-
矿质营养是植物生长发育的物质基础。氮素作为植物生长发育过程中需求量较大、影响较大的矿质元素之一,被称为生命元素[1-2]。氮不仅是蛋白质的重要组成成分,也是核酸、磷脂、光合色素等多种生物大分子的重要组分[3]。由于氮素在植物生命活动中所处的特殊地位,探究氮素营养对植物生长的影响一直是农业领域的研究热点。JIANG C Q等[4]的研究结果表明,烟草在高氮和低氮处理下的生物量均低于对照,增加供氮量可在一定程度上提高烟草叶片的叶绿素含量和光合能力。邢倩等[5]在对冬小麦的研究中发现,氮、磷、钾3种大量元素中,氮素的缺乏对冬小麦净光合速率的影响较大。BOUSSADIA O等[6]发现,较低的供氮水平使不同品种橄榄的生物量、叶片氮含量、叶绿素含量、光合速率均显著下降。相似的研究结果在对玉米、棉花、咖啡、木薯等的研究中也得以体现[7-10]。上述研究结果表明,缺氮胁迫影响作物的生长,从而影响作物的产量,因此说明及时监测和诊断植物生长发育中氮素营养的供应水平,对指导作物增产生产有积极意义。通过叶绿素荧光仪可测定植物叶片的光合能力,借此可判断作物的供氮水平。叶绿素荧光仪在测定植物叶片光合能力中具有快捷、灵敏、无损伤等特点,而被广泛应用于植物营养供应的监测中[11]。
棕榈科植物广泛分布于热带及亚热带地区,在热带经济作物中占据着十分重要的地位[12]。槟榔(Areca catechu L.)是棕榈科槟榔属植物,在我国有着一千多年的栽培历史,是海南省重要的特色经济作物之一[13]。近些年随着槟榔在海南的大面积推广种植,很多地区形成了栽种密度大、物种单一的槟榔种植体系,且不注重水肥管理及土壤改良,造成土壤耕性下降和肥力不足,导致槟榔植株无法获得充足养分,使得槟榔生长受到抑制、抗性降低、收获期变短、产量品质下降[14]。这给槟榔种植、加工等行业的健康发展造成障碍。优化水肥管理、提高土壤肥力、培育健壮种苗是提高槟榔生产水平的有效措施。氮素作为大量元素之一,氮肥的施用是提高土壤肥力、培育壮苗中的重要一环。目前,氮素对槟榔生长发育影响的相关研究鲜有报道。笔者利用叶绿素荧光仪和光合仪测定缺氮条件下槟榔苗叶片的光合特性,并依据测定结果探索针对槟榔缺氮的便捷、有效的诊断方法,旨在为槟榔育苗中合理施用氮肥提供理论依据。
HTML
-
供试槟榔苗为‘热研一号’槟榔,由中国热带农业科学院椰子研究所刘立云研究员与黄丽云博士提供。选取长势一致的1~2叶龄槟榔幼苗,经2周水培适应和平衡后,分别培养于全素营养液(CK)(表1)和缺氮营养液(-N)中。全素营养液配方见表1。每日保持通气泵充气2次(30 min·次)。培养地点:海南大学海甸校区温室大棚(自然光照)。培养3个月后,观测CK、-N处理的槟榔苗的生物量,和自新叶向下依次3片叶片(叶序号:L1,L2,L3)的光合指标、叶绿素荧光参数、叶绿素含量和叶片组织显微结构。1个处理取样3株苗,重复3次。
母液 组分 摩尔质量/(g·mol−1) 母液浓度/1000×(mmol·L−1) 使用浓度/(mmol·L−1) 母液含量/1000×(g·L−1) 1 KNO3 101.1 1500 1.5 151.65 Ca(NO3)2·4H2O 236.15 1200 1.2 283.38 MgCl2 95.21 25 0.025 2.38 NH4NO3 80.04 400 0.4 32.02 K2SO4 174.27 300 0.3 52.28 MnSO4·H2O 169.01 1.5 1.5×10−3 0.254 ZnSO4·7H2O 287.55 1.5 1.5×10−3 0.431 2 CuSO4·5H2O 249.71 0.5 0.5×10−3 0.125 H2MoO4 161.95 0.14 0.14×10−3 0.02 MgSO4·7H2O 246.48 500 0.5 123.24 (NH4)2SO4 132.4 300 0.3 39.72 3 Fe-EDTA(Na2) 367.1 40 0.04 14.68 4 NaB4O7·10H2O 381.37 2.5 2.5×10−3 0.95 5 KH2PO4 136.09 250 0.25 34.02 Table 1. Formula of total nutrient solution
-
将新鲜槟榔苗分别按地上部、地下部拍照记录,并在称取鲜质量后,放入烘箱(40 ℃, 12 h)烘干至恒重后,称干质量。
-
称取新鲜叶片0.1 g,放入干净的研钵中,加入1 mL丙酮以及适量石英砂和碳酸钙粉末帮助研磨(研磨过程在冰上进行),研磨过程中,再加入3 mL 80%丙酮研磨至样品变为白色,将白色样品放入黑暗环境下静置5 min,然后用滤纸过滤,最后将滤液用80%丙酮定容至5 mL。使用UV721分光光度计分别测量645,663 nm处的吸光度,每个样品重复3次。按照Arnon(1949)的方法计算叶绿素含量。数据分析采用DunCan检测。
叶绿素a = 12.7A663−2.69A645;
叶绿素b = 22.9 A645−4.68 A663。
-
使用CIRAS-3 型便携式光合仪,于2019−12−15(晴天)上午10:00−11:00,测定叶片净光合速率、胞间CO2浓度、气孔导度等光合参数。将槟榔苗暗适应30 min后,使用Dual-Pam-100测定叶片PSⅡ的最大光化学效率Fv/Fm和实际光化学效率 Y(Ⅱ)、光化学淬灭参数qP、非光化学淬灭参数NPQ等。采用IBM SPSS Statistics 20中的单因素方差分析选项对数值进行统计分析。
-
剪取叶片中部约3 cm×5 cm见方,置于FAA固定液中固定12 h,采用树脂半薄切片法制片。用leica 2235切片机切片,切片厚度为5 μm。切片经甲苯胺蓝-O(TBO)染色后,置于Nikon生物光学显微镜下观察并拍照。每个处理挑选3张完整切片,每张切片随机选取10个视野,用ImageJ1.43软件分别测定叶片厚度、叶片上下表皮细胞厚度、叶肉细胞间隙面积、单位面积视野内叶肉细胞数量(叶肉细胞密度)。采用ImageJ1.43对细胞大小等参数进行测量,并使用IBM SPSS Statistics 20中的单因素方差分析及独立样本t检测进行统计分析。
1.1. 实验材料
1.2. 生物量测定
1.3. 叶绿素含量测定
1.4. 叶片光合参数及叶绿素荧光参数测定
1.5. 叶片显微结构观测
-
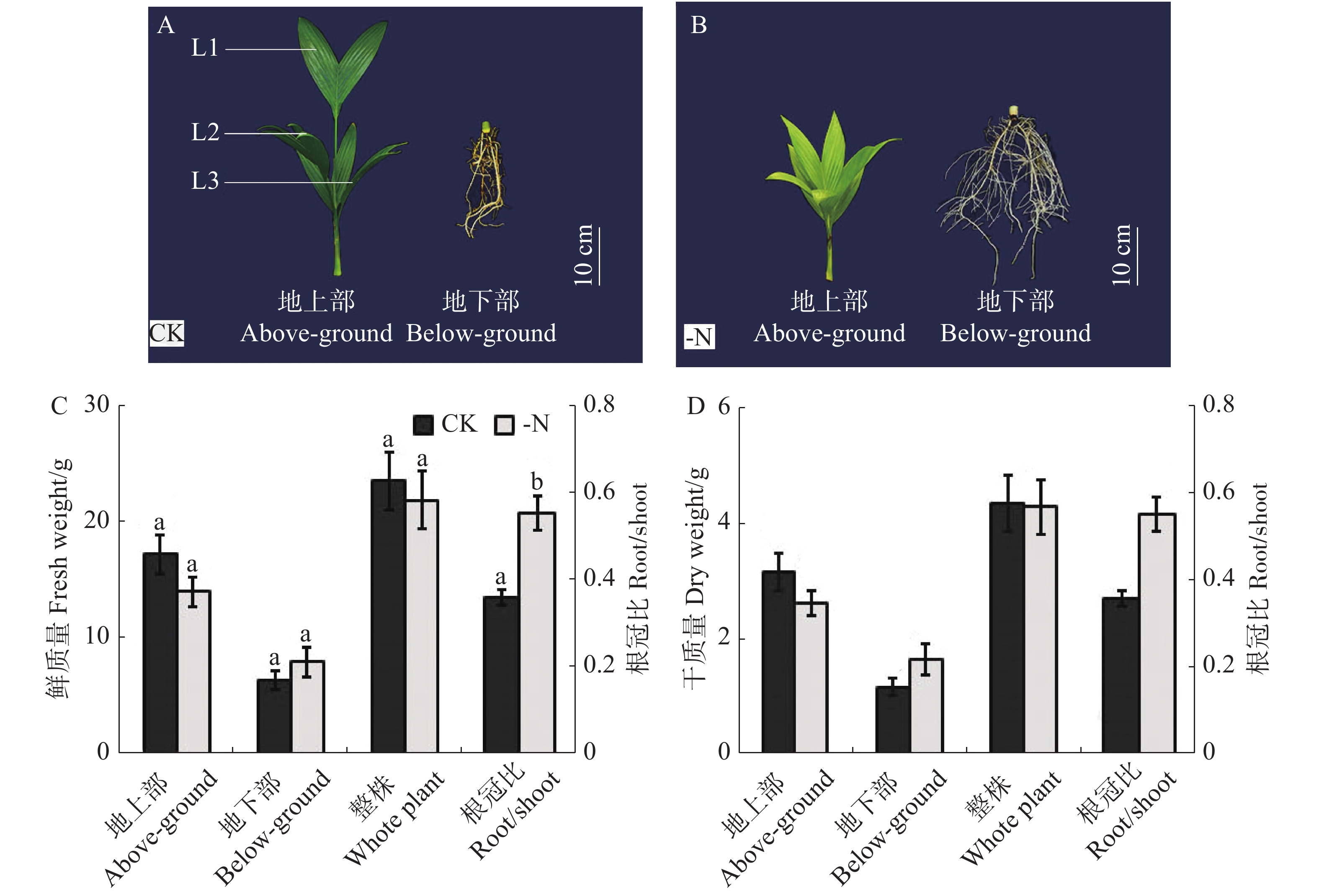
缺氮对槟榔苗生长状态的影响如图1-A,B所示,与对照相比,缺氮处理的槟榔苗生长受到显著影响。缺氮胁迫下槟榔苗地上部生长受到抑制,植株矮小,叶色变黄,而地下部根系发达。槟榔苗生物量分析结果(图1-C,D)显示,缺氮处理(-N)下槟榔苗地上部鲜质量和干质量均低于对照(CK),地下部鲜质量及干质量则均高于对照,但差异均不显著。而缺氮处理下槟榔苗鲜质量和干质量的根冠比均显著高于对照(图1-C、D)。
-
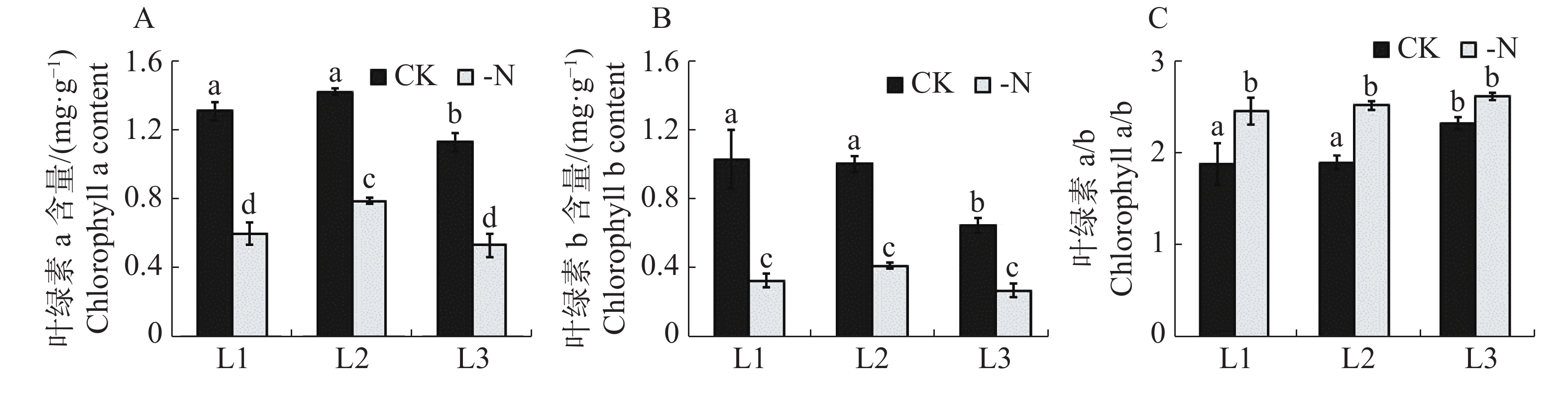
对照和缺氮处理的槟榔苗各叶序叶片的单位鲜质量的叶绿素含量及组分的测定结果(图2)显示,缺氮处理下的槟榔苗L1,L2,L3的叶绿素a和叶绿素b含量均显著低于CK相同叶序叶片 (图2-A,B)。与CK相比,缺氮处理下槟榔苗L1,L2,L3的叶绿素a含量分别降低了54.2%,44.5%,52.9%;叶绿素b分別下降了67.8%,58.4%,58.0%。与CK相比,-N处理的槟榔苗各叶序叶片叶绿素a与叶绿素b比值(a/b)均有上升,且在L1,L2叶序上差异达到显著水平(图2-C)。该结果表明,在缺氮胁迫下槟榔苗植株各叶序叶片叶绿素a和叶绿素b含量均显著降低,且叶绿素b比叶绿素a含量降低幅度更大。
-
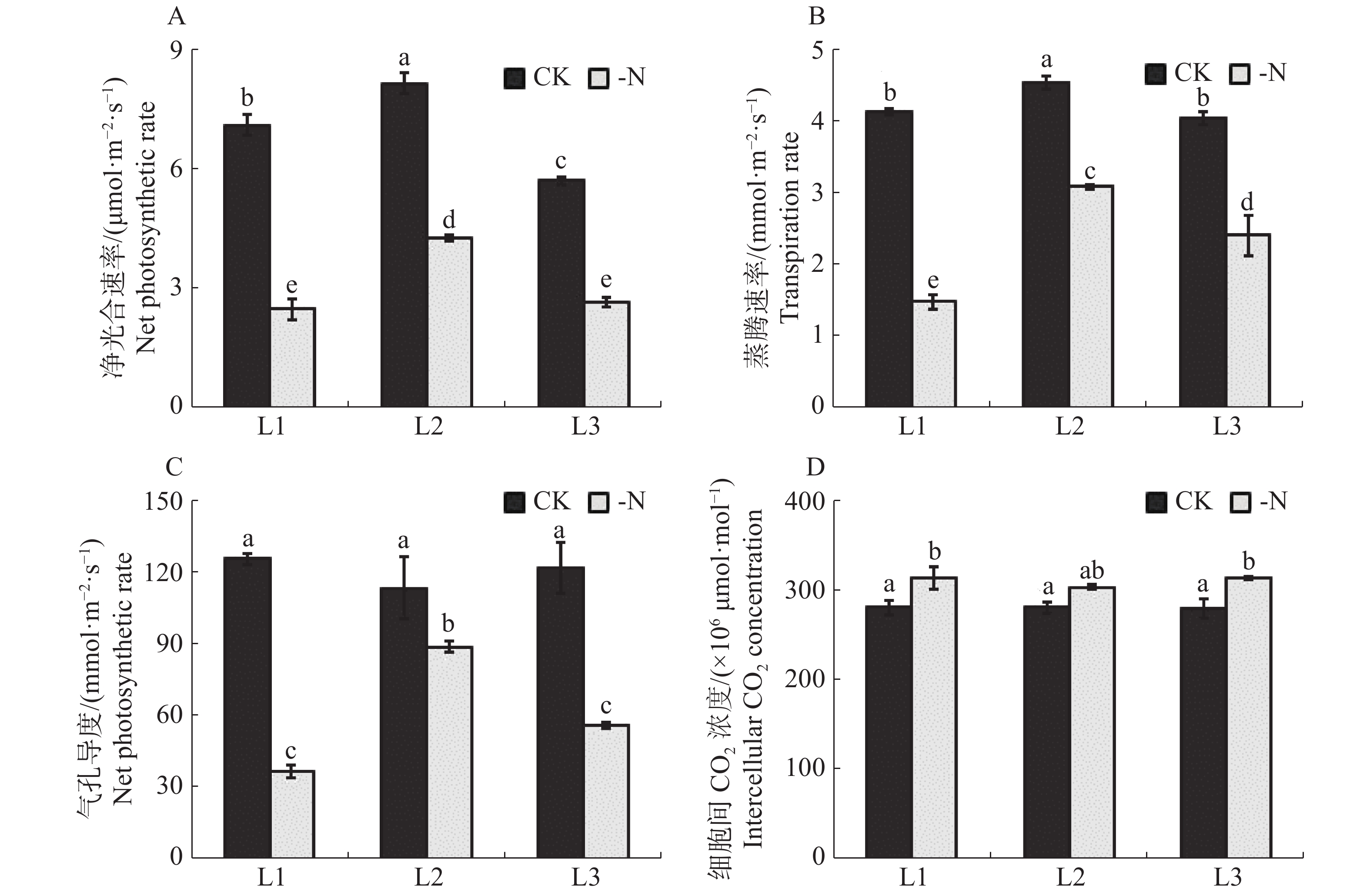
缺氮处理下的槟榔苗各叶序叶片净光合速率均显著低于对照(图3-A)。与CK相比,-N处理的槟榔苗L1,L2,L3净光合速率分别降低了65.0%,47.7%,53.2%。在相同处理下,槟榔苗L2净光合速率最高,CK和-N处理的槟榔苗L2的净光合速率分别达到了8.1,4.2 μmol·m−2·s−1。缺氮胁迫对槟榔苗叶片蒸腾速率的影响与净光合速率情况相同,-N处理的槟榔苗L1,L2,L3蒸腾速率较CK极显著降低了64.3%,32.1%,40.4%(图3-B)。这说明缺氮对槟榔苗各叶序叶片净光合速率及蒸腾速率均造成显著影响。
槟榔苗叶片气孔导度对缺氮胁迫的响应与其净光合速率和蒸腾速率对缺氮胁迫的响应情况类似。缺N处理的槟榔苗各叶序叶片气孔导度均显著低于对照(图3-C)。胞间CO2浓度的变化主要受叶片气孔导度和光合消耗的影响。叶片胞间CO2浓度与光合能力密切相关。缺氮处理下的槟榔苗各叶序叶片细胞间CO2浓度均显著高于对照(图3-D),这表明缺氮处理下,槟榔幼苗各叶序叶片碳同化能力均低于对照。
-
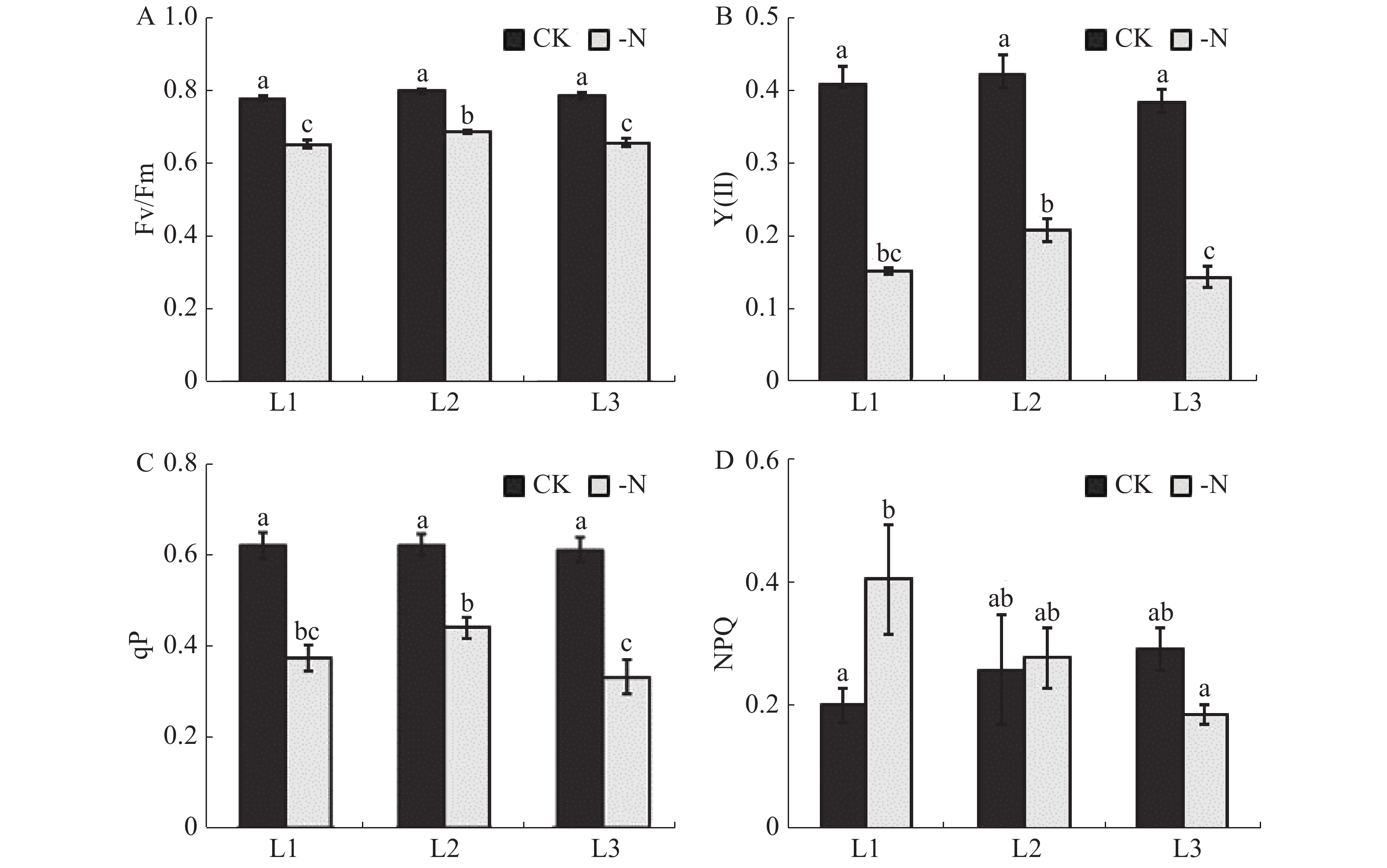
在缺氮处理下的槟榔苗L1,L2,L3各叶片的 Fv/Fm、Y(Ⅱ)、光化学淬灭参数qP值均极显著低于对照(CK)。与CK相比,-N处理的槟榔苗L1,L2,L3叶片的Fv/Fm分别降低16.1%,13.8%,16.4%;Y(Ⅱ)分别降低62.4%,50.3%,62.2%;qP分别降低39.9%,29.4%,和45.8%(图4-A,B、C)。这表明,缺氮条件下,槟榔幼苗叶片受到明显的光抑制,PSⅡ反应中心进行光化学反应的效率显著降低。非光化学淬灭参数NPQ反映了PS II天线色素吸收的光能不能用于光合电子传递而以热的形式耗散掉的部分。在本实验中,-N处理的槟榔苗L1叶片NPQ值显著高于CK槟榔苗L1叶片,而L2和L3差异不显著(图4-D)。
-
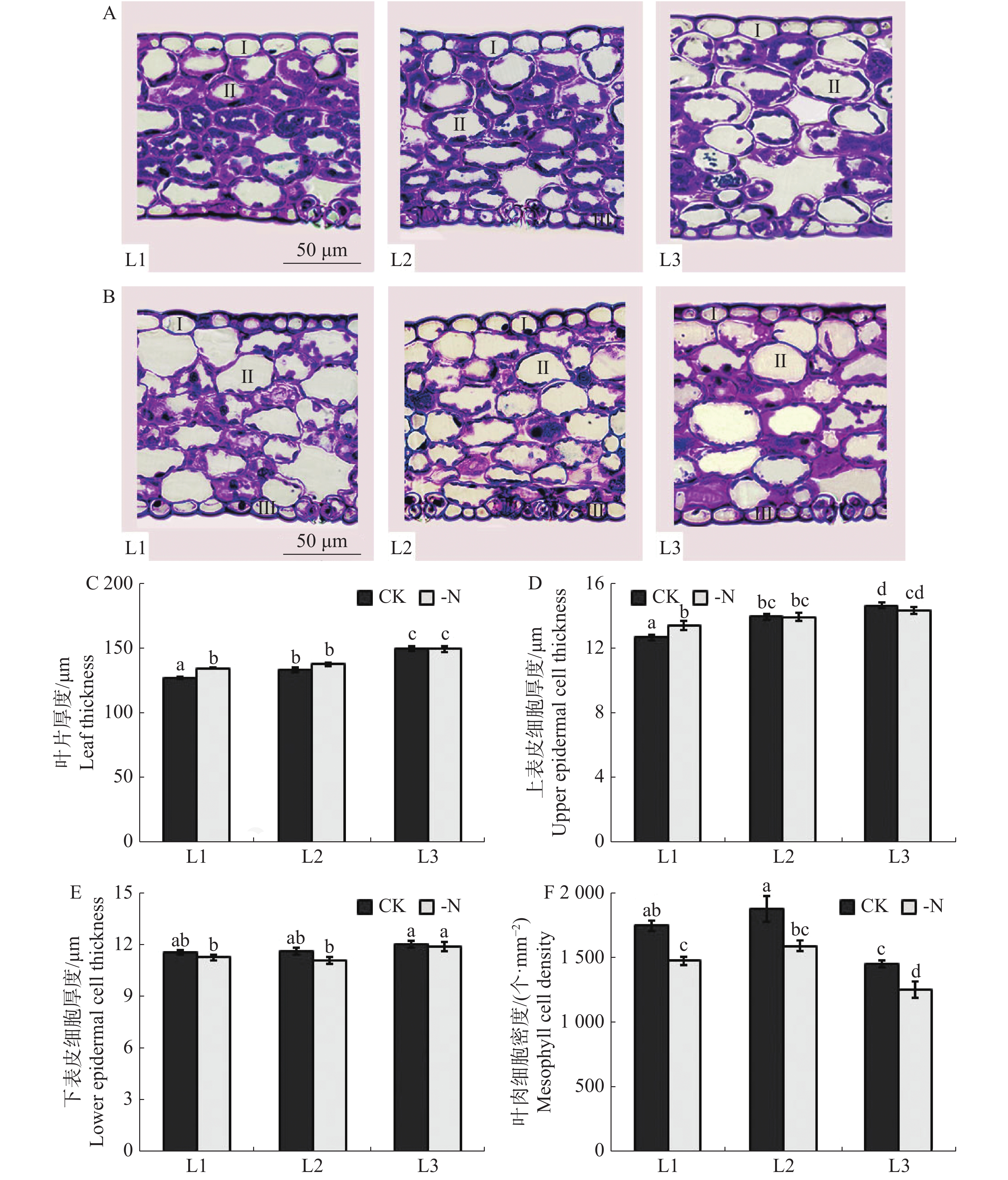
由槟榔苗叶片显微图片(图5-A,B)可见,缺氮胁迫对槟榔苗各叶序叶片显微结构影响显著,主要表现为缺氮处理下槟榔苗叶片叶肉细胞较对照排列松散,细胞内叶绿体含量显著降低。对显微图片进一步测量结果(图5-C,D、E)表明,缺氮处理下,槟榔幼苗L1叶片厚度、上表皮细胞厚度均显著高于CK,下表皮细胞厚度显著低于CK;L2下表皮细胞厚度显著低于CK而叶片厚度、上表皮细胞厚度较CK均无显著差异;L3上表皮细胞显著低于CK,而叶片厚度、下表皮细胞厚度与CK无显著差异。槟榔幼苗各叶序叶片叶肉密度均受缺氮处理影响(图5F)。缺氮处理下L1,L2,L3叶肉细胞密度相较于CK分别降低了15.7%,15%,13.8%,且差异显著。
2.1. 缺氮对槟榔苗生长状态的影响
2.2. 缺氮对槟榔幼苗叶片叶绿素含量的影响
2.3. 缺氮对槟榔幼苗叶片光合参数的影响
2.4. 缺氮对槟榔幼苗叶片荧光参数的影响
2.5. 缺氮对叶片显微结构的影响
-
叶片的含氮量可以有效反映植物的供氮水平。大多数植物叶片中的氮素约有一半被分配到光合机构中,其中的1/3被投入到以叶绿素a、b为主的光能捕获系统中[15]。本实验结果显示,缺氮处理下槟榔苗各叶片叶绿素a和叶绿素b含量均显著低于CK相同叶序叶片。刘迁杰等[16]、寸竹等[17]、何亮珍等[18]的研究中同样发现氮素的缺乏会导致植物叶片叶绿素含量的显著下降。氮素是叶绿素及其相关合成酶的原料,而氮素的缺乏导致叶绿素的合成受阻,因此缺氮会直接导致叶片叶绿素的含量降低[19]。
缺氮胁迫不但影响了槟榔苗叶片叶绿素含量,而且对叶绿素组分也造成了显著影响。缺氮处理下槟榔苗各叶序叶片叶绿素a/b显著上升,这意味着缺氮胁迫导致槟榔苗叶片中叶绿素a比例上升,叶绿素b比例下降。在叶绿素组分中,叶绿素a主要是起到激活反应中心的作用,而有少量叶绿素a负责吸收和传递光能;绿素b作为捕光天线,主要作用是吸收和捕获光能[20]。MU X H等[21]的研究结果显示,在缺氮条件下玉米叶片捕光系统中的氮素比例会降低。在缺氮胁迫下植物光合速率降低,捕光天线吸收的光能不能完全用于CO2固定,剩余的激发能会通过热耗散、光呼吸、环式电子传递等途径耗散,而不能及时耗散的激发能会结合O2产生单线态氧自由基,过多的氧自由基可能损伤PSⅡ从而产生光抑制[22-23]。本研究结果显示,缺氮胁迫下槟榔苗各叶序叶片光化学淬灭参数qP值显著低于对照组。这说明缺氮胁迫导致槟榔苗叶片发生了一定程度的光抑制。有研究结果表明,强光适应下的叶片叶绿素 a/b相较于遮荫叶片高[24-25]。槟榔作为典型的热带作物,在缺氮胁迫下可能采取降低叶绿素含量和减少叶绿素b组分的策略来减少光能捕获,防止过剩激发光对光合机构造成损害。本研究还发现缺氮组非光化学淬灭参数NPQ显著高于对照组,这表明缺氮组槟榔苗将激发光更多的通过热耗散的形式消耗,减少过剩激发光对光合机构造成损害,达到光保护的效果。
叶绿素含量的丰缺影响着光能捕获、传递、电子传递效率等整个光反应过程[20]。JIANG C D等[22]的研究结果表明,增加氮素供应量能够有效提高天线色素捕捉光能的效率和PSⅡ反应中心的开放比例。在对水稻、玉米、香榧、佛手等的诸多研究中,均证实缺氮显著影响植物叶片光合作用的各个阶段[26-29]。结合本研究结果缺氮处理的叶片实际光化学效率Y(Ⅱ)、净光合速率与对照组相比的下降比例,可初步判断缺氮对槟榔苗叶片光合效率的影响主要受光能捕获、电子传递效率等光反应活性的限制。
在本研究中,缺氮处理使槟榔幼苗地上部及整株生物量降低,而地下部生物量升高,且根冠比显著高于对照,这表明槟榔幼苗在缺氮胁迫下地上部的生长受到了抑制,而根系的生长发育得到了促进。武勇军[30]在对缺氮、复氮条件下玉米幼苗根系的研究中发现,缺氮处理后的根系总长度、根表面积、分根数要比正常处理的数值更大。在对不同品种粳稻的研究中同样发现,缺氮处理下粳稻的根冠比明显提高[26]。COOMES D A等[31]和BIJLSTMA R J等[32]的研究结果证明,土壤缺氮时,植物会向根部投入较多的碳同化物,促进根系的生长以获得足够营养。此外,本研究在对不同供氮水平下槟榔苗叶片显微结构的观测及统计中发现,缺氮胁迫下的槟榔苗顶端叶片L1的叶片厚度与上表皮细胞厚度均显著高于对照。而在对其他植物的研究[33-34]中发现,氮素或其他矿质元素的缺乏均会导致植物叶片厚度、叶片表皮细胞厚度不同程度的降低。马慧丽等[35]发现,强光适应下的植物叶片厚度会显著升高。这表明,槟榔在缺氮胁迫下还可能通过提高叶片厚度及上表皮细胞厚度来更好地适应海南高光强的生长环境。缺氮胁迫对槟榔叶片超微结构造成的影响尚不清楚,有待进一步研究。
本研究结果表明,缺氮胁迫使槟榔苗叶片叶绿素合成受抑制,PSⅡ反应中心活性下降,光合效率降低,导致整株生物量下降;在缺氮条件下,槟榔苗会通过调整地上部与地下部的生物量的分配以维持根系与地上部的生长平衡,同时槟榔苗可能通过采取降低叶绿素b在光合色素中的比例及提高叶片厚度和上表皮细胞厚度等策略来增强热耗散机制,从而适应热带高温、强光的生存环境。
致谢:中国热带科学研究院椰子研究所刘立云博士与黄丽云博士为本实验提供‘热研一号’槟榔幼苗,一并感谢。











 DownLoad:
DownLoad: