-
香蕉(Musa spp.)起源于亚洲东南部,被联合国粮农组织认定为第4大粮食作物[1-2]。世界上约有130个种植香蕉的国家,中国是世界上最早种植香蕉的国家之一。我国香蕉主要种植区域分布在广东、广西、海南、福建、云南和台湾[3]。香蕉具有生长周期短和产量高等特点,但同样伴随着大量的香蕉秸秆等农业废弃物[4]。有研究表明,长期的秸秆还田对土壤细菌群落的丰富度与多样性具有积极影响[5]、能改变土壤微生物群落结构[6]。不同秸秆还田方式也会影响土壤微生物的群落结构或群落组成,如土壤微生物的群落结构随着还田量和沟埋深度的增加而变化[7],还田秸秆的新鲜程度也影响着土壤微生物群落等[8]。香蕉秸秆含有蛋白质、纤维素、维生素和某些微量元素[9-10],是一种营养丰富的植物资源,然而有关香蕉秸秆不同还田方式对土壤微生物群落影响的研究鲜见报道。土壤微生物在农业生态系统中发挥着不可替代的作用[11],土壤微生物群落还能影响作物的生长[12],同时土壤微生物也是影响土壤质量的主要因素之一。研究表明,土壤微生物的组成改变土壤中C的含量[13],土壤微生物动态变化与土壤中N和C的含量具有相关性[14],而且土壤酶活性是由微生物丰度决定的[15]。顾美英等[16]发现,不同秸秆还田方式对土壤微生物数量影响差异显著。不同秸秆还田方式均能提高土壤微生物活性和丰富度指数[17]。笔者通过研究香蕉秸秆在不同模式下还田对土壤中微生物群落的影响,旨在找出适宜的还田模式,为香蕉秸秆还田技术提供理论依据。
HTML
-
土壤采集于海南省临高县红星农场香蕉园(位于北纬19°47′N,东经109°35′E,海南岛的西北部),采集深度为0~20 cm。该蕉园土壤有机质含量为2.33 g·kg−1、pH7.53、碱解氮96.48 mg·kg−1、有效磷115.42 mg·kg−1、速效钾477.05 mg·kg−1。将采集后的土壤放置于海南大学试验基地(北纬30°52′N,东经121°54′E)并用1 cm孔径的筛子筛净土壤,将其完全混合,随后装入3 kg容量的花盆中。试验所用香蕉秸秆采自于临高县红星农场香蕉园,该蕉园种植品种为巴西蕉(Musa nana Lour.),香蕉秸秆为收获期砍掉的新鲜、健康秸秆(C/N为29.45∶1)。随后,将秸秆运回海南大学试验基地,将其粉碎。
-
本研究在海南大学试验基地进行,采用盆栽试验,根据不同的秸秆还田方法,还田量以蕉园耕作层的实际秸秆还田量计算,分为3个处理(每个处理20次重复)。处理设计:土壤(3 kg)+无香蕉秸秆还田作为对照(CK);土壤(3 kg)+新鲜、粉碎香蕉秸秆(150 g),充分混合,作为秸秆还田掩埋处理(T);土壤(3 kg)+新鲜、粉碎香蕉秸秆(150 g),将香蕉秸秆覆盖到土壤上,作为香蕉秸秆自然还田(F)。试验模拟田间灌溉环境,人为定期浇水。
-
待秸秆完全腐解后,在花盆内0~20 cm深度随机采集土壤样品(每个处理7个重复)。将每个样品采集的重复土样充分混合。将土样过2 mm筛,一部分保存在−20 ℃冰箱用于后续的DNA提取。
-
使用PowerSoil DNA Isolation Kits(MoBio Laboratories Inc.,Carlsbad, USA)按照生产协议提取土壤DNA,每个处理设置5个重复。同时(NanoDrop 2000, thermosscientific, USA)测定DNA的浓度和质量(A260/A280的比率)。细菌16S rRNA(V3+V4)区域采用引物:5′-ACTCCTACGGGA GGCAGCA-3′和5′–GGACTACHVGGGTWTCTAAT-3′,真菌ITS1区域采用引物:5′-CTTGGTCATTTAGA GGAAGTAA-3′和5′-GCTGCGTTCTTCATCGATGC-3′。
-
一个分类单元内的序列数除以该组内序列的总数就是相对丰度。在相同的深度随机对序列重新采样(16S rRNA基因的6 415个基因序列和ITS的4 514个基因序列),以标准化采样。利用Excel 2010基于OUT计算微生物门水平与属水平相对丰度。利用MOTHUR在处理间OTU丰度矩阵构建聚类分析。在OTU丰度矩阵的基础上计算微生物丰富度(SChao1)和多样性(HShannon),并利用IBM SPSS 20.0单因素方差分析(one-way analysis of variance)。利用MOTHUR基于Jaccard相似度分析处理间微生物群落组成相似差异(PCoA),利用R ggplot2软件包进行主坐标分析(PCoA)可视化。
1.1. 材料
1.2. 试验设计
1.3. 采样方法
1.4. 测定方法
1.5. 分析方法
-
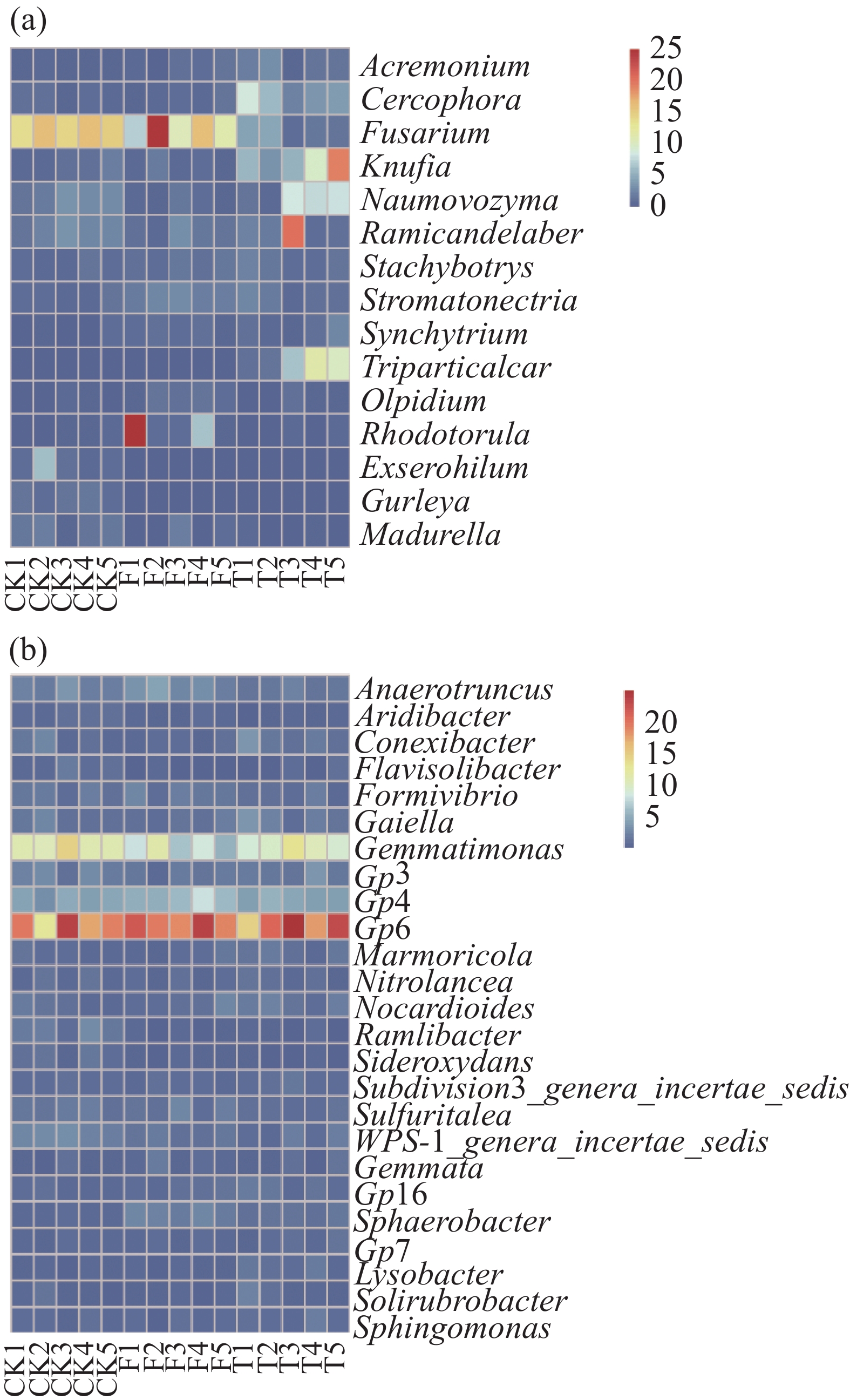
3个处理(CK、F、T)的微生物群落(图1)都包含相同的真菌门(Ascomycota、Basidiomycota、Chytridiomycota、Glomeromycota、Microsporidia、Zygomycota),其中Ascomycota的相对丰度最高,分别为81.1%、54.7%、68.3%。3个处理在细菌门水平(Acidoacteria、Actinobacteria、Bacteroidetes、candidate division WPS-1、Chloroflxi、Firmicutes、Gemmatimonadetes、Planctomycetes、Proteomcetes、Verrucomicrobia、Others)中,Acidoacteria、Actinobacteria、Proteomcetes为优势菌种,且相对丰度均大于10%。图2是相对丰度大于1%的微生物分类属,真菌属水平分析结果表明,T、F、CK处理的Fusarium相对丰度分别为2.6%、14.3%、15.5%。在细菌最丰富的分类属中,各处理中GP6为优势菌种,且相对丰度均大于18%。
-
单因素方差分析(ANOVA)结果(表1)表明,不同秸秆还田处理间细菌群落丰富度(Chao1)和多样性(Shannon)存在显著差异。与无香蕉秸秆还田处理(CK)相比,掩埋还田处理(T)的土壤微生物群落的丰富度和多样性最显著。与香蕉秸秆自然还田处理(F)相比,掩埋还田处理(T)的土壤微生物群落的丰富度更高,但不同处理间真菌群落丰富度和多样性差异不显著。
处理 Treatments 群落丰富度 Community richness 群落多样性 Community diversity Chao1 ACE Shannon Invsimpson 16S rRNA CK 3 832.78±116.90c 3 865.18±92.21c 6.54±0.18b 220.55±91.28a F 3 999.52±153.05b 4 035.82±113.97b 6.70±0.03a 267.79±26.92a T 4 393.32±78.78a 4 480.35±32.31a 6.76±0.07a 266.50±37.83a ITS CK 839.97±60.92ab 839.77±57.59ab 4.40±0.24a 29.21±5.72a F 799.36±38.18b 795.57±36.08b 4.26±0.33a 21.29±7.69a T 924.46±116.94a 931.55±96.38a 4.21±0.24a 21.55±8.53a 注:不同字母表示不同处理间差异显著(P < 0.05)。
Notes: Values followed by different letters mean significant difference between treatments (P < 0.05).Table 1. Soil microbial community richness and diversity between treatments
-
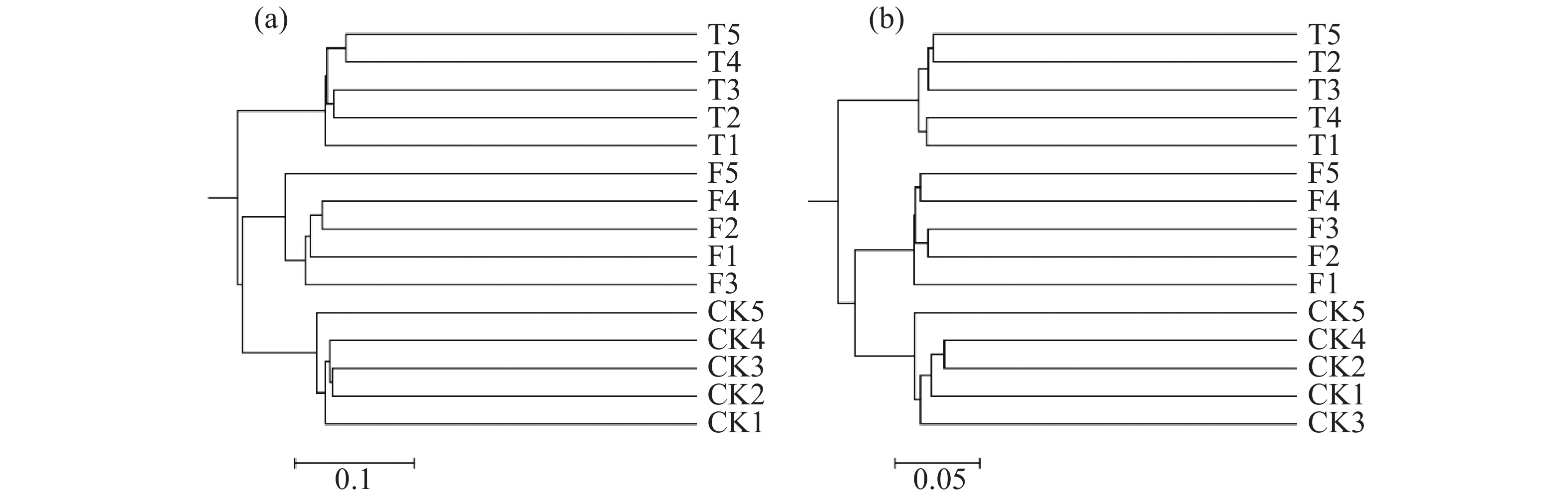
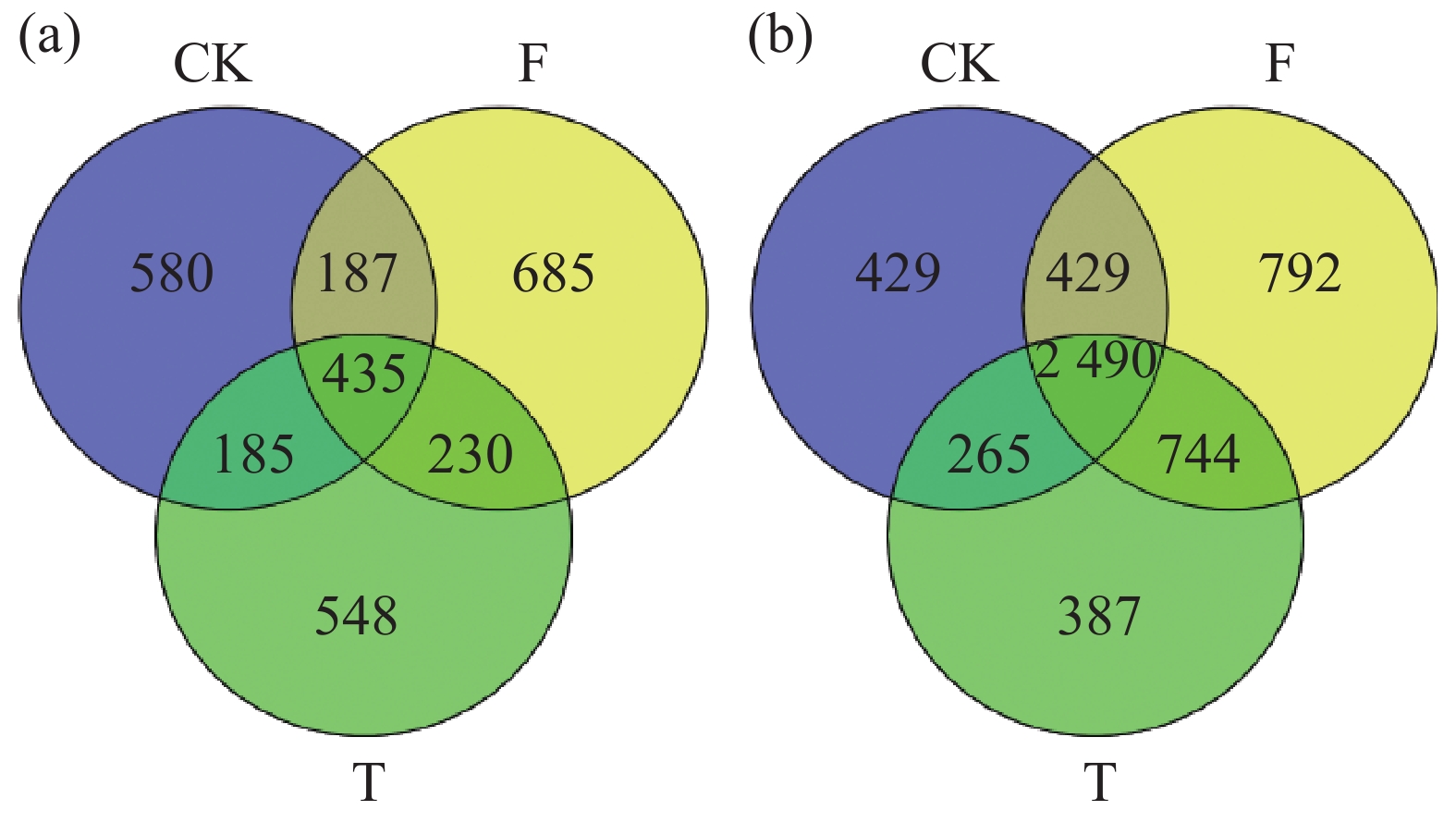
聚类分析结果(图3)表明,15个土壤样品的微生物群落群落结构明显分为3组,分别为3个香蕉秸秆还田处理。利用主坐标分析(图4)不同香蕉秸秆还田处理对土壤微生物群落的影响。从图4-a可知,真菌群落的前两个主坐标解释了各样本的34.37%的变化。第1主坐标(PCoA1)T和F处理的真菌群落与CK处理分离良好,第2个主坐标(PCoA2)结果表明,3个处理之间明显分离。细菌群落的前两个主坐标解释了各样本的35.99%的变化,且3个处理间的群落分离效果与真菌群落的分离效果相似(图4-b)。土壤真菌与细菌OTU分布结果(图5)表明:CK、F、T处理真菌独有OTU数量与细菌独有OTU数量分别为580、685、548和429、792、387。CK与F,T处理真菌共有OTU数量和细菌共有OTU数量分别为187、185和429、265,F与T真菌和细菌共有OTU数量分别为230、744。
2.1. 土壤微生物群落组成
2.2. 土壤微生物群落的丰富度与多样性
2.3. 土壤微生物群落结构
-
秸秆还田作为一种农业管理策略,对土壤微生物活性有积极的影响[18]。在本研究中,不同香蕉秸秆还田管理对土壤微生物群落组成和结构有显著差异。3个处理中,Ascomycota为优势真菌门,CK、F、T处理的Basidiomycota的相对丰度分别为4.7%、7.5%、6%。Basidiomycota在自然界中起着主要分解纤维素的作用[19],因此,香蕉秸秆还田为Basidiomycota在土壤中提供了有益环境,使其相对丰度增高。T处理Fungi_Unidentified的相对丰度分别比CK和F增高了12.8%和13.7%,可能由于T处理香蕉秸秆的分解比F更彻底,造成分解产物方面的差异从而影响Unidentified_Fungi的相对丰度[20]。在细菌门分类中,以Acidobacteria和Proteobacteria(图1-b)为优势菌,同时与CK相比,2种秸秆还田处理均能使Acidobacteria的相对丰度增加,这与CHEN等人[21]的研究结果一致。与CK相比,F和T处理土壤中Actinobacteria的相对丰度分别增加了3.2%和1.9%,Actinobacteria是一种非常重要的分解者[22],这也解释了秸秆还田处理可使其相对丰度增高的原因,同时,一些放线菌同样对土壤病原真菌起到拮抗作用[23]。本研究中,真菌分类属结果表明(图2-a),T处理的Frusarium相对丰度最低为2.6%,最高为CK(15.5%),而F处理的Frusarium相对丰度与CK处理相比并无明显降低,这一结果说明,香蕉秸秆掩埋处理可能起到了降低病原菌的作用,并且对土壤健康有积极影响,但香蕉秸秆掩埋处理使土壤中Frusarium相对丰度降低的理论依据还需要进一步研究。
本研究中,香蕉秸秆还田方式土壤细菌群落丰富度和多样性存在显著差异,其中,T处理细菌群落的丰富度最高,并且T和F的多样性均与CK存在显著差异。细菌群落的变化是多种复杂因素共同作用的结果[24-25],而本研究中秸秆还田方式为唯一的因素。但各处理的真菌群落丰富度和多样性并无差异,说明香蕉秸秆还田对细菌群落丰富度和多样性的影响远高于真菌。基于OTU组成的聚类分析、主坐标分析、OTU数量分布结果均显示,T处理的土壤微生物群落结构与F处理相近且与CK处理明显不同。并且,无论是在第1主坐标还是第2主坐标上,秸秆掩埋处理均显著与对照分开。因此,相比于表面覆盖,香蕉秸秆的掩埋处理(T)更容易改变土壤微生物群落结构。
本研究结果表明,相比于香蕉秸秆不还田处理(CK)和香蕉秸秆自然还田处理(F),香蕉秸秆掩埋还田处理(T)对土壤微生物群落具有更积极的影响。香蕉秸秆还田为某些具有分解功能的微生物提供了有益的土壤环境,并且香蕉秸秆掩埋还田处理(T)降低了土壤中Frusarium的相对丰度,提高了细菌群落多样性与丰富度。因此,在本研究条件下,香蕉秸秆掩埋还田处理(T)为适宜、安全的秸秆还田方式。












 DownLoad:
DownLoad: