原菌菌丝块转接至含有不同碳源的查氏培养基上。碳源分别是葡萄糖、蔗糖、麦芽糖、乳糖、甘露醇、淀粉和果糖,以不加碳源为对照,各处理重复5皿,28 ℃恒温培养,7 d后观察菌落生长情况,十字交叉法测量菌落直径。
-
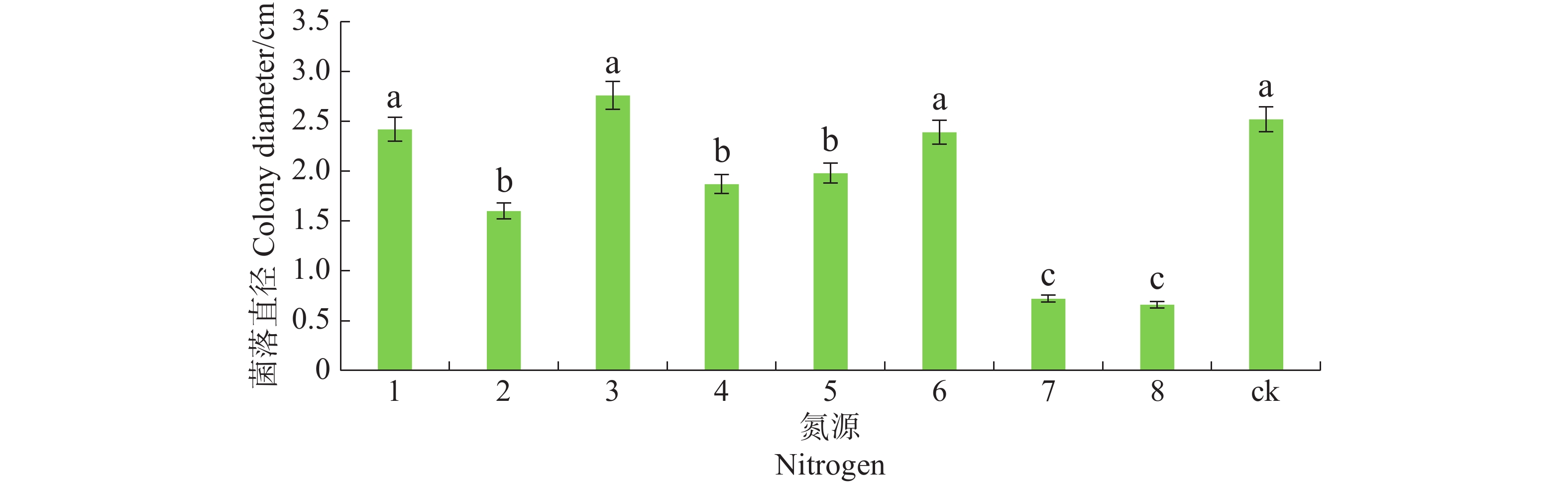
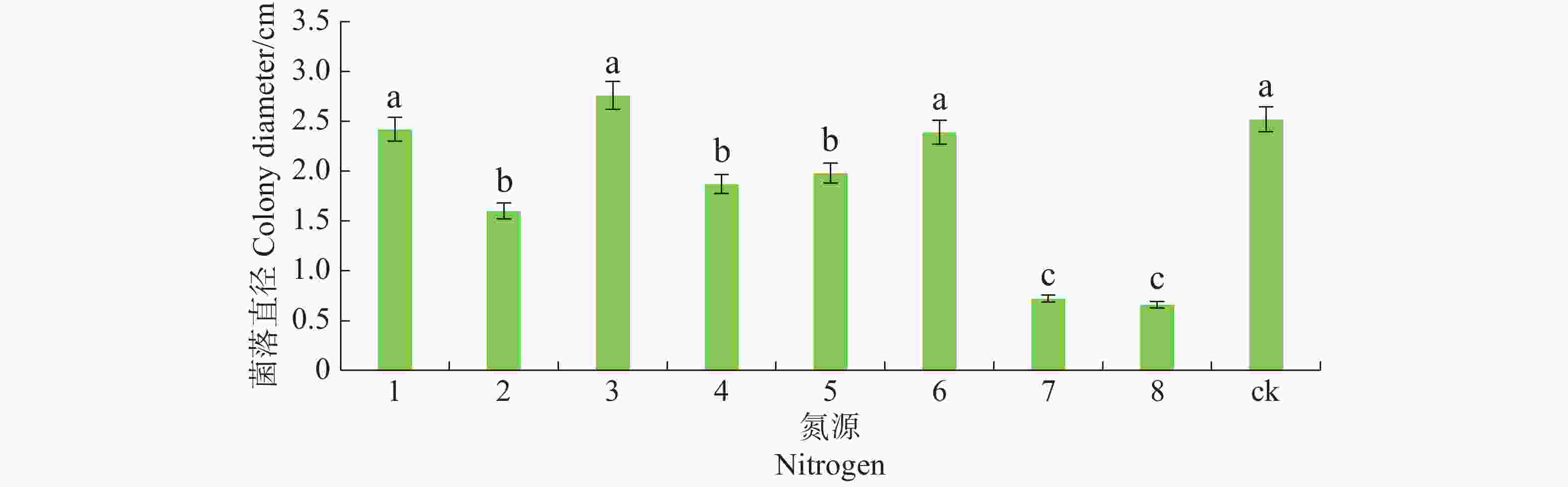
将6 mm病原菌菌丝块转接至含有不同氮源的查氏培养基上。氮源分别为蛋白胨、牛肉膏、酵母膏、脲、磷酸氢二铵、硝酸钠、氯化铵和硫酸铵,以不加氮源为对照,各处理重复5皿,28 ℃恒温培养,7 d后观察菌落生长情况,十字交叉法测量菌落直径。
-
将接有6 mm病原菌菌丝块的PDA平板分别置于24 h全光照、24 h全黑暗、12 h光照12 h黑暗、16 h光照8 h黑暗(长日照)、16 h黑暗8 h光照(短日照)5种光照处理下,各处理重复5皿,28 ℃恒温培养,7 d后观察菌落生长情况,十字交叉法测量菌落直径。
-
将6 mm病原菌菌丝块置于盛有100 mL PD液体培养基的250 mL三角瓶中,每瓶装3个菌丝块,设3种培养方式:28 ℃恒温静置、28 ℃恒温摇床振荡以及28 ℃恒温振荡12 h静置12 h,其中摇床振荡转速梯度分别设置为50、100、150、200 r·min−1,各处理重复3瓶,7 d后称取菌丝的干、湿质量。
-
病原菌在PDA培养基上培养7 d后,加入40 mL无菌水,用灭菌三角棒刮取病原菌菌丝,制成菌悬液;分别取5 mL至各灭菌试管中,分别置于室温(27.2 ℃),40,45,50,55,60,65,70 ℃的水浴中,分别水浴5,10,15,20 min,共32个处理,期间缓缓摇动试管,以保证温度均匀。水浴后立即取出用冷水降温,取0.5 mL菌悬液涂于PDA培养基上,各处理重复5皿,28 ℃恒温培养,7 d后观察生长情况。
-
对数据进行单因素方差分析,并用Duncan法比较均值间的差异显著性,以上所有统计过程均用Excel 2019和Spss软件26.0.0.0完成。
1.1. 病原菌的分离与培养
1.2. 致病性测定
1.3. 病原菌鉴定
1.3.1. 形态学鉴定
1.3.2. 分子生物学鉴定
1.4. 病原菌生物学特性测定
1.4.1. 培养基对菌丝生长的影响
1.4.2. 温度对菌丝生长的影响
1.4.3. pH对菌丝生长的影响
1.4.4. 碳源对菌丝生长的影响
1.4.5. 氮源对菌丝生长的影响
1.4.6. 光照对菌丝生长的影响
1.4.7. 通气条件对菌丝生长的影响
1.4.8. 致死温度
1.5. 数据分析与处理
-
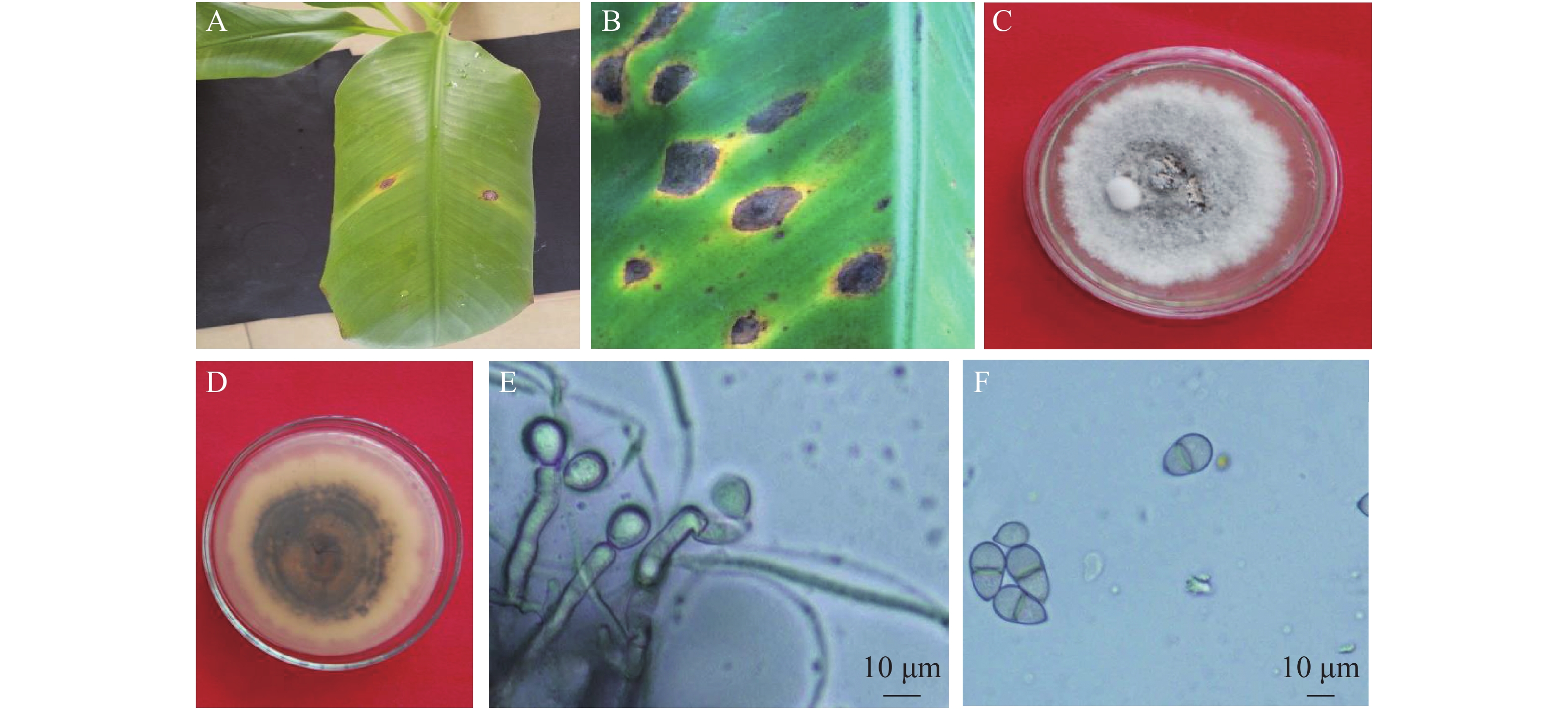
采用组织分离法从发病香蕉叶片分离获得6株纯化的真菌菌株,菌落形态观察及镜检表明,6株分离菌形态特征一致,为同一种真菌。接种后第3 d,叶片刺伤处(6个菌丝块接种点)均开始发病,发病率为100%,而PDA琼脂块接种的对照叶片均未发病。菌丝块接种点病斑为短椭圆形,随叶脉方向扩展,外围具黄色晕圈,中间灰褐色,有轮纹,其发病症状(图1A)与田间发病症状相似(图1B)。根据柯赫氏法则,从接种发病的香蕉叶片上重新分离到与原接种菌株形态特征一致的真菌,确认6株分离菌株为香蕉灰纹病的致病菌。
-
病原菌在PDA培养基上的菌落正面呈圆形,灰白色,绒毛状,边缘不整齐,有轮纹;基底中菌丝灰褐色至灰黑色,质地紧密,生长旺盛(图1C);菌落背面呈黑褐色,同心轮纹状,边缘明显,有淡黄色晕圈(图1D)。经显微镜观察发现,分生孢子梗褐色、直立、无分枝;分生孢子梗顶端突出膨大,产孢(图1E);分生孢子双胞、倒卵形,隔膜处有缢缩,透明或微褐色,孢子顶端具脐点(图1F)。根据病原菌的培养性状与形态学特征,参考HERNÁNDEZ-RESTREPO等[12]、戚佩坤[16]及PLOETZ等[22]的描述,将海南的香蕉灰纹病病原初步鉴定为暗双孢属真菌(Neocordana sp.)。
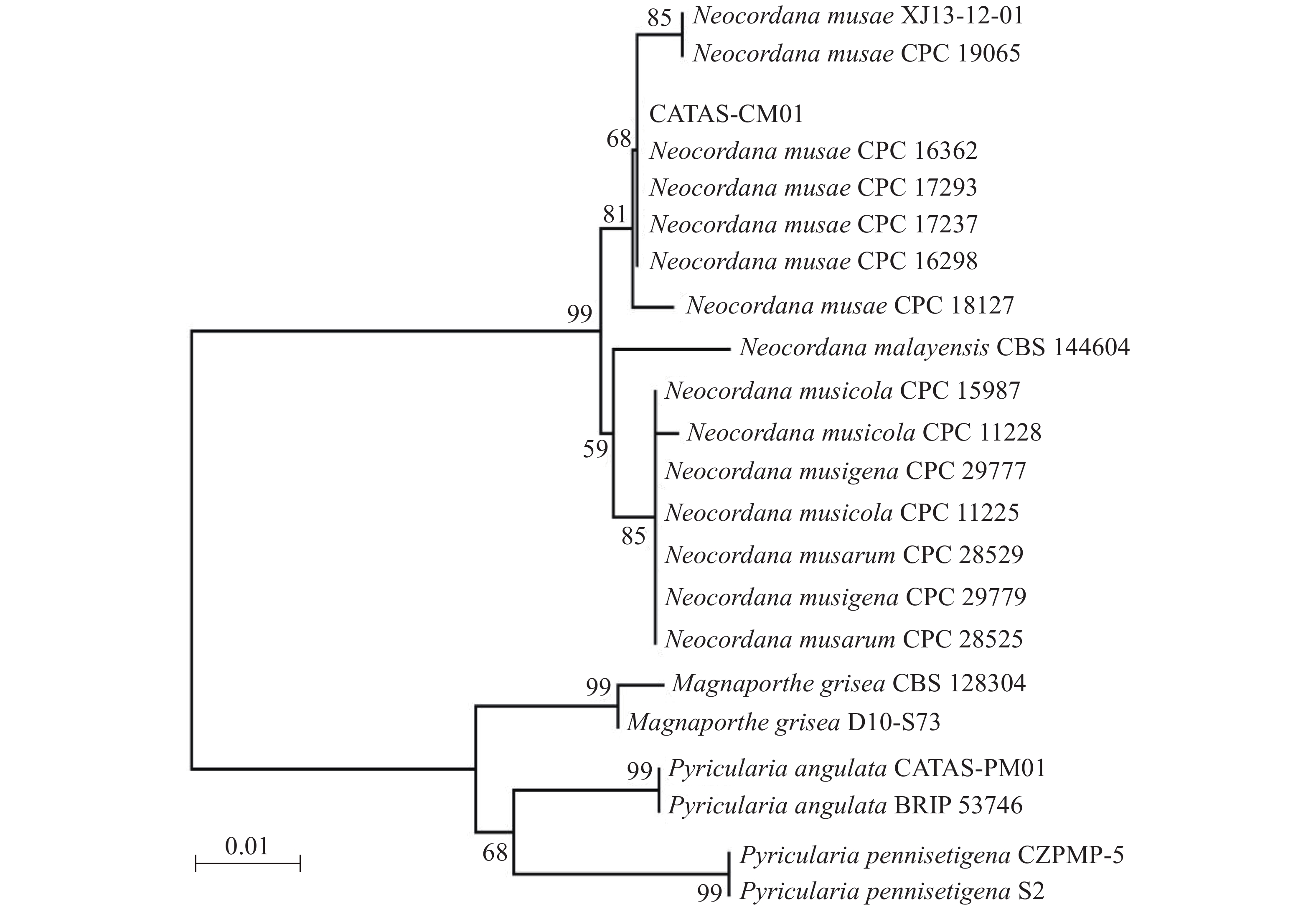
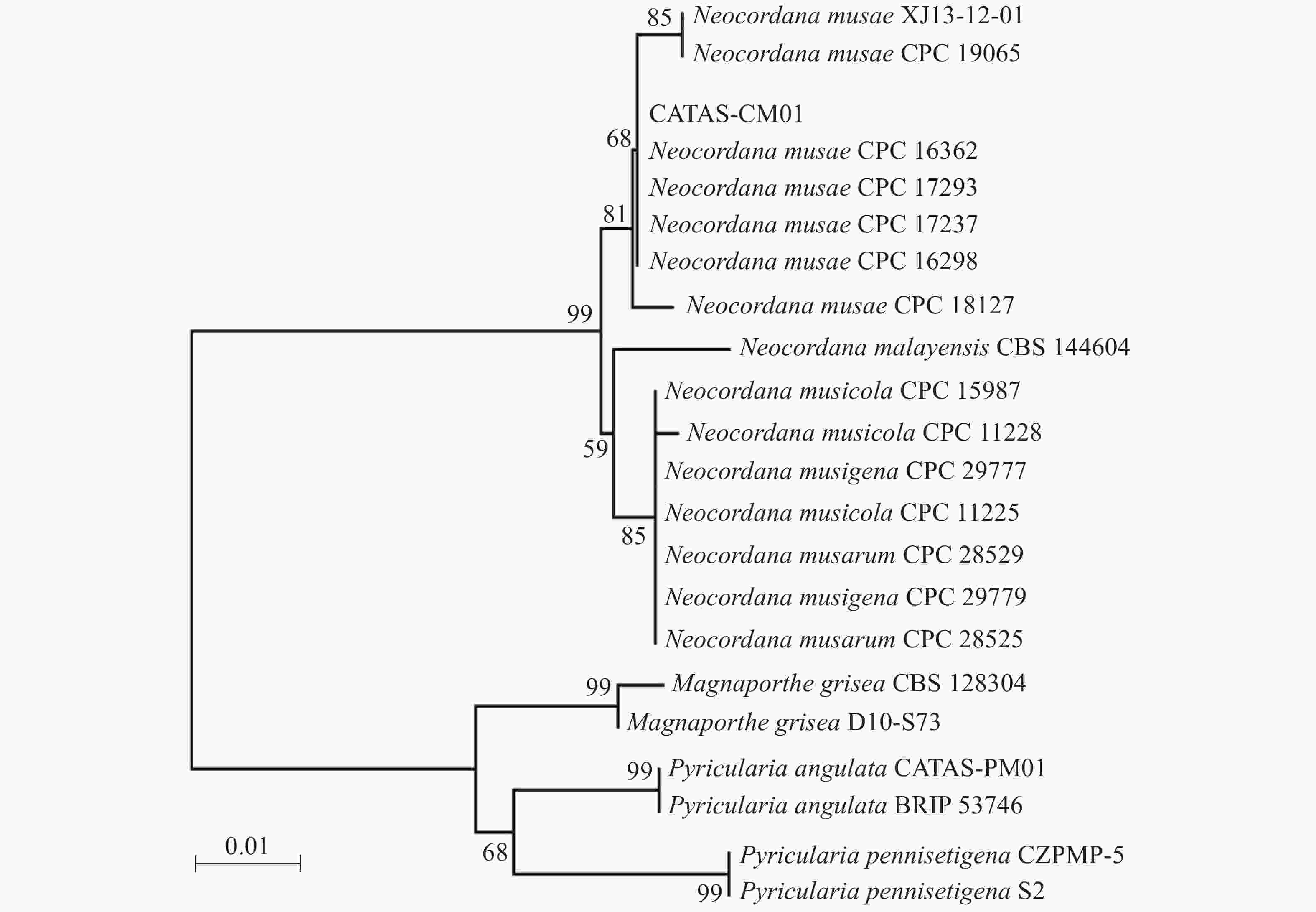
提取6株病原菌的基因组DNA,利用rDNA-ITS通用引物对ITS1/ITS4进行PCR扩增,PCR产物经测序及序列多重比对,发现6株病原菌的rDNA-ITS区扩增片段长度均为575 bp,且相似性为100%。选择1号菌株的ITS序列提交到GenBank(登录号:MN960387),并命名为CATAS-CM01。经GenBank数据库Blantn比对分析,CATAS-CM01与GenBank中公布的Neocordana musae(LN713276-LN713278,LN713280-LN713282,KP770138)相应片段最相近,相似性达99.28%~99.65%,与N. malayensis(MK442593)、N. musarum(KY173424-KY173425)、N. musigena(KY979749-KY979750)及N. musicola(LN713283-LN713285)相应片段序列相似性为95.6%~98.58%,而与其他属菌株同源性较低,约87%的相似性。以Magnaporthe grisea(登录号:MH864859、FN555111)、Pyricularia pennisetigena(登录号:MN889450、MH412638)、Pyricularia angulata(登录号:MT071757、JF719830)作为外群构建菌株CATAS-CM01的rDNA-ITS基因系统发育树,结果显示CATAS-CM01与香蕉暗双孢菌云南分离株XJ13-12-01(登录号:KP770138)及6株N. musae模式菌株CPC 19605、CPC 16362、CPC 17293、CPC 17237、CPC 16298和CPC 18127(登录号:N713276-LN713278,LN713280-LN713282)以81%的置信度聚在同一分支,遗传距离最近(图2)。结合6株病原菌的形态学特征,最终将病原菌鉴定为香蕉暗双孢菌Neocordana musae( Cordana musae)[12]。
-
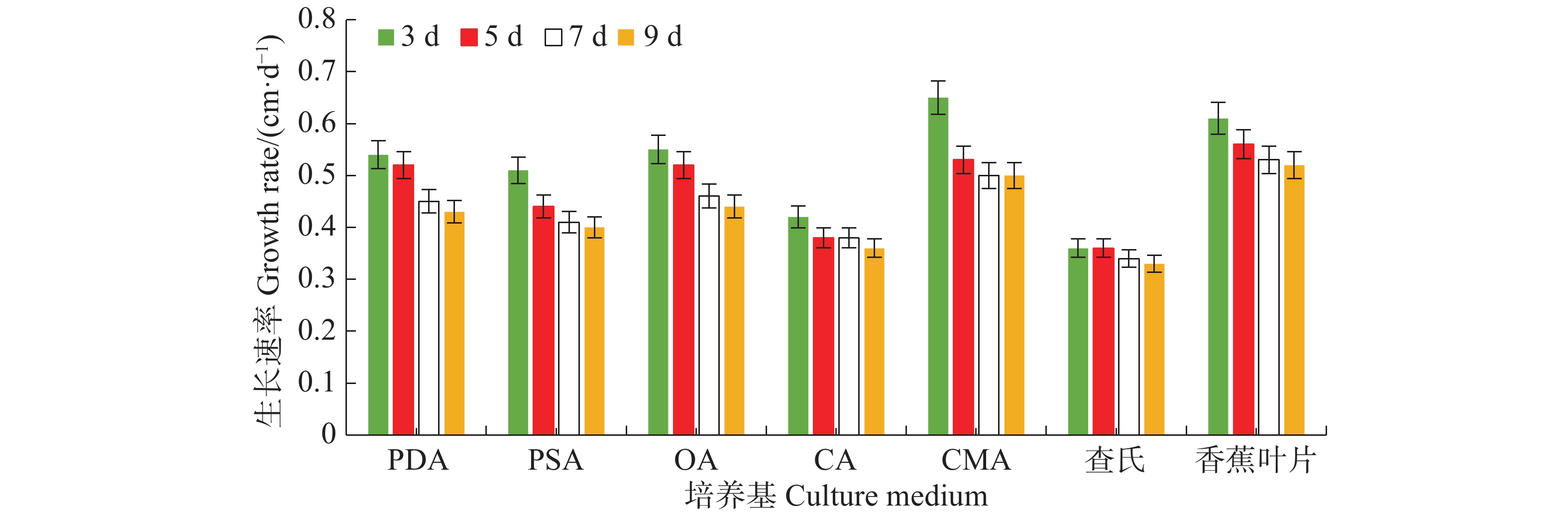
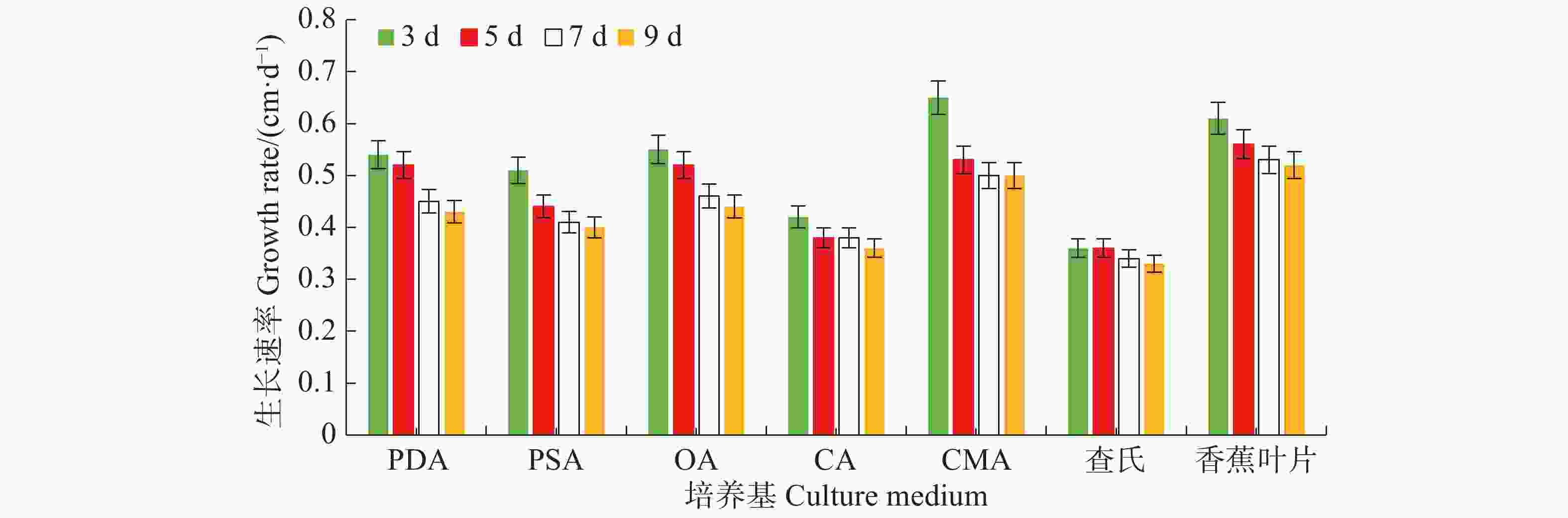
病原菌在7种培养基上均能生长,其中生长最快的是LEDA和CMA培养基,平均生长速率分别为0.56 cm·d−1和0.55 cm·d−1,满皿时间分别为18 d和19 d,菌落边缘规则,但气生菌丝轻薄;其次是OA培养基和PDA培养基,平均生长速率均为0.49 cm·d−1,满皿时间为21 d,菌落边缘虽不规则,但气生菌丝较紧密,PDA培养基上的菌落有轮纹;生长较慢的培养基依次为PSA、CA和CDA,平均生长速率分别为0.44、0.39、0.35 cm·d−1,满皿时间分别为23、26、29 d(表1)。OA、PDA、LEDA及CMA培养基上的菌落平均生长速率无显著差异(P<0.05),结合菌落形态及满皿时间,最适合菌落生长的培养基为OA和PDA。病原菌菌丝的生长速率随天数的增加而逐渐减慢,在7 d时各培养基上菌丝的生长速率逐渐趋于稳定(图3)。
培养基
Medium菌落直径/cm Colony diameter 平均生长速率/ (cm·d−1)
Average growth rate满皿时间/d
Day of full dish3 d 5 d 7 d 9 d PDA 1.63± 0.05 bc 2.58±0.20 bc 3.14±0.11 bc 3.89±0.13 bc 0.49±0.03ab 21 PSA 1.54±0.10 c 2.18±0.14 cd 2.90±0.11 cd 3.56±0.14 cd 0.44±0.02 bc 23 OA 1.65±0.11 bc 2.58±0.20 ab 3.20±0.26 bc 4.00±0.27 bc 0.49±0.03 ab 21 CA 1.27±0.05 d 1.91±0.07 d 2.65±0.10 de 3.25±0.13 de 0.39±0.01 cd 26 CMA 1.95±0.05 a 2.67±0.09 ab 3.53±0.08 ab 4.47±0.08 ab 0.55±0.07 a 19 CDA 1.09±0.09 d 1.82±0.12 d 2.37±0.16 e 2.93±0.25 e 0.35±0.01 d 29 LEDA 1.84±0.08 ab 2.80±0.10 a 3.69±0.14 a 4.66±0.15 a 0.56±0.04 a 18 注:表中数据均为3次重复的平均值。同列数据后小写字母不同者表示在5%水平时差异显著(Duncan氏新复极差检验),下同。
Note: The data are the average of the 3 replicates. Different lowercase letters in the same column show significant difference at a 5% level (Duncan’s multiple-range test), similarly hereinafter.Table 1. Effect of different culture mediums on mycelial growth of N. musae
-
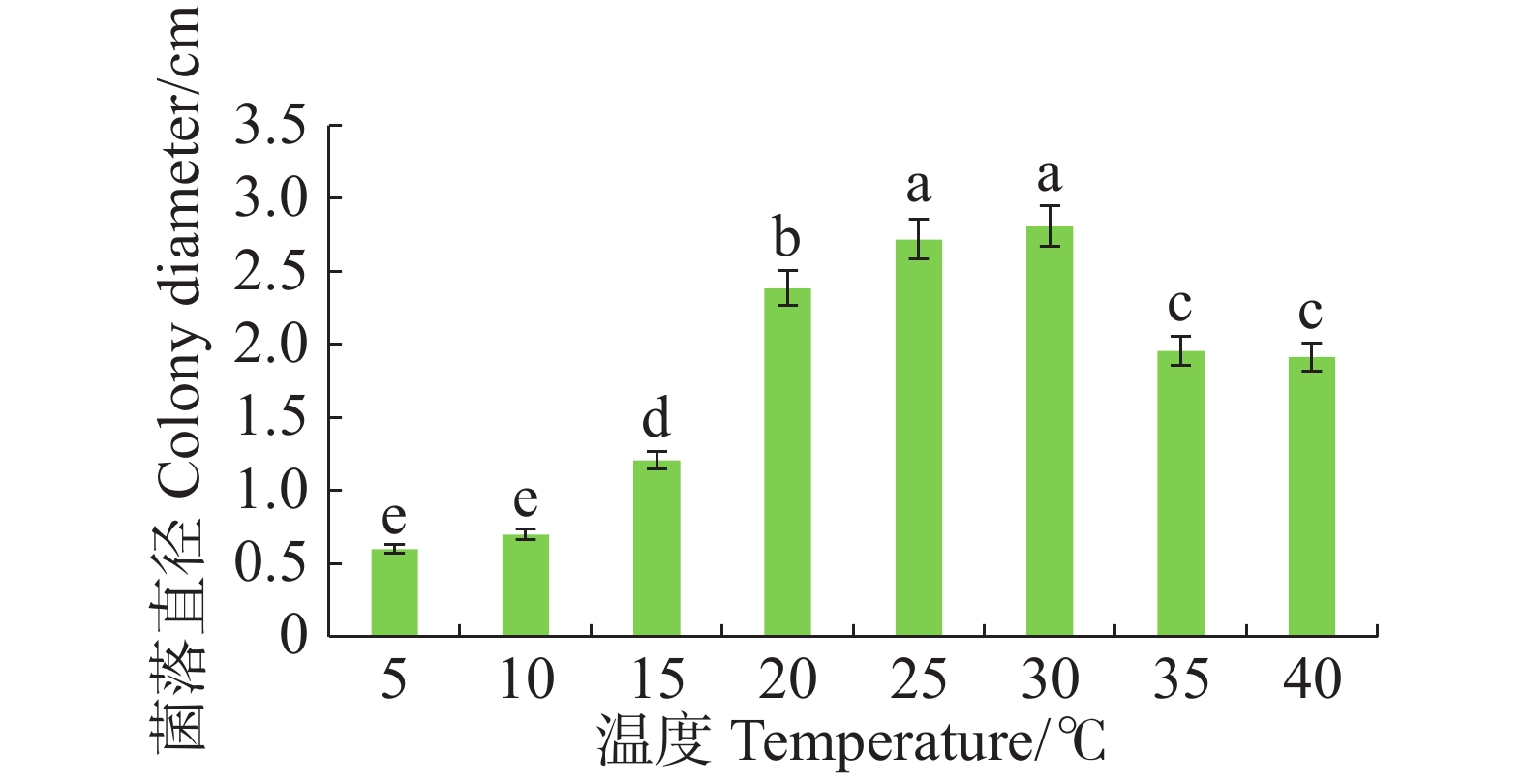
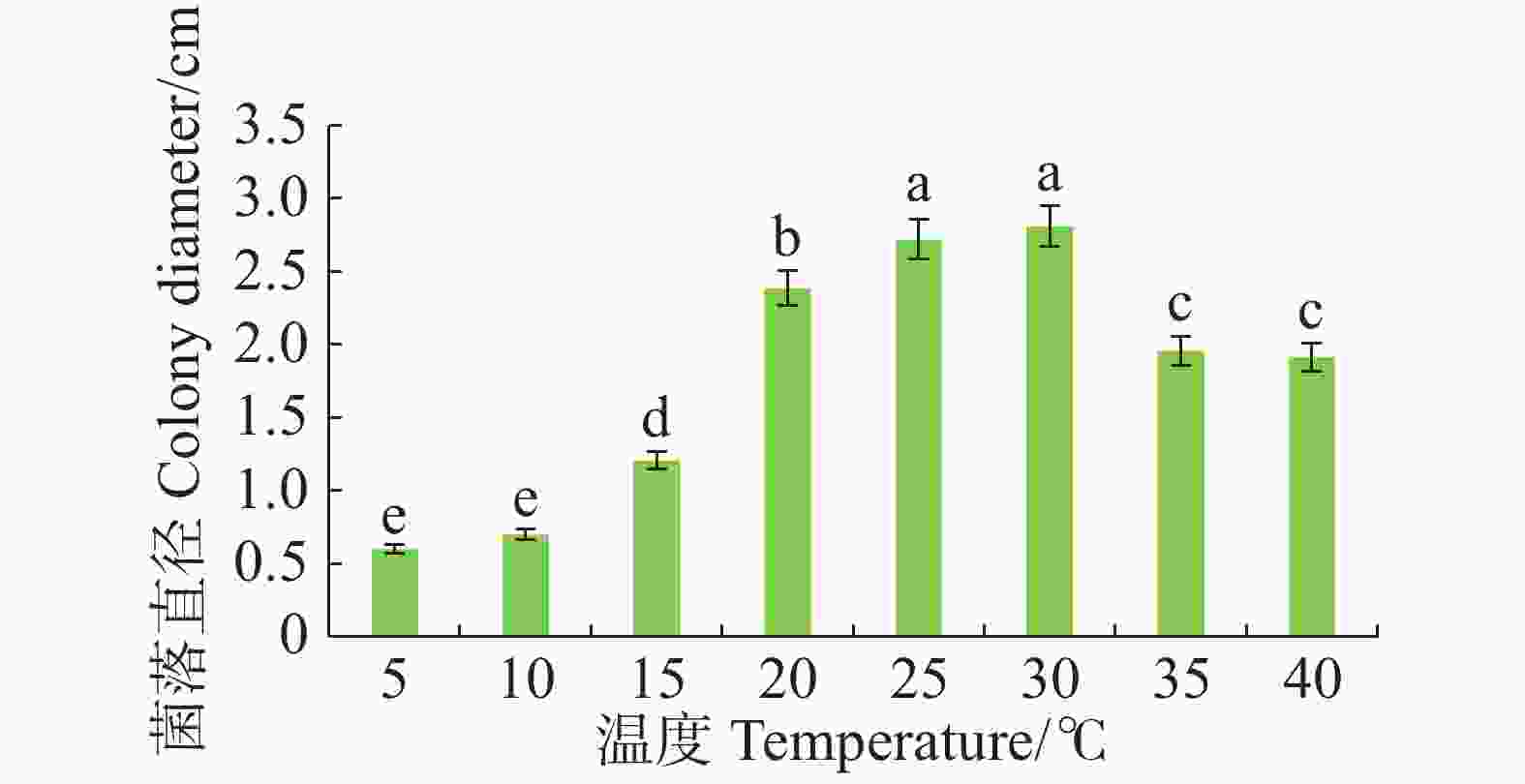
病原菌在10~40 ℃范围内均能生长,适宜生长温度为25~30 ℃,与其他处理温度有显著差异(P<0.05),且最适生长温度为30 ℃。温度≤15 ℃或者≥35 ℃时,病原菌生长受抑制(图4)。
-
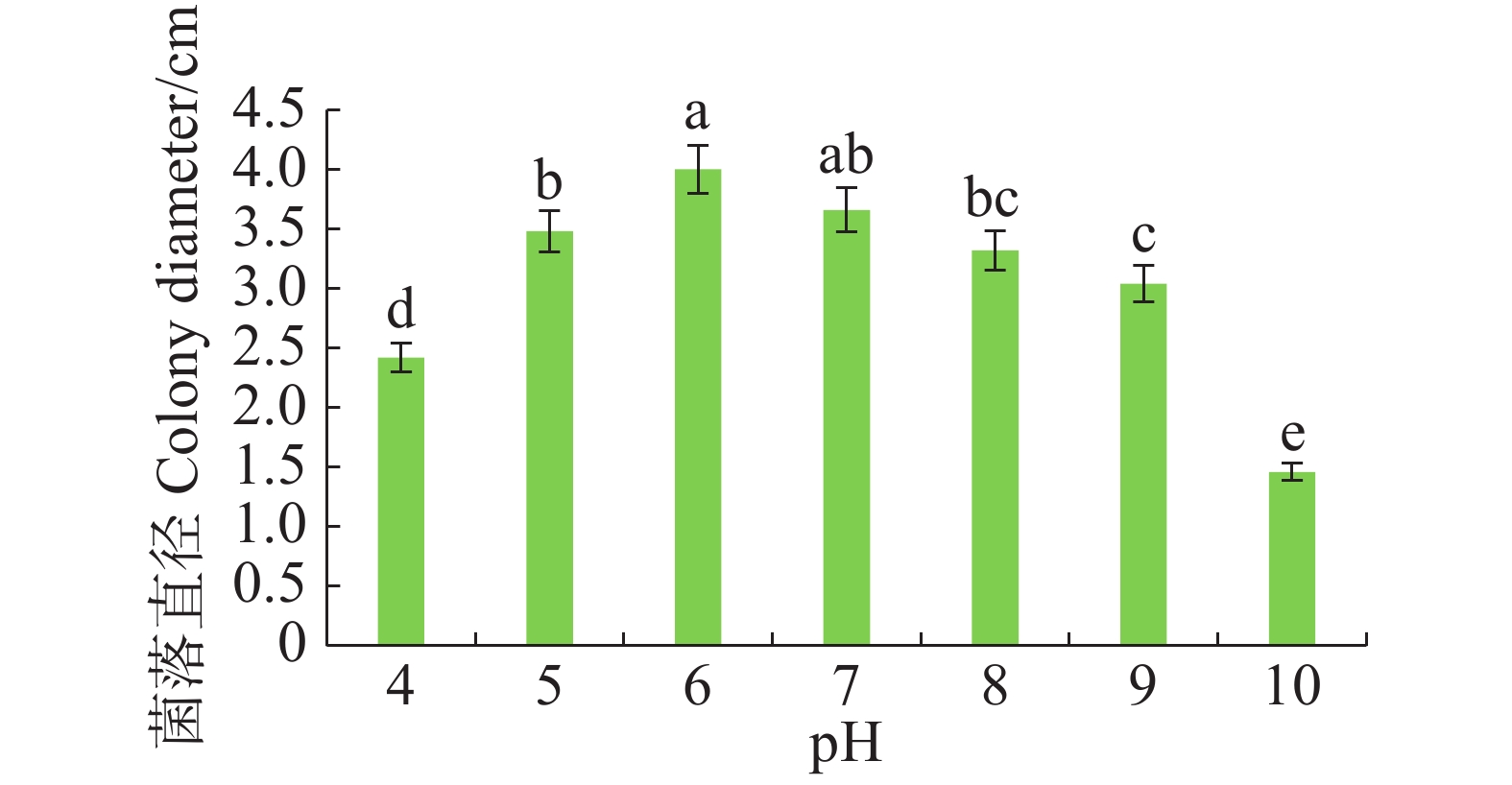
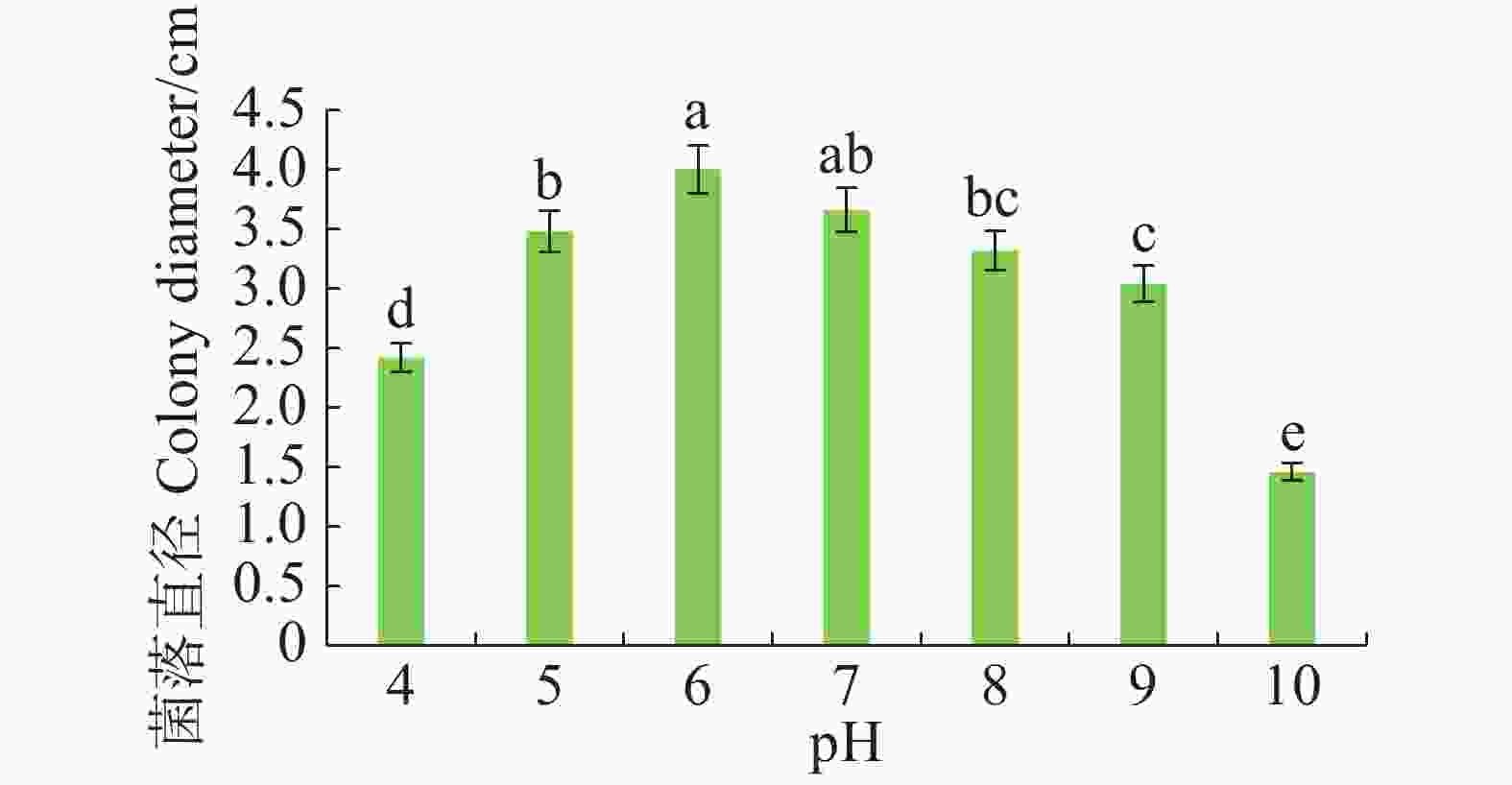
病原菌在pH 4~10范围内均可生长,pH 5~7时生长较好,pH 为6时生长最佳,与pH 7差异不显著,但与其他pH有显著差异(P<0.05)。pH大于8时,菌丝扩展受抑制(图5)。
-
病原菌在不同碳源的培养基上均可生长,其中对乳糖利用效果最好,与其他碳源有显著差异(P<0.05),其次是蔗糖和淀粉,果糖利用效果最差(图6)。
-
病原菌在不同氮源的培养基上均能生长,其中对蛋白胨、酵母膏及硝酸钠利用较好,与其他氮源有显著差异(P<0.05),且以酵母膏最佳,其次是脲、磷酸氢二铵和牛肉膏,氯化钠和硫酸铵的利用最差(图7)。
-
病原菌在5种光照条件下均能生长,其中24 h全光照条件下菌落直径最大,为4.06±0.14 a(a、b、c、d表示差异显著性,下同);其次是16 h光照8 h黑暗(长日照)和12 h光照12 h黑暗,菌落直径分别为3.80±0.05 ab,3.39±0.03 c;在24 h全黑暗条件下菌落直径最小,为2.96±0.09 d;8 h光照16 h黑暗(短日照)条件下菌落直径为3.53±0.08 bc。全黑暗与全光照及光暗交替培养有显著差异,说明光照利于菌丝生长。
-
通气与不通气对菌丝生长均有一定影响,在4种转速梯度中,均以24 h振荡培养的菌丝湿质量、干质量最大,与其他处理差异最显著(P<0.05),其中以转速200 r·min−1时,菌丝湿质量、干质量分别达4.50 g和2.91 g;其次是12 h振荡12 h静置;24 h静置处理条件下,菌丝湿质量、干质量最低(表2)。
转速/(r·min−1) 处理 Treatment 菌丝平均质量 Average mycelia mass/g 湿质量 Wet mass 干质量 Dry mass 50 24 h 静置 24 h quiet 0.85±0.05 c 0.25±0.08 b 24 h 振荡 24 h shaking 2.14±0.04 a 0.55±0.04 a 12 h 振荡12 h 静置 12 h shaking and 12 h quiet 1.49±0.03 b 0.36±0.03 b 100 24 h 静置 24 h quiet 0.77±0.07 c 0.18±0.04 b 24 h 振荡 24 h shaking 2.53±0.37 a 0.86±0.05 a 12 h 振荡12 h 静置 12 h shaking and 12 h quiet 1.76±0.20 b 0.68±0.02 b 150 24 h 静置 24 h quiet 0.86±0.05 b 0.22±0.02 c 24 h 振荡 24 h shaking 3.29±0.18 a 2.15±0.17 a 12 h 振荡12 h 静置 12 h shaking and 12 h quiet 3.24±0.07 a 1.68±0.06 b 200 24 h 静置 24 h quiet 0.93±0.08 c 0.33±0.09 c 24 h 振荡 24 h shaking 4.50±0.41 a 2.91±0.41 a 12 h 振荡12 h 静置 12 h shaking and 12 h quiet 2.87±0.20 b 1.73±0.21 b Table 2. Effect of ventilation condition on mycelial growth of N. musae
-
病原菌菌丝在60 ℃下水浴10 min后仍能在PDA平板上正常生长,但在60 ℃及以上温度处理15 min后不能生长,说明病原菌菌丝的致死温度为60 ℃ 10 min(表3)。
处理 Treatment/min 温度 Temperature/℃ 室温 (Room temperature) 40 45 50 55 60 65 70 5 + + + + + + + − 10 + + + + + + − − 15 + + + + + − − − 20 + + + + + − − − Table 3. The lethal temperature on mycelial growth of N. musae
2.1. 病原菌分离与致病性测定
2.2. 病原菌鉴定
2.3. 病原菌生物学特性测定
2.3.1. 培养基对菌丝生长的影响
2.3.2. 温度对菌丝生长的影响
2.3.3. pH对菌丝生长的影响
2.3.4. 碳源对菌丝生长的影响
2.3.5. 氮源对菌丝生长的影响
2.3.6. 光照对菌丝生长的影响
2.3.7. 通气条件对菌丝生长的影响
2.3.8. 致死温度测定
-
香蕉灰纹病是香蕉生长过程中重要的叶部病害之一,病害流行时可导致香蕉减产30%~50%[5],引起香蕉灰纹病的病原有6种[10-15]。Hernández-Restrepo等[12]依据形态学特征和LSU及ITS序列分析等相关数据,提出将香蕉灰纹病病原C. musae[10]、C. johnstonii[11]、C. musicola及其相关种从Cordana属中分出来,归入新属Neocordana。此命名代表了该菌的最新分类进展,已得到部分研究者的承认[13-15],但目前也有部分国内外研究者仍用Cordana属描述香蕉灰纹病病原菌[6, 23-24]。
传统真菌病害的鉴定主要是根据病害症状特征、病原真菌的形态学及生物学等特性进行,但有些植物病原真菌在不同培养条件下形态变异较大,给真菌的分类鉴定带来了一定的难度。真菌DNA的碱基组成遗传稳定,不易受环境影响,而且在其生活史的任何阶段均可获得,其中rDNA的ITS区段既具保守性,又在科、属、种水平上均有特异性序列的特性,对ITS区序列进行PCR扩增、测序以及序列分析是鉴定真菌的常用方法。RENSKE等[25]认为,通过比对真菌ITS区,序列相似性大于99%时,可鉴别为相同种;序列相似性大于95%且小于99%时,可鉴别为相同属;序列相似性小于95%时,可鉴别为相同科。本研究对分离菌株的ITS序列进行比对分析并构建了系统发育树,依据Renske等[25]研究结论结合分离菌株的培养性状、形态学特征及其柯赫氏法则验证,将海南香蕉灰纹病的病原鉴定为香蕉暗双孢菌Neocordana musae。
为了便于病害的预测预报及科学防治,笔者进一步研究了该病原菌的生物学特性,确认该病原菌最适培养基为PDA培养基和OA培养基,最适生长温度为30 ℃,最适生长pH为6,可利用多种碳源、氮源,光照、通气利于菌丝生长,致死条件为60 ℃ 10 min。这与该病原菌适应环境能力较强的研究结论基本一致[18, 23]。
另外,本实验供试菌株在40 ℃也能正常生长,致死条件为60 ℃ 10 min,与番华彩等[18]对在云南分离的香蕉暗双孢菌生物学特性的研究结果存在差异;进一步的系统进化树分析也发现,代表菌株CATAS-CM01与来自墨西哥培养型模式菌株(CPC 16362、CPC 16298)、澳大利亚培养型模式菌株(CPC 17237、CPC 17293)遗传距离较近,处于同一小分支,而与国内云南分离株(XJ13-12-01)遗传距离较远。以上研究表明,香蕉灰纹病菌的生物学特性随地域的不同有一定差异,这可能是该病原菌在不同区域具有不同的生态适应性,具体原因还有待进一步研究。本研究仅探讨了培养基、温度、pH、碳氮源、光照及通气等因素对香蕉暗双孢菌菌丝生长的影响,因此结果具有一定的局限性。下一步,本实验室将开展病原菌孢子产生与萌发条件及病原菌致病性分化与遗传变异等方面的观测与研究,探讨香蕉灰纹病菌群体遗传特征,以期为海南香蕉灰纹病的监测预报和综合防治提供更多理论依据。






 DownLoad:
DownLoad: