-
被子植物自从白垩纪中期以来,几乎占据了所有的地球上现存陆地生态系统的植被,包括了近1万个属,200 000 ~ 300 000 种植物,接近植物界的一半,是出现最晚,种类最多,分布最广的植物类群[1]。被子植物在人类进化过程中,给人类提供了食物、医药、纤维和木材等衣食住行的必需品,是人类发展所必须的物质基础,为此其起源和演化一直是研究的焦点之一[2]。被子植物起源的研究长期以来都集中在多系和单系方面的争论,而被子植物和其他种子植物的关系的讨论则相对较少。长期以来,被子植物的起源和早期演化主要是通过信息不清晰的化石证据、现存的被子植物之间的不确定的关系和形态学上被子植物和其他种子植物直接无法解释的差异[1]来论证,到了20世纪60~80年代,该研究才有显著的突破。一是发现了木兰纲是被子植物的基部类群之一[3-5];二是基于对早期白垩纪被子植物化石的花粉、叶片和生殖器官结构的研究,了解了早期被子植物演化的过程[6-10]。现在对被子植物起源的研究主要集中于早期被子植物进化的方式、过程和系统发育的分析,以及基于形态学和分子生物学相结合的方法等方面,这些研究成果使研究者对过去的许多争论变得清晰。一些新的关于白垩纪被子植物的花和花粉化石证据和新的关于被子植物形态结构的研究进一步弄清了被子植物的单系起源,木兰纲是基部类群之一,认为买麻藤目应该是被子植物的姊妹群[1]。新的基于分子生物学证据的APG II系统已经建立[11],并在逐步完善,研究者已经认识到无油樟(Amborella)、睡莲科(Nymphaeales)和八角目(Illiciales)−苞被木科(Trimeniaceae)−木兰藤科(Austrobaileyaceae)是早期被子植物进化的第一步,其中无油樟是其他所有被子植物的姊妹群[2],但是仍然未给出买麻藤目与被子植物的关系,不过也有研究者认为买麻藤目更加近于针叶裸子植物,与被子植物不是姊妹群[2]。植物的次生代谢产物是植物的重要进化特征和性状,但买麻藤目植物的化学成分研究相对较少,只有早期总结过的化学成分类型[12]。被子植物的化学成分研究较多,且类型多样复杂,但作为研究被子植物起源证据的研究相对较少。由于长期缺少对买麻藤目植物的化学成分中的关键化学成分类型进行系统的分离和整理,而只是认为被子植物与买麻藤目植物存在着某种关系,却无法系统地关联起来与现有的形态结构、化石和分子生物学证据进行互相佐证。因此,笔者从经典分类学、化学分类学、古植物学、现代分子生物学角度梳理被子植物起源于买麻藤目的相关证据,旨在解释买麻藤目与被子植物的关系及其起源。
HTML
-
买麻藤目(Gnetales)现存3个属[13]:麻黄属Ephedra (约40种,分布于干旱和半干旱地区)、买麻藤属Gnetaum(大约42种,分布于热带雨林和矮树林)和百岁兰属Welwischia(一种Welwischia mirabilis,分布于非洲纳米比亚沙漠)(图1)。早期的植物学家发现买麻藤目有大量不同于裸子植物,又同于被子植物的特征,如都具有导管和筛管的结构、对生或互生的叶序、叶非针形或退化、平行或网状叶脉、较多的腋芽、胚胎发育过程中具有营养细胞[14-15]。后来还发现麻黄属和买麻藤属植物具有双受精现象并形成胚乳[16-17]。从这些特征可看到买麻藤目植物应该与被子植物关系更加近,而应该远于裸子植物,但是买麻藤目植物很长一段时间被认为是单一演化的单系类群,其根本原因在于其胚珠裸露,没有包被,而这正是定义被子植物的根本性依据,因此,笔者认为研究胚珠包被的进化过程,控制和决定在植物胚的形成过程中子房的形成和胚珠包被的关键性的基因,以及这些基因在被子植物物种进化过程当中的作用,成为了解被子植物演化的重要关键点之一。
-
与苔藓植物,维管植物化学成分最大的不同是买麻藤目植物含有大量的丹宁和木脂素,现存的蕨类和裸子植物利用这些酚性成分作为植食动物消化酶的有效抑制剂[18]。蕨类植物和裸子植物在抵御植食动物取食的时候才和动物产生化学成分的交流,裸子植物传粉靠风,传播种子的时候才需要鸟[19]。直到本内苏铁的出现,昆虫才开始介入植物的传粉[20]。一般来说,这种植物和动物的交流程度在泥炭纪至白垩纪都是非常浅的,但是到了白垩纪,昆虫、鸟类和哺乳动物开始大量介入植物的传粉和种子传播过程,并一起共同进化的时候,被子植物就发生了急剧且持续性的演化,这些特殊的化学成分却是连接动物行为和植物之间共同进化的物质媒介[21]。这种被子植物化学成分多样性对被子植物和昆虫等动物产生和演化都是至关重要的,而根据这一理论,植物次生代谢产物的合成和多样化是植物类群成功演化的重要条件之一[22]。这种化学成分的多样性是被子植物强大的氧化还原潜力来实现的,从而使生物合成的途径逐渐突破了裸子植物到被子植物的界限[23]。这些氧化还原过程伴随各种各样的取代、耦合等,从而使被子植物中出现新的结构更复杂的化学成分,但是仍然可以看到整个合成途径的主体以及合成那些新的成分的前体,以及在演化过程中一脉相承的痕迹。很早就有人认识到种子植物化学进化的观点,认为买麻藤目植物应该更近于被子植物而不是裸子植物[12],但是限于当时的条件没有能够从买麻藤目中分离到苄基异喹啉类生物碱和大量菧类成分。笔者通过分析近年来关于买麻藤目中重要的苄基异喹啉生物碱和菧类,能够很好地验证买麻藤目植物更近于被子植物这一观点。
-
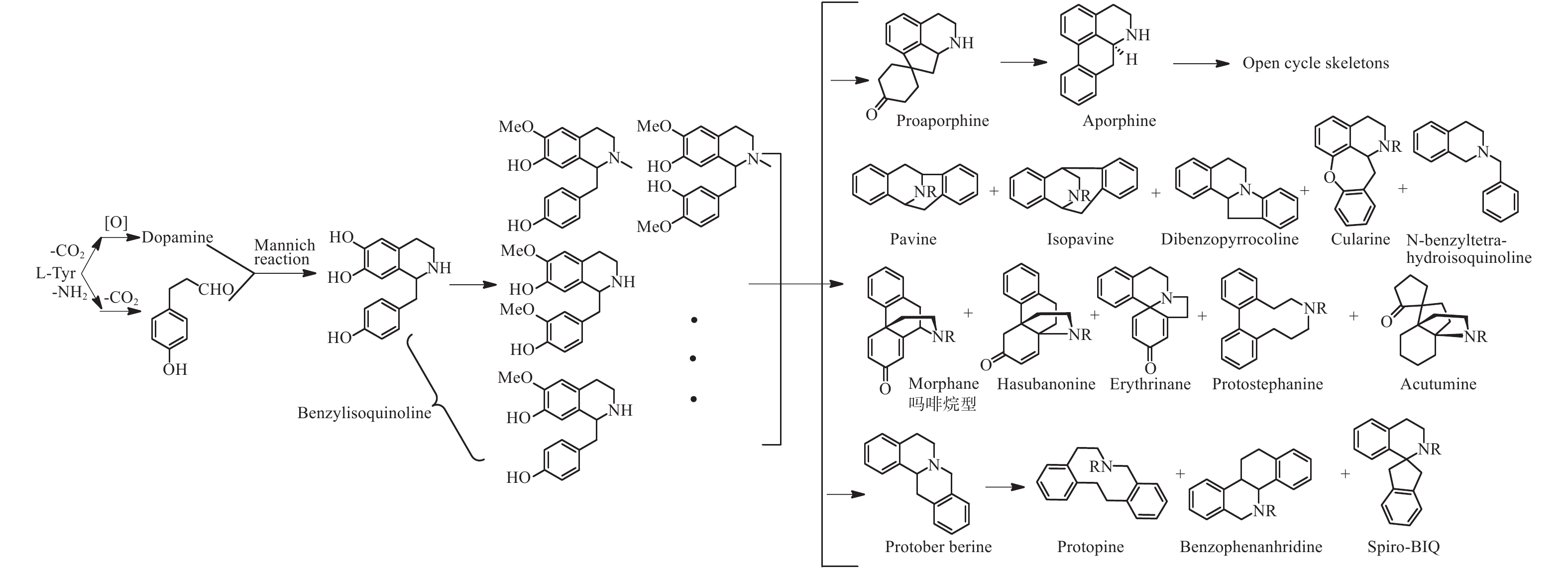
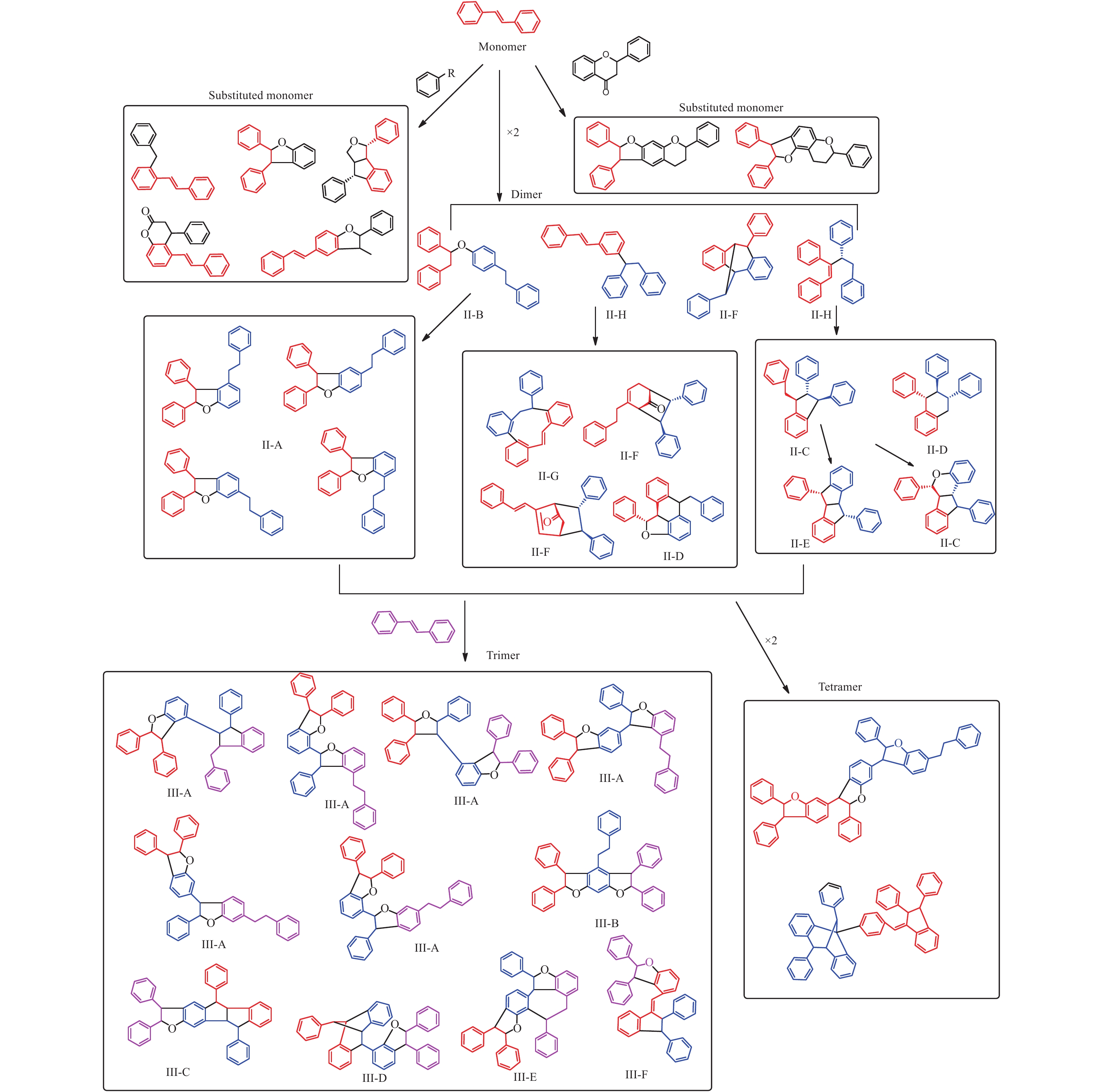
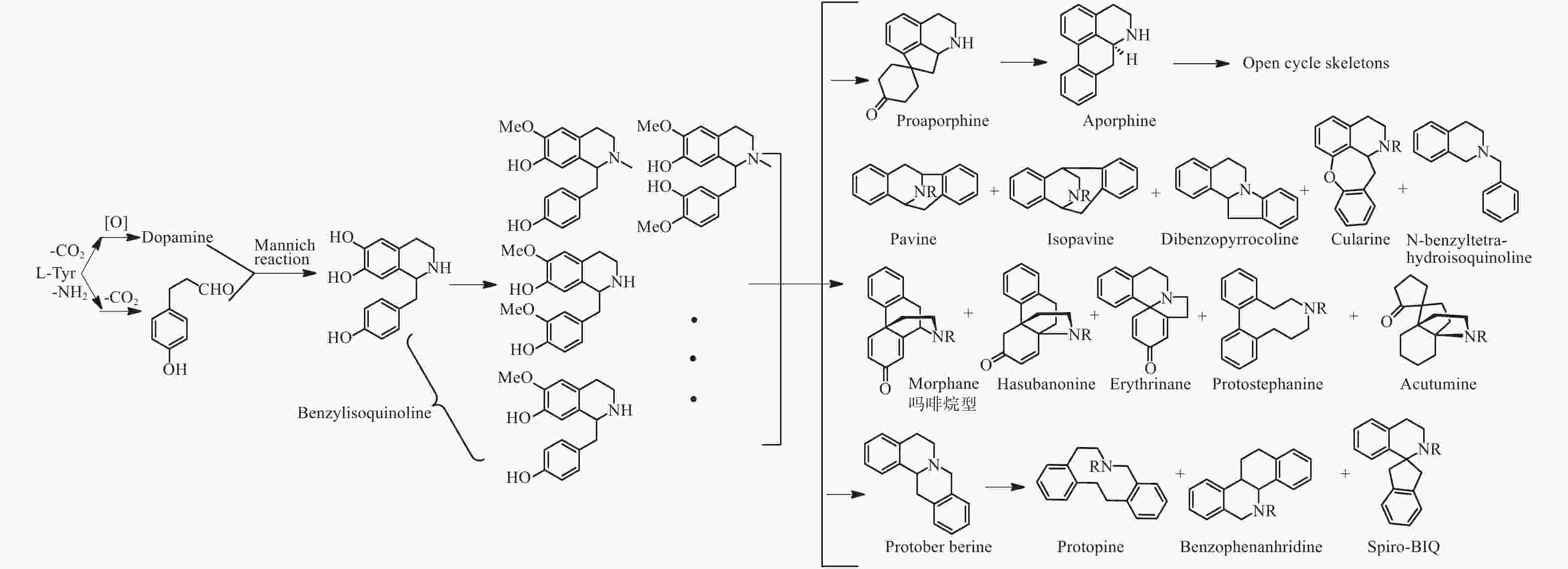
苄基异喹啉生物碱是在被子植物中广泛存在的一类生物碱,其合成途径主要是来源于两分子的酪氨酸,经过脱羧、氧化和Mannich类似反应生成去甲乌药碱和乌药碱,并在此基础上合成原小檗碱型、阿朴菲型、吗啡烷型、普罗托品型和苯菲啶型等骨架类型生物碱并两个相同的或不同的骨架连接形成二聚体(图2)[24]。据2005年统计电子数据库《Dictionary of Natural Products》一共涉及超过1 800个次的苄基异喹啉类生物碱,涉及23个被子植物科,其中以位于被子植物基部类群的木兰科、番荔枝科、樟科、防己科、小檗科等木兰亚纲的科居多[25],且结构类型变化多样,除了甲乌药碱,乌药碱等简单苄基异喹啉生物碱广泛分布外,结构类型涉及所有已知的苄基异喹啉生物碱骨架,且有大量的二聚体[26]。而在裸子植物中三尖杉属植物中有报道苯乙基异喹啉类生物碱,由于其合成途径与苄基异喹啉完全不同,在此不做讨论。
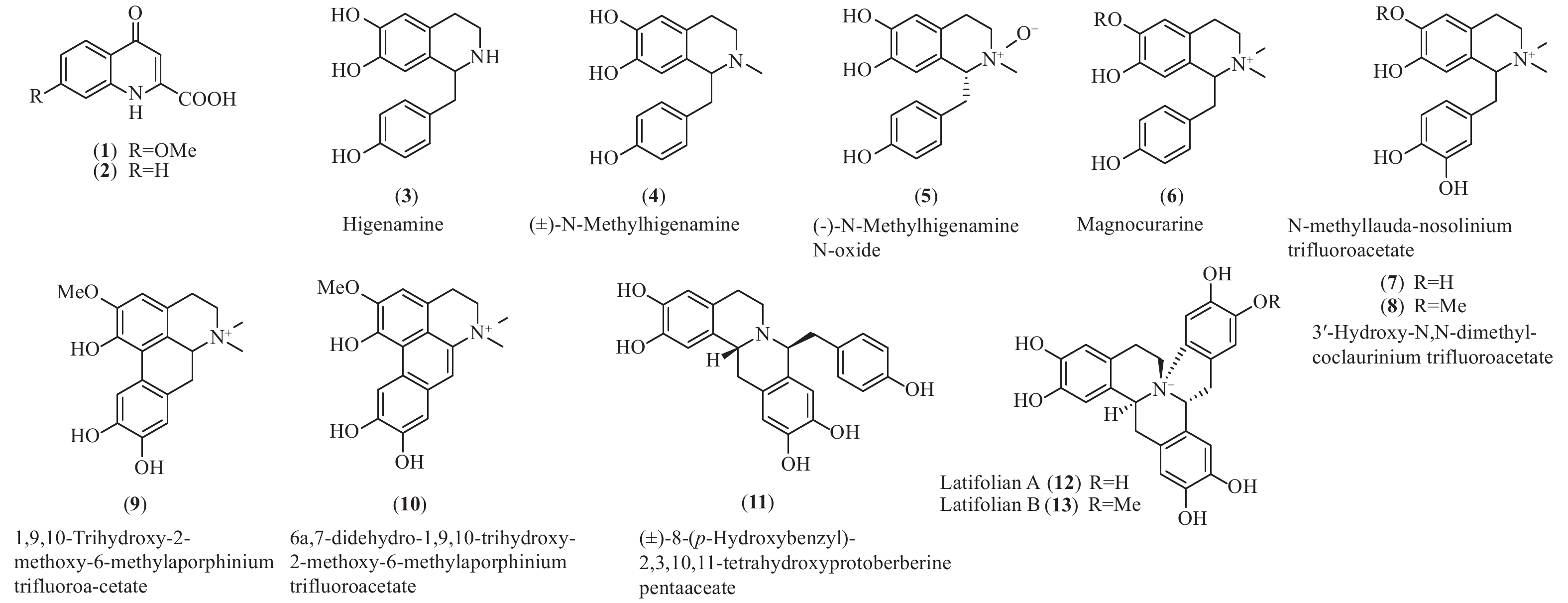
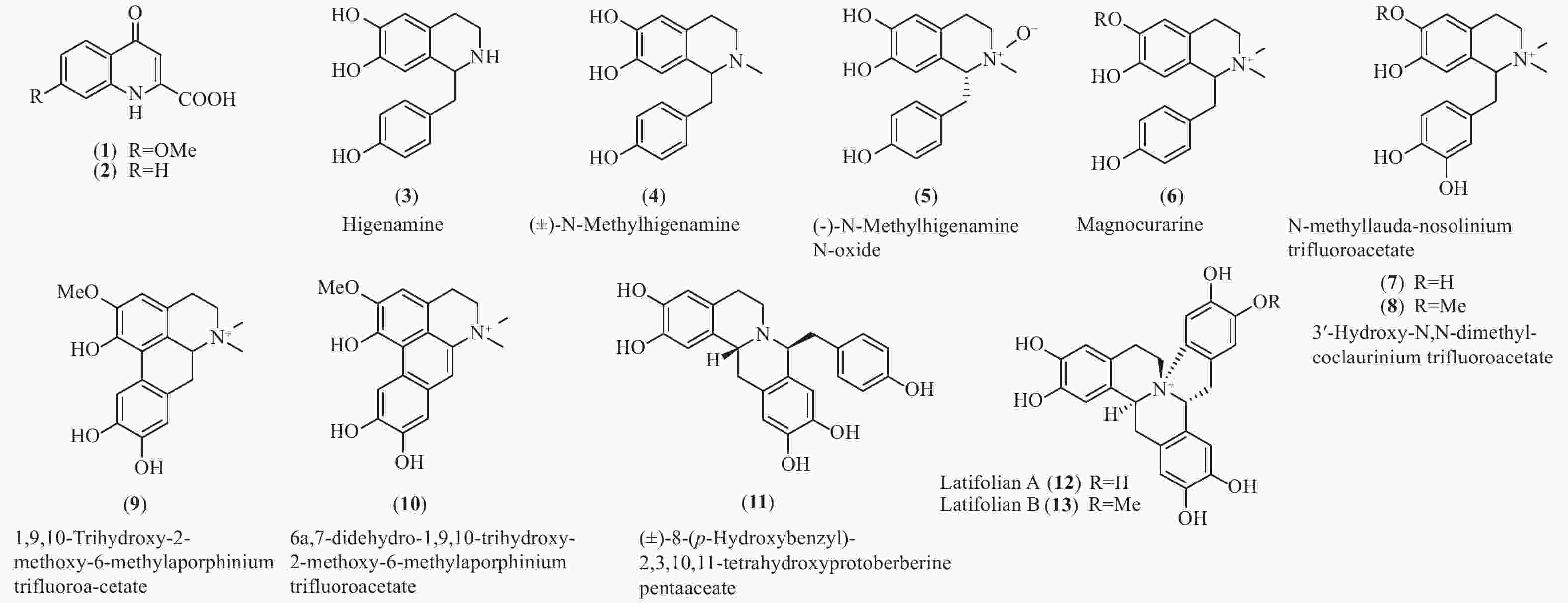
买麻藤目中只有买麻藤属报道有苄基异喹啉类生物碱,而麻黄属中报道的多是不同生物合成来源的苯乙胺类生物碱和少量喹啉类生物碱(1和2)[27-28],百岁兰属未见报道。通过分析买麻藤属的生物碱的结构和数量(图3和表1),不难发现,买麻藤属植物中的苄基异喹啉类生物碱结构相对简单,多为简单类型如苄基异喹啉类(3−8)、阿朴菲类(9和10)和原小檗碱类(11−13),可以明显的看出苄基异喹啉类生物碱在买麻藤目植物向被子植物进化的过程中,应该是起始于买麻藤目,并在被子植物中苄基异喹啉类生物碱的类型及其生物合成途径得以演化和发展。
来源植物
Plant source化学成分
Constituent部位
Plant partsEphedra alata. Ephedrolone (1) 全株Whole plants[27] E. transitoria Transtorine (2) 地上部分Aerial part[28] Gnetum parvifolium Higenamine (3) 茎Stem[29- 30] (±)-N-Methylhigenamine (4) 藤茎Liana[30] (-)-N-Methylhigenamine N-oxide (5) 藤茎Liana[30] (±)-8-(p-Hydroxybenzyl)- 2,3,10,11-tetrahydroxyprotoberberine pentaaceate (11) 藤茎Liana[30] G. latifolium Latifolian A (12) 藤茎叶Vine[31] Latifolian B (13) 藤茎叶Vine[31] G. montanum Magnocurarine (6) 藤茎叶Vine[32] N-Methyllauda-nosolinium trifluoroacetate (7) 藤茎叶Vine[32] 3′-Hydroxy-N,N-dimethyl-coclaurinium trifluoroacetate (8) 藤茎叶Vine[32] 1,9,10-Trihydroxy-2-methoxy-6-methylaporphinium trifluoroa-cetate (9) 藤茎叶Vine[32] 6a,7-Didehydro-1,9,10-trihydroxy-2-methoxy-6-methylaporphinium trifluoroacetate (10) 藤茎叶Vine[32] Latifolian A (12) 藤茎叶Vine[32] Table 1. Quilolines and benzylisoquinoline alkaloid isolated from Gnetales plants
-
菧类是种子植物中大量存在的一类酚性成分,其结构从简单的菧类单体、到二聚体、三聚体等等,甚至八聚体都有分布。通过调查文献发现,在裸子植物中,特别是针叶植物中只发现有少量的简单菧类单体类成分如白皮杉醇(piceatanol 19),赤松素(pinosylvine 27),(E)-3,5-dimethoxystilbene,(Z)-3,5-dimethoxystilbene,3-hydroxy-5-methoxybibenzyl和(E)-3-hydroxy-5-methoxystilbene[33]。亦有报道上新世(Pliocene)华山松Pinus armandii木质部的化石中也分离到简单的菧类成分(E)-3,5-dimethoxystilben和(Z)-3,5-dimethoxystilbene[34]。
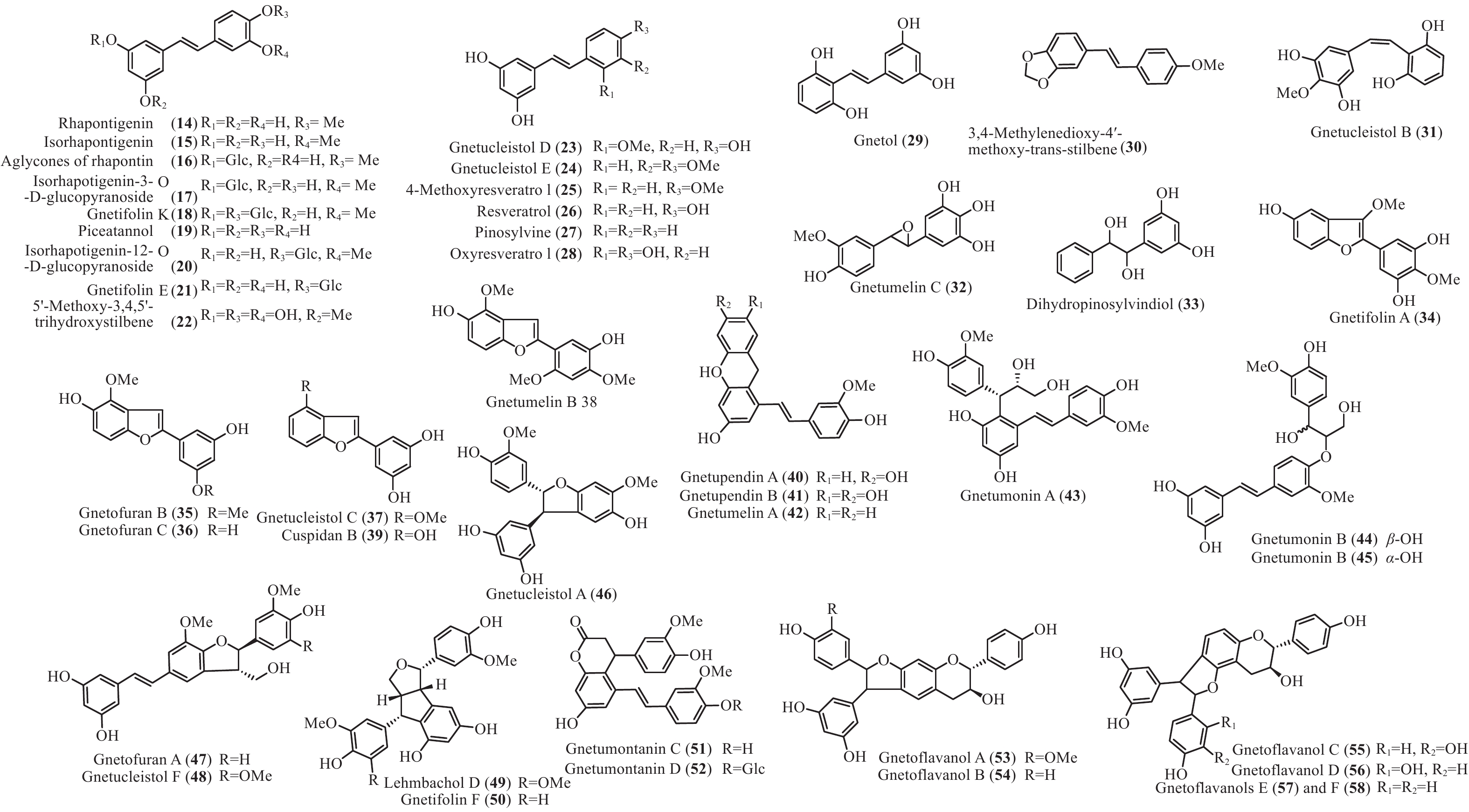
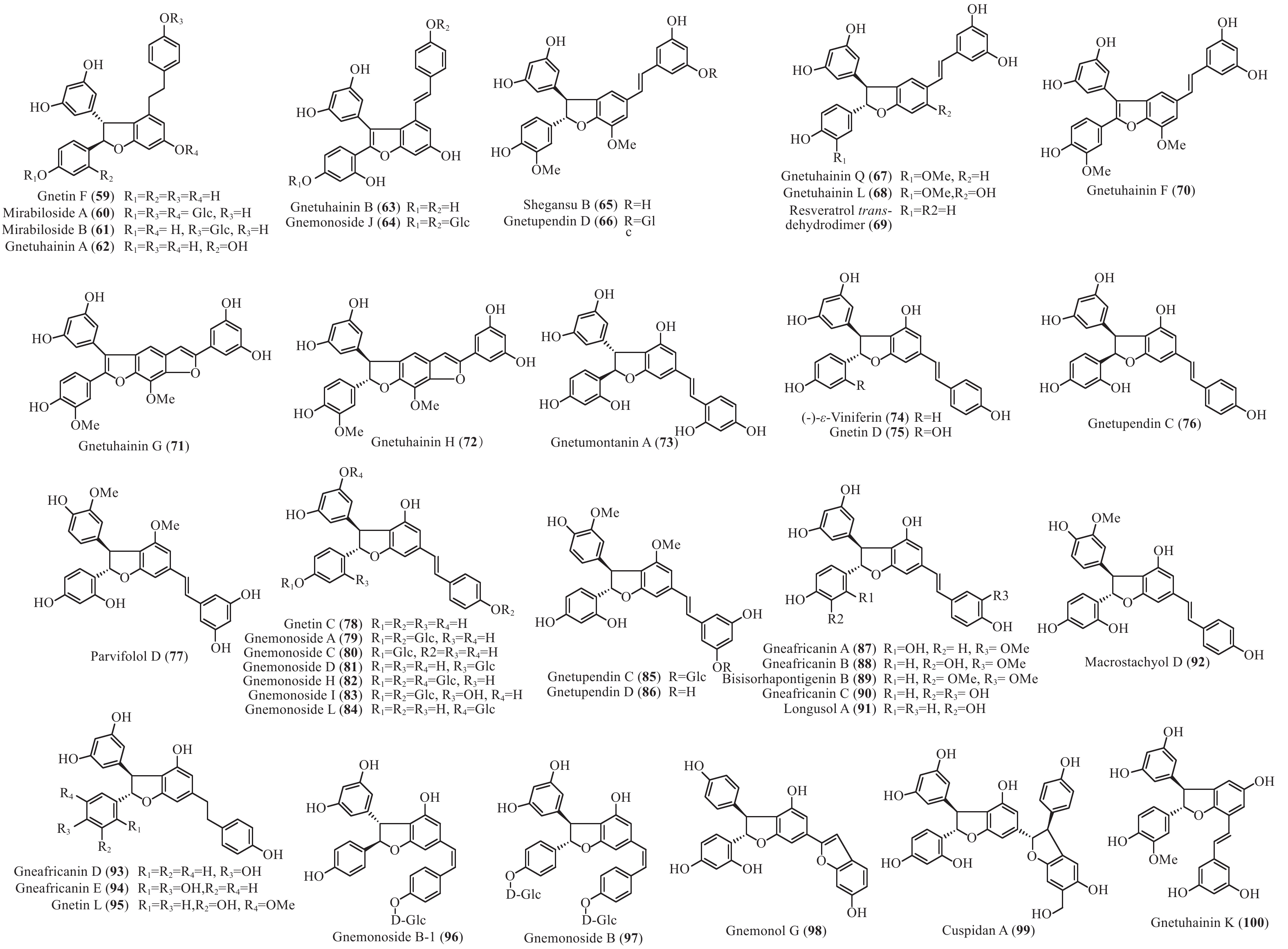
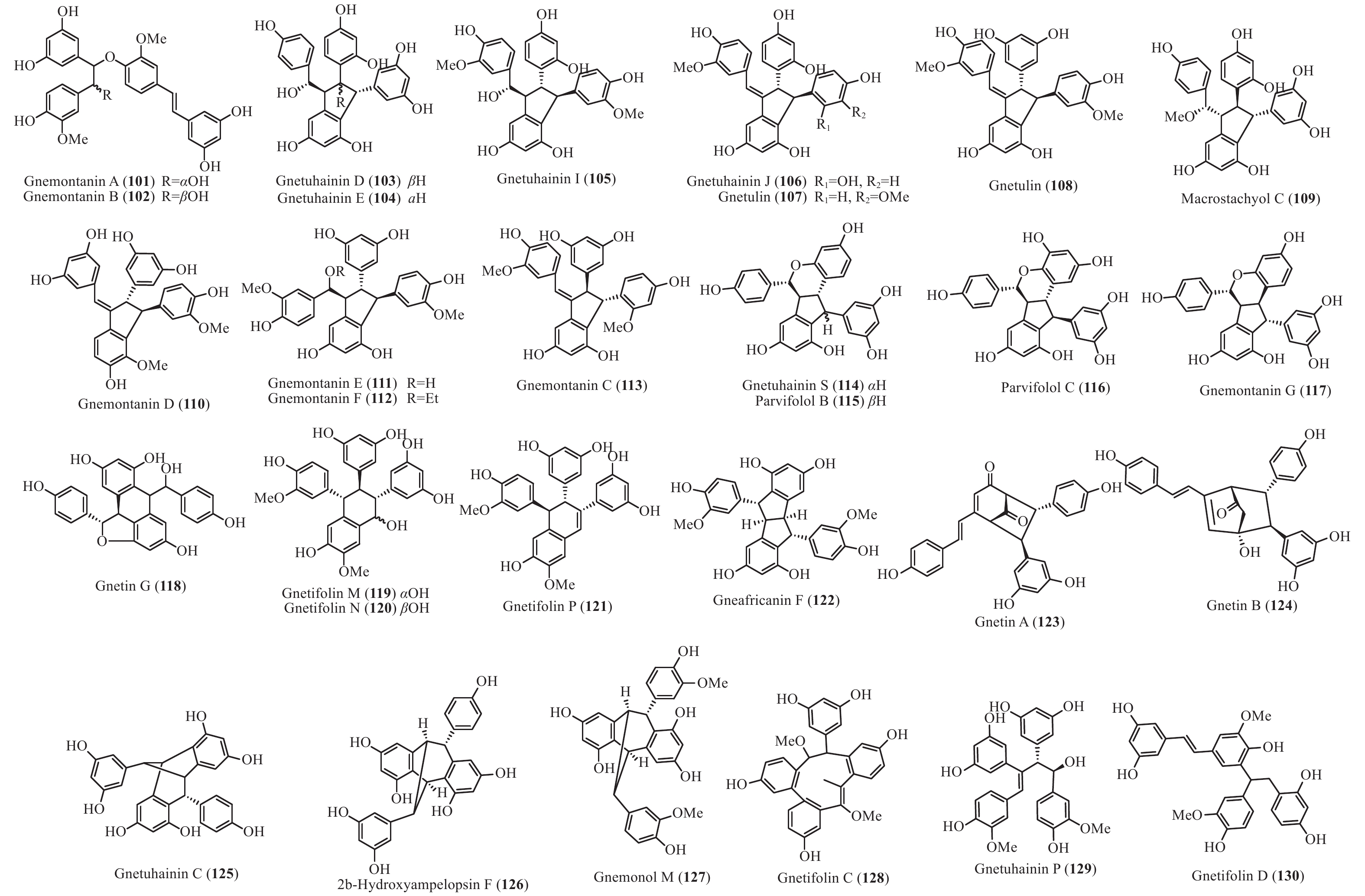
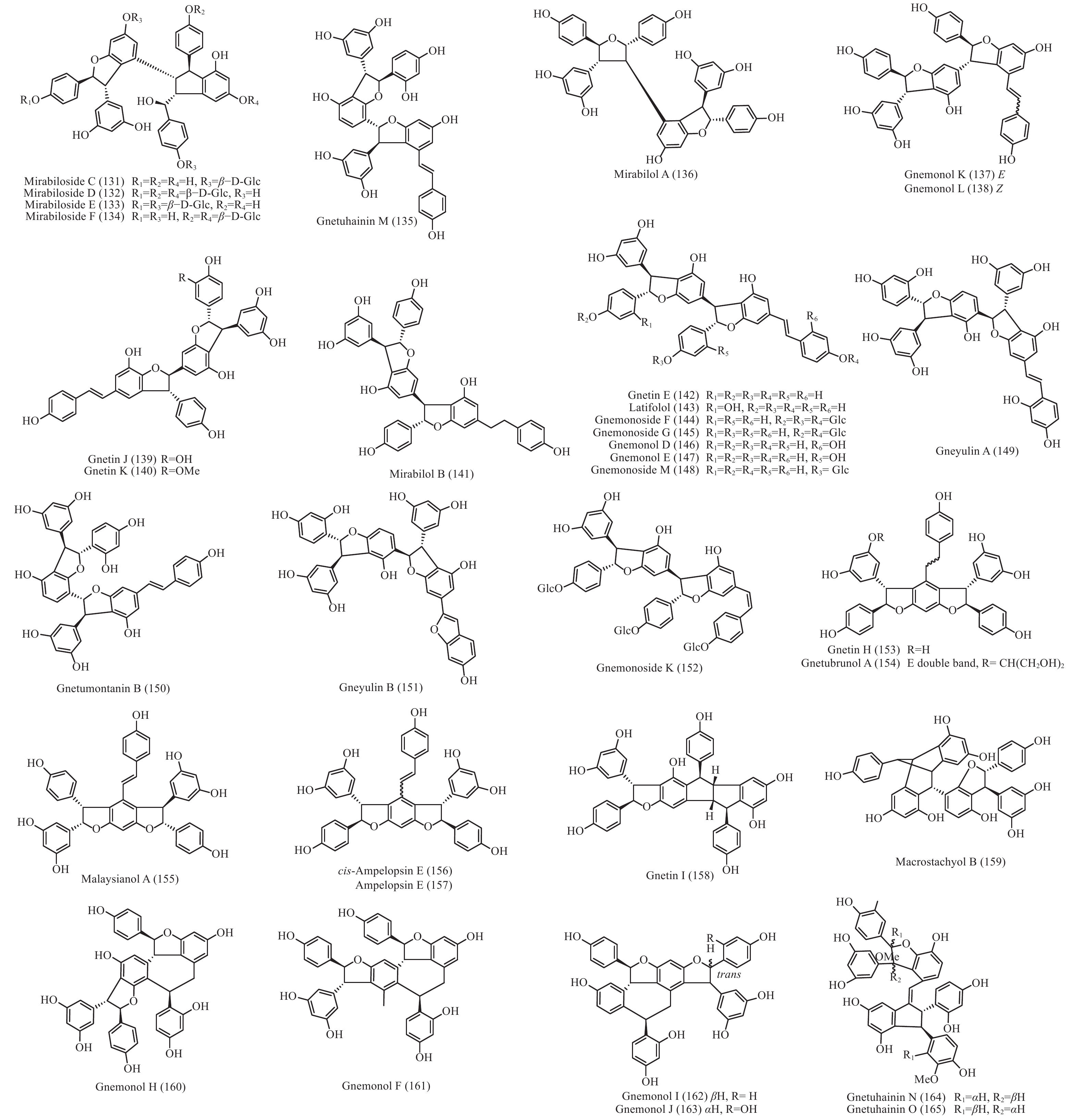
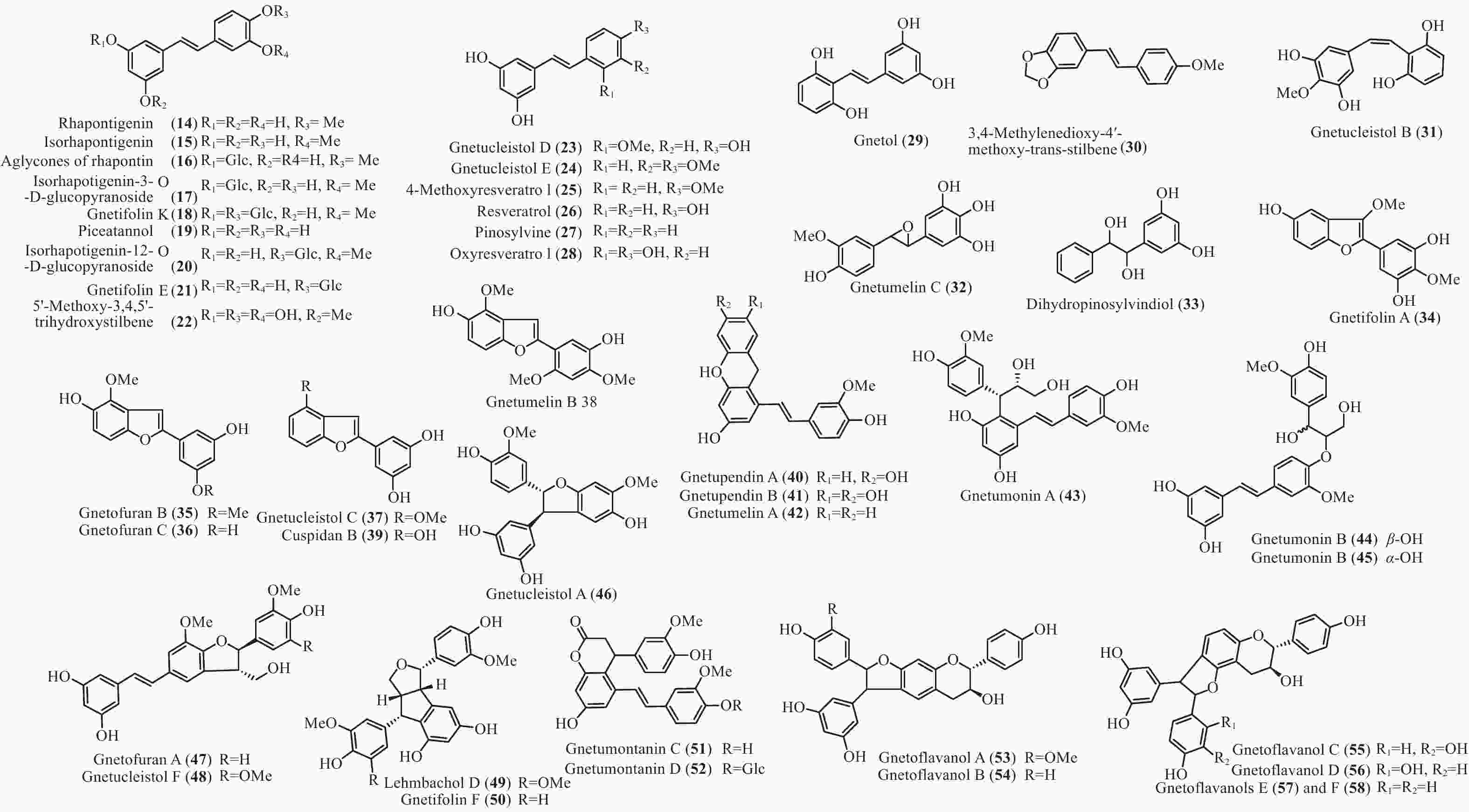
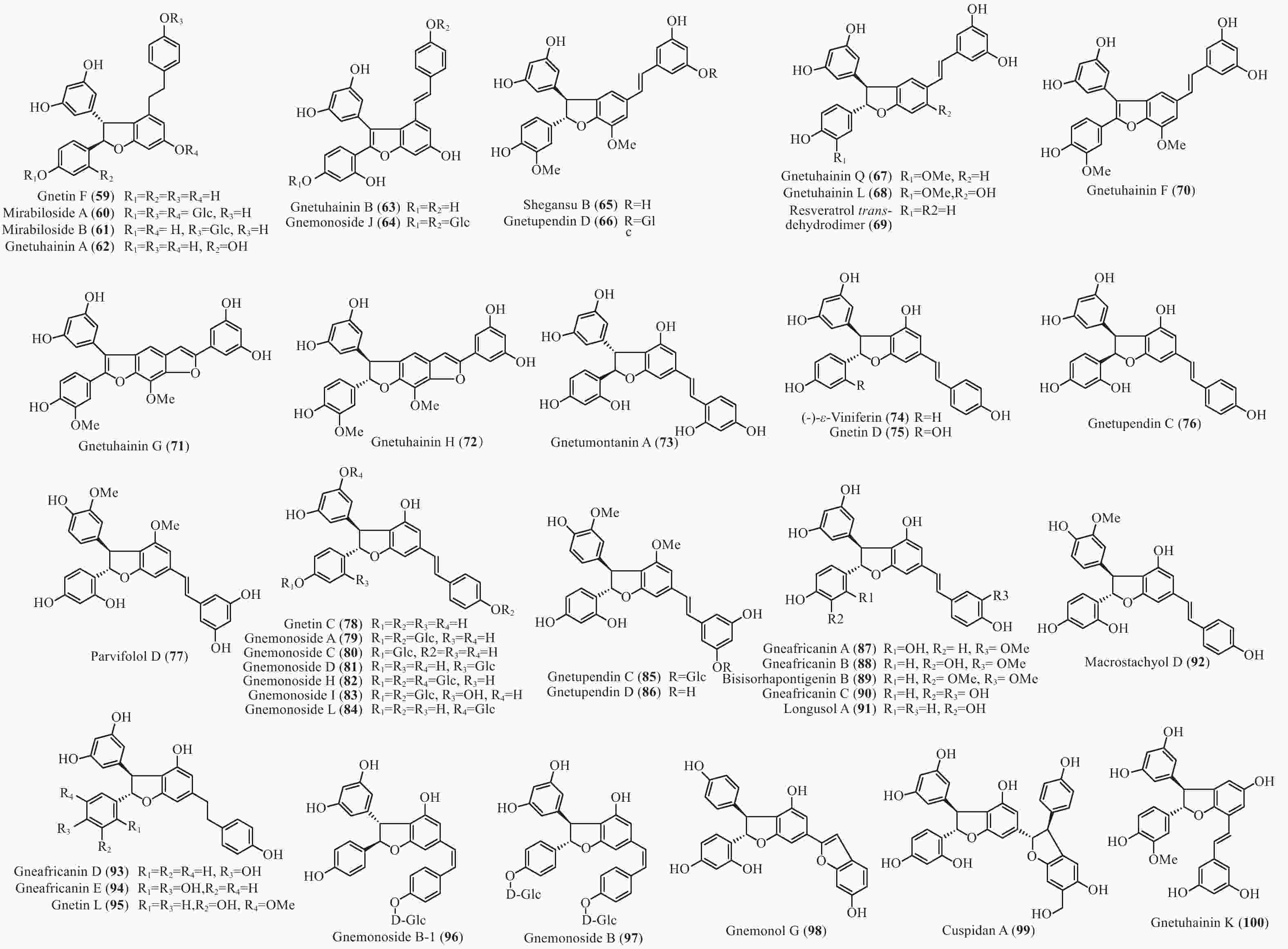
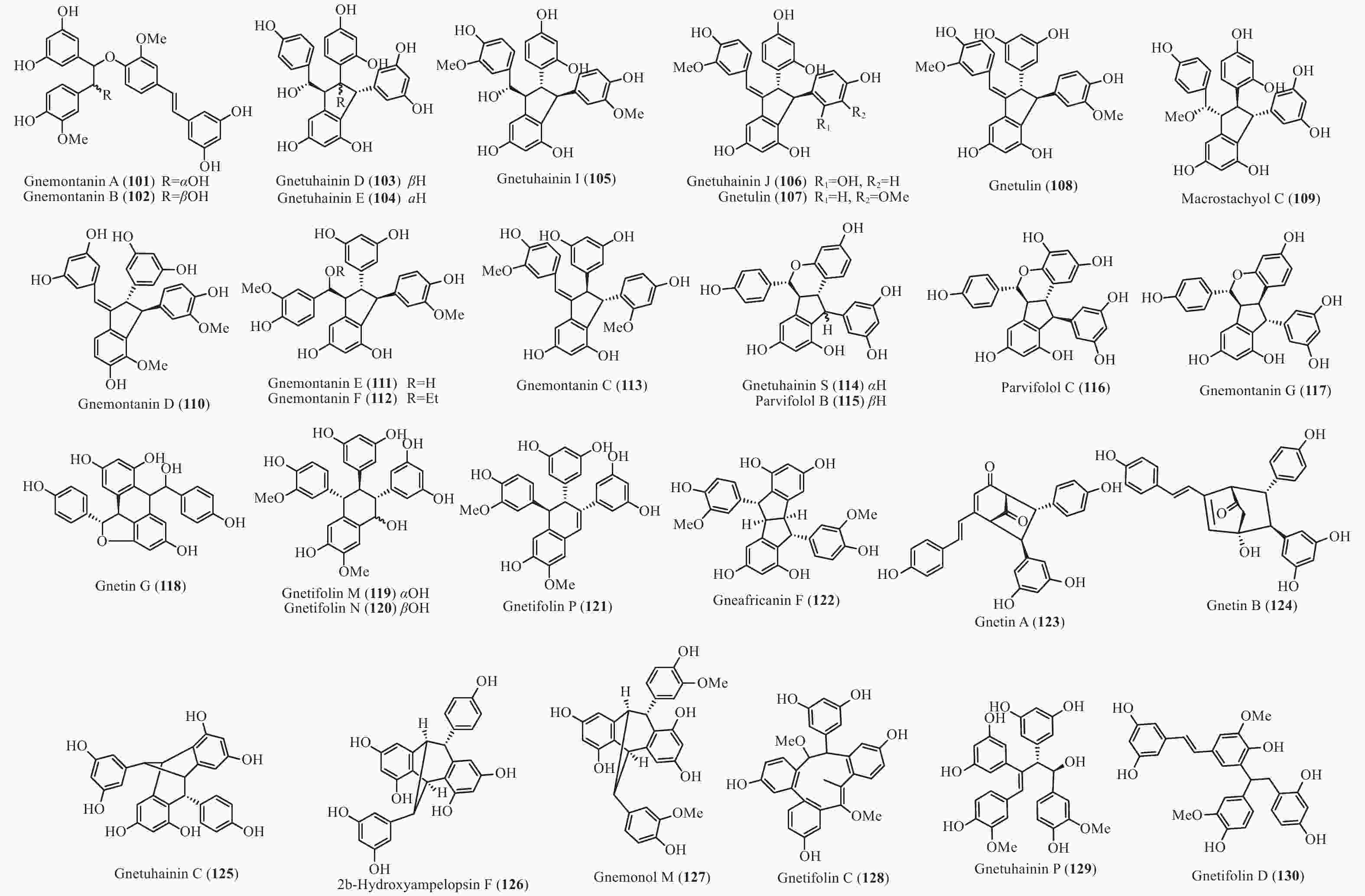
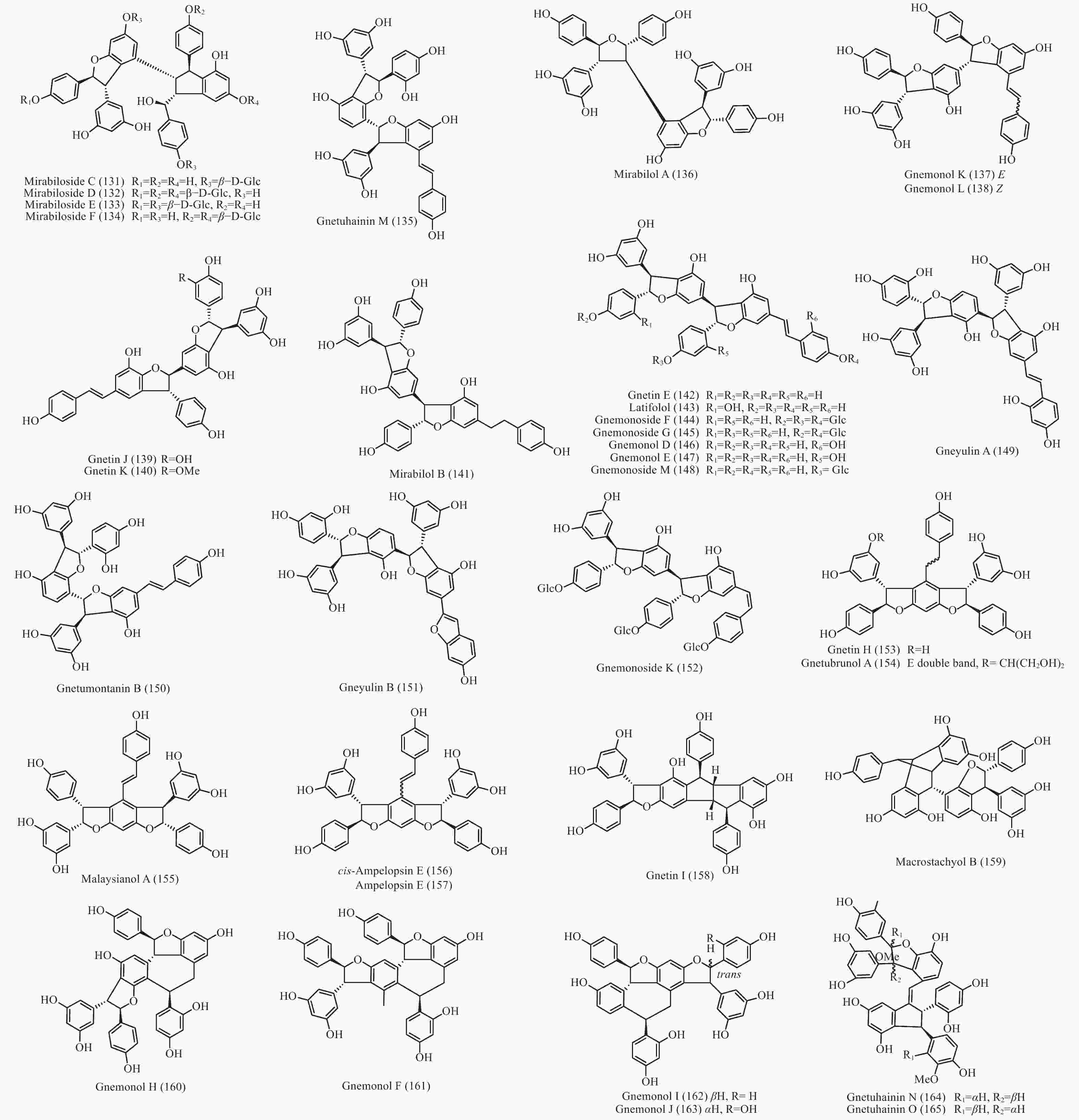
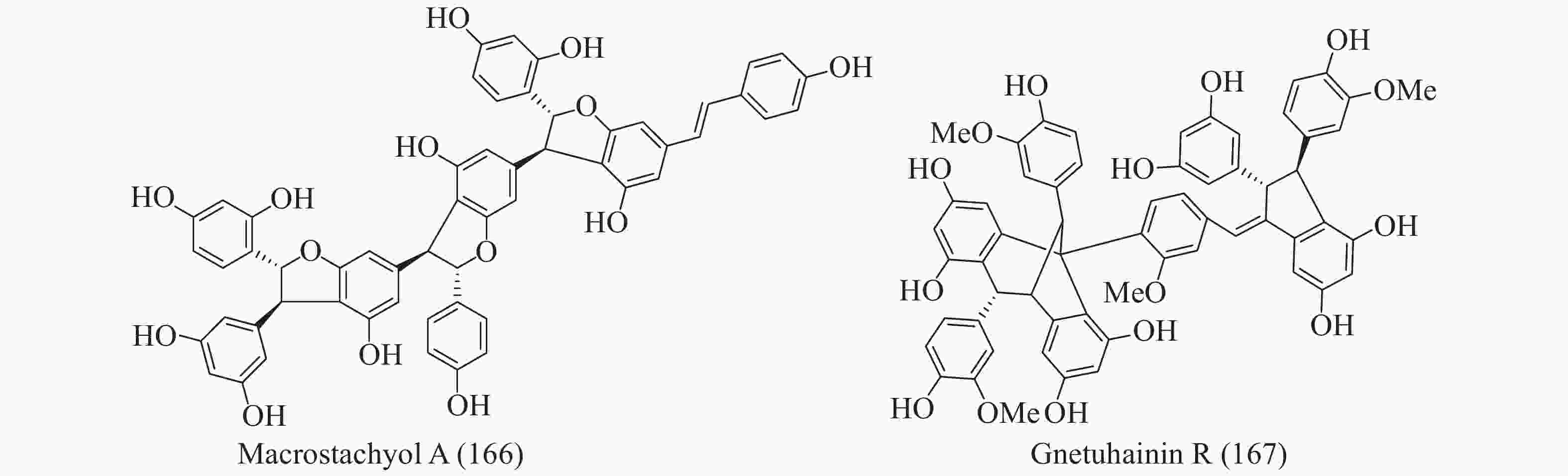
买麻藤目中报道的菧类成分主要来自百岁兰属和买麻藤属,买麻藤属由于现存种类较多,至2018年5月,通过Scifinder数据库检索发现,有菧类成分研究的买麻藤属植物含变种有19个,百岁兰属植物1个,所以报道的买麻藤目菧类成分也特别多,共150个(表2)。化合物类型包括了简单菧类I、二聚菧类II、三聚菧类III和四聚菧类IV(图4-8)。简单的菧类I成分41个(图4),主要是菧类的单体及其苷(14−39),苄基或苯基取代物(40−42),与苯丙素的聚合物(43−52)和与黄烷的聚合物(53−58),其中部分化合物如丹叶大黄素(rhapontigenin 14)、异丹叶大黄素(isorhapontigenin 15)、白皮杉醇(piceatannol 19)、白藜芦醇(resveratrol 26)、买赤松素(pinosylvine 27)和买麻藤醇(gnetol 29)等,作为合成菧类聚合物的前体广泛分布;二聚菧类成分最多有106个,根据其结构差异分为8类:II-A苯并呋喃型有最多42个(59−100), 结构见图5,II-B简单氧连接型(101−102),II-C苯并环丙烷型 (103-117),II-D四氢萘型 (118−121),II-E并二茚型 (122),II-F桥环型 (123−127),II-G大环型 (128)和简单碳连接型II-H(129和130),结构见图6;三聚菧类(结构见图7)成分相对较复杂,共有35个(131−165),将其分为5类:III-A双苯并呋喃型(131−152), III-B苯并双呋喃型 (153−157),III-C苯并呋喃-并二茚型(158),III-D苯并呋喃-桥环型(159),III-E苯并环庚烷型 (160−163),III-F苯并呋喃-苯并环丙烷型 (164−165);四聚菧类IV(结构见图8)有2个(166和167)。其不同结构类型的生源关系进行了推导和关联,结构见图9。菧类的生物合成途径应该是起始于简单菧类,如丹叶大黄素(rhapontigenin 14)和白藜芦醇(resveratrol 26)等,两两结合形成不同骨架类型的二聚体,并在二聚体基础上加入新的菧类单元合成三聚体,或二聚体两两结合合成四聚体。
来源植物
Plant source化学成分
Constituent类型
Type部位
Plant partsWelwischia mirabilis Gnetin F (59) Dimer 木质部Wood[35], 茎、根stem and root[36] Gnetin G (118) Dimer 木质部Wood[35], 茎、根stem and root[37] Gnetin H (153) Trimer 木质部Wood[35] Gnetin I (158) Trimer 木质部Wood[35], 茎、根stem and root[36] Mirabiloside A (60) Dimer 茎、根stem and root[36] Mirabiloside B (61) Dimer 茎、根stem and root[36] Mirabilol A (136) Trimer 茎、根stem and root[36] Mirabilol B (141) Trimer 茎、根stem and root[36] Gnetin C (78) Dimer 茎、根stem and root[36] Gnetin E (142) Trimer 茎、根stem and root[36] Mirabiloside C (131) Trimer 茎、根stem and root[37] Mirabiloside D (132) Trimer 茎、根stem and root[37] Mirabiloside E (133) Trimer 茎、根stem and root[37] Mirabiloside F (134) Trimer 茎、根stem and root[37] Resveratrol (26) Monomer 茎、根stem and root[37] Gnemonoside B-1 (96) Dimer 茎、根stem and root[37] Gnetum africanum Gneafricanin A (87) Dimer 茎stem[38] Gneafricanin B (88) Dimer 茎stem[38] Bisisorhapontigenin B (89) Dimer 茎stem[38] Longusol A (91) Dimer 茎stem[38] Gnetin C (78) Dimer 茎stem[38] Gnetin D (75) Dimer 茎stem[38] Gnetin E (142) Trimer 茎stem[38] Gnetifolin E (21) Monomer 茎stem[38] Gnetol (29) Monomer 茎stem[38] Isorhapontigenin (15) Monomer 茎stem[38] Resveratrol (26) Monomer 茎stem[38] Gneafricanin C (90) Dimer 茎stem[39] Gneafricanin D (93) Dimer 茎stem[39] Gneafricanin E (94) Dimer 茎stem[39] Gneafricanin F (121) Dimer 茎stem[39] Gnemonoside H (82) Dimer 茎stem[40] Gnemonoside I (83) Dimer 茎stem[40] Gnemonoside J (64) Dimer 茎stem[40] Gnetoflavanol A (53) Monomer 茎stem[41] Gnetoflavanol B (54) Monomer 茎stem[41] Gnetoflavanol C (55) Monomer 茎stem[41] Gnetoflavanol D (56) Monomer 茎stem[41] G. brunonianum Gnetubrunol A (154) Trimer 藤茎Liana[42] Shegansu B (65) Dimer 藤茎Liana[42] Resveratrol (26) Monomer 藤茎Liana[42] Isorhapontigenin (15) Monomer 藤茎Liana[42] Gnetifolin E (21) Monomer 藤茎Liana[42] Isorhapontigenin-12-O-D-glucopyranoside (20) Monomer 藤茎Liana[42] G. cleistostachyum Gnetucleistol D (23) Monomer 藤茎Liana[43] Gnetucleistol E (24) Monomer 藤茎Liana[43] Rhapontigenin (14) Monomer 藤茎Liana[43] Isorhapontigenin (15) Monomer 藤茎Liana[43] 4-Methoxyresveratrol (25) Monomer 藤茎Liana[43] Pinosylvine (27) Monomer 藤茎Liana[43] Gnetucleistol A (46) Monomer 藤茎Liana[44] Gnetucleistol B (31) Monomer 藤茎Liana[44] Gnetucleistol C (37) Monomer 藤茎Liana[44] Gnetifolin A (34) Monomer 藤茎Liana[44] Resveratrol (26) Monomer 藤茎Liana[44] Piceatannol (19) Monomer 藤茎Liana[44] Gnetucleistol F (48) Monomer 藤茎Liana[45] Gnetofuran A (47) Monomer 藤茎Liana[45] Lehmbachol D (49) Monomer 藤茎Liana[45] Gnetifolin F (58) Monomer 藤茎Liana[45] Gnetumontanin C (51) Monomer 藤茎Liana[45] Shegansu B (65) Dimer 藤茎Liana[45] Gnetupendin B (41) Monomer 藤茎Liana[45] Gnetol (29) Monomer 藤茎Liana[45] G. cuspidatum Isorhapontigenin (15) Monomer 茎stem[46] Rhapontigenin (14) Monomer 茎stem[46] Aglycones of rhapontin (16) Monomer 茎stem[46] Isorhapotigenin-3-O-D-glucopyranoside (17) Monomer 茎stem[46] Cuspidan A (99) Dimer 树皮Bark[47] Cuspidan B (39) Monomer 树皮Bark[47] G. gnemon Gnemonol G (95) Dimer 根root[48] Gnemonol H (160) Trimer 根root[48] Gnemonol I (164) Trimer 根root[48] Gnemonol J (163) Trimer 根root[48] Ampelopsin E (157) Trimer 根root[48] cis-Ampelopsin E (156) Trimer 根root[48] Gnetin C (78) Dimer 根root[48], 胚乳endosperm[49], 果实fruit[50] Gnetin D (75) Dimer 根root[48] Gnetin E (142) Trimer 根root[48], 果实fruit[50] Gnemonol D (146) Trimer 根root[51] Gnemonol E (147) Trimer 根root[51] Gnemonol F (161) Trimer 根root[51] Gnemonol K (137) Trimer 根root[52] Gnemonol L (138) Trimer 根root[52] Gnemonol M (127) Dimer 根root[52] Gnemonoside K (152) Trimer 根root[52] (-)-ε-viniferin (74) Dimer 根root[52] Gnetol (29) Monomer 根root[52] Isorhapontigenin (15) Monomer 根root[52] Gnetifolin E (21) Monomer 根root[52], 果实fruit[50] Isorhapontigenin-3-O-D-glucopyranoside (17) Monomer 根root[52] Resveratrol (26) Monomer 根root[52], 胚乳endosperm[49], 果实fruit[50] Gnemonoside A (79) Dimer 根root[52], 胚乳endosperm[49] Gnemonoside B (97) Dimer 根root[52] Gnemonoside F (144) Trimer 根root[52] Latifolol (143) Trimer 根root[52] Gnetoflavanol E (57) Monomer 茎stem[41] Gnetoflavanol F (58) Monomer 茎stem[41] Gnetin L (95) Dimer 胚乳endosperm[49] Gnemonoside C (80) Dimer 胚乳endosperm[49], 果实fruit[50] Gnemonoside D (81) Dimer 胚乳endosperm[49], 果实fruit[50] Gnemonoside L (84) Dimer 果实Fruit[50] Gnemonoside M (148) Trimer 果实Fruit[50] G. gnemonoides Gnemonoside A (79) Dimer 茎stem[53] Gnemonoside B (97) Dimer 茎stem[53] Gnemonoside C (80) Dimer 茎stem[53] Gnemonoside D (81) Dimer 茎stem[53] Resveratrol (26) Monomer 茎stem[53] Gnetin C (78) Dimer 茎stem[53] Gnetin D (75) Dimer 茎stem[53] Gnetin E (142) Trimer 茎stem[53] Gnemonoside F (144) Trimer 茎stem[40] Gnemonoside G (145) Trimer 茎stem[40] Gnemonoside H (82) Dimer 茎stem[40] Gneyulin A (149) Trimer 树皮Bark[54] Gneyulin B (151) Trimer 树皮Bark[54] G. klossii Gnetofuran A (47) Monomer 茎stem[55] Gnetofuran B (35) Monomer 茎stem[55] Gnetofuran C (36) Monomer 茎stem[55] Dihydropinosylvindiol (33) Monomer 茎stem[55] Gnetifolin F (50) Monomer 茎stem[55] Isorhapontigenin (15) Monomer 茎stem[55] Gnetulin (108) Dimer 茎stem[55] Gnetin E (142) Trimer 茎stem[55] Gnetin C (78) Dimer 茎stem[55] Latifolol (143) Trimer 茎stem[55] Gnetol (29) Monomer 茎stem[55] (-)-ε-Viniferin (74) Dimer 茎stem[55] Resveratrol (26) Monomer 茎stem[55] G. hainanense Gnetuhainin A (62) Dimer 藤茎Liana[56] Gnetuhainin B (63) Dimer 藤茎Liana[56] Gnetuhainin C (124) Dimer 藤茎Liana[56] Gnetuhainin D (103) Dimer 藤茎Liana[56] Gnetuhainin E (104) Dimer 藤茎Liana[56] Resveratrol trans-dehydrodimer (69) Dimer 藤茎Liana[56] Resveratrol (26) Monomer 藤茎Liana[56] Oxyresveratrol (28) Monomer 藤茎Liana[56] (-)-ε-Viniferin (71) Dimer 藤茎Liana[56] Gnetuhainin F (70) Dimer 藤茎Liana[57] Gnetuhainin G (71) Dimer 藤茎Liana[57] Gnetuhainin H (72) Dimer 藤茎Liana[57] Gnetuhainin I (105) Dimer 藤茎Liana[57] Gnetuhainin J (106) Dimer 藤茎Liana[57] Gnetulin (108) Dimer 藤茎Liana[57] Rhapontigenin (14) Monomer 藤茎Liana[57] Isorhapontigenin (15) Monomer 藤茎Liana[57] Gnetol (29) Monomer 藤茎Liana[57] Gnetuhainin P (129) Dimer 藤茎Liana[58- 59] Gnetuhainin M (135) Trimer 藤茎Liana[60] Gnetuhainin N (164) Trimer 藤茎Liana[60] Gnetuhainin O (165) Trimer 藤茎Liana[60] Gnetuhainin Q (67) Dimer 藤茎Liana[59] Gnetuhainin K (100) Dimer 藤茎Liana[59] Gnetuhainin L (68) Dimer 藤茎Liana[59] Gnetuhainin R (167) Tetramer 藤茎Liana[61] Gnetuhainin S (114) Dimer 藤茎Liana[61] G. latifolium Latifolol (143) Trimer 茎stem[62] Gnetin C (78) Dimer 茎stem[62] Gnetin D (75) Dimer 茎stem[62] Gnetin E (142) Trimer 茎stem[62] (-)-ε-Viniferin (74) Dimer 茎stem[62] Resveratrol (26) Monomer 茎stem[62] G. leyboldii Gnetin A (123) Dimer 茎stem[63- 64] Gnetin B (124) Dimer 茎stem[64] Gnetin C (78) Dimer 茎stem[64] Gnetin D (75) Dimer 茎stem[64] Gnetin E (142) Trimer 茎stem[64] G. macrostachyum Macrostachyol A (166) Tetramer 根root[65] Macrostachyol B (159) Trimer 根root[65] Macrostachyol C (109) Dimer 根root[65] Macrostachyol D (82) Dimer 根root[65] Gnetuhainin S (parvifolol A) (114) Dimer 根root[65] Resveratrol (26) Monomer 根root[65] Latifolol (143) Trimer 根root[65] Gnetol (29) Monomer 根root[65] Isorhapontigenin (15) Monomer 根root[66] Bisisorhapontigenin B (89) Dimer 根root[65] G. montanum Gnetifolin M (119) Dimer 藤茎Liana[67-68] Gnetifolin N (120) Dimer 藤茎Liana[67] Gnetifolin E (21) Monomer 藤茎Liana[69] Gnetol (29) Monomer 藤茎Liana[69] Isorhapontigenin-3-O-D-glucopyranoside (17) Monomer 藤茎Liana[69] (-)-ε-Viniferin (74) Dimer 藤茎Liana[69] Gnetifolin C (128) Dimer 藤茎Liana[69] Resveratrol (26) Monomer 藤茎Liana[68] Gnemontanin A (101) Dimer 茎stem[70] Gnemontanin B (102) Dimer 茎stem[70] Gnemontanin C (113) Dimer 茎stem[70] Gnemontanin D (110) Dimer 茎stem[70] Gnemontanin E (111) Dimer 茎stem[70] Gnemontanin F (112) Dimer 茎stem[70] Gnemontanin G (117) Dimer 茎stem[70] Gnetuhainin P (129) Dimer 茎stem[70] Gnetuhainin I (105) Dimer 茎stem[70] Gnetumonin A (43) Monomer 茎stem[71] Gnetumonin B (44) Monomer 茎stem[71] Gnetumonin C (45) Monomer 茎stem[71] (−)-Gnetucleistol F (48) Monomer 茎stem[71] Gnetupendin A (40) Monomer 茎stem[71] (+)-Gnetofuran A (47) Monomer 茎stem[71] G. montanum f. megalocarpum Gnetumontanin A (73) Dimer 藤茎Liana[72] Gnetumontanin B (150) Trimer 藤茎Liana[72] Gnetumontanin C (51) Monomer 藤茎Liana[72] Gnetumontanin D (52) Monomer 藤茎Liana[72] Pinosylvine (27) Monomer 藤茎Liana[72] Gnetifolin A (34) Monomer 藤茎Liana[72] Isorhapontigenin (15) Monomer 藤茎Liana[72] Resveratrol (26) Monomer 藤茎Liana[72] Gnetol (29) Monomer 藤茎Liana[72] Gnetupendin B (41) Monomer 藤茎Liana[72] Shegansu B (65) Dimer 藤茎Liana[72] (-)-ε-Viniferin (74) Dimer 藤茎Liana[72] Gnetulin (105) Dimer 藤茎Liana[72] Gnetin D (75) Dimer 藤茎Liana[72] Gnetuhainin M (135) Trimer 藤茎Liana[72] Isorhapotigenin-3-O-D-glucopyranoside (17) Monomer 藤茎Liana[72] Isorhapotigenin-12-O-D-glucopyranoside (20) Monomer 藤茎Liana[72] Gnetumelin A (42) Monomer 藤茎Liana[73] Gnetumelin B (38) Monomer 藤茎Liana[73] Gnetumelin C (32) Monomer 藤茎Liana[73] Oxyresveratrol (28) Monomer 藤茎Liana[73] Rhapontigenin (15) Monomer 藤茎Liana[73] Gnetifolin E (21) Monomer 藤茎Liana[73] Gnetifolin K (18) Monomer 藤茎Liana[73] Gnetifolin M (119) Dimer 藤茎Liana[73] Gnetofuran B (35) Monomer 藤茎Liana[73] Pinosylvine (27) Monomer 藤茎Liana[73] G. microcarpum Malaysianol A (155) Trimer 藤茎Liana[66] Gnetol (29) Monomer 藤茎Liana[66] Gnetucleistol C (37) Monomer 藤茎Liana[66] Gnetucleistol D (23) Monomer 藤茎Liana[66] Resveratrol (26) Monomer 藤茎Liana[66] (-)-ε-Viniferin (71) Dimer 藤茎Liana[66] G. parvifolium Isorhapontigenin (15) Monomer 茎stem[74- 75] Resveratrol (26) Monomer 茎stem[74- 75] Gnetifolin A (34) Monomer 茎stem[74] Gnetifolin C (128) Dimer 藤茎Liana[75] Gnetifolin D (130) Dimer 藤茎Liana[75] Gnetifolin E (21) Monomer 藤茎Liana[75] Gnetifolin F (50) Monomer 藤茎Liana[76] Pinosylvine (27) Monomer 藤茎Liana[75] Isorhapotigenin-3-O-D-glucopyranoside (17) Monomer 藤茎Liana[75] Gnetol (29) Monomer 藤茎Liana[75] Gnetifolin K (18) Monomer 藤茎Liana[77] (-)-ε-Viniferin (74) Dimer 藤茎Liana[78] Gnetuhainin S (parvifolol A) (114) Dimer 藤茎Liana[79] Parvifolol B (115) Dimer 藤茎Liana[79] Parvifolol C (116) Dimer 藤茎Liana[79] Parvifolol D (77) Dimer 藤茎Liana[79] 2b-Hydroxyampelopsin F (126) Dimer 藤茎Liana[79] Gnetifolin P (121) Dimer 藤茎Lianas[80] Gnetuhainin E (104) Dimer 藤茎Lianas[80] Shegansu B (65) Dimer 藤茎Lianas[80] Gnetulin (108) Dimer 藤茎Lianas[80] (-)-Gneafricanin F (121) Dimer 藤茎Lianas[80] Lehmbachol D (49) Monomer 茎stem[81] G. pendulum Gnetupendin A (40) Monomer 藤茎Liana[82] Gnetupendin B (41) Monomer 藤茎Liana[82] Resveratrol (26) Monomer 藤茎Liana[82] Isorhapontigenin (15) Monomer 藤茎Liana[82] Shegansu B (65) Dimer 藤茎Liana[82] Gnetupendin C (75) Dimer 藤茎Liana[83] Gnetupendin D (65) Dimer 藤茎Liana[84] Gnetulin (108) Dimer 藤茎Liana[84] Gnetin D (75) Dimer 藤茎Liana[84] Isorhapontigenin-3-O-D-glucopyranoside (17) Monomer 藤茎Liana[84] Gnetupendin C (76) Dimer 藤茎Liana[84] Gnetupendin D (66) Dimer 藤茎Liana[84] G. schwackeanum Gnetin C (78) Dimer 果实Fruit[64] Gnetin E (142) Trimer 果实Fruit[64] G. ula 5′-Methoxy-3,4,5'-trihydroxystilbene (22) Monomer 茎stem[85- 86] Gnetol (29) Monomer 茎stem[86] 3,4-Methylenedioxy-4′-methoxy-trans-stilbene (30) Monomer 茎stem[86] Gnetulin (108) Dimer 木质部Wood[87] G. venosum Resveratrol (26) Monomer 果核Fruit kernel[88] Isorhapontigenin (3-methoxyresveratrol) (15) Monomer 果核Fruit kernel[88] Gnetin C (78) Dimer 果核Fruit kernel[88] Gnetin E (142) Trimer 果核Fruit kernel[88] Gnetin J (139) Trimer 果核Fruit kernel[88] Gnetin K (140) Trimer 果核Fruit kernel[88] Table 2. Stilbenes isolated from Gnetales plants
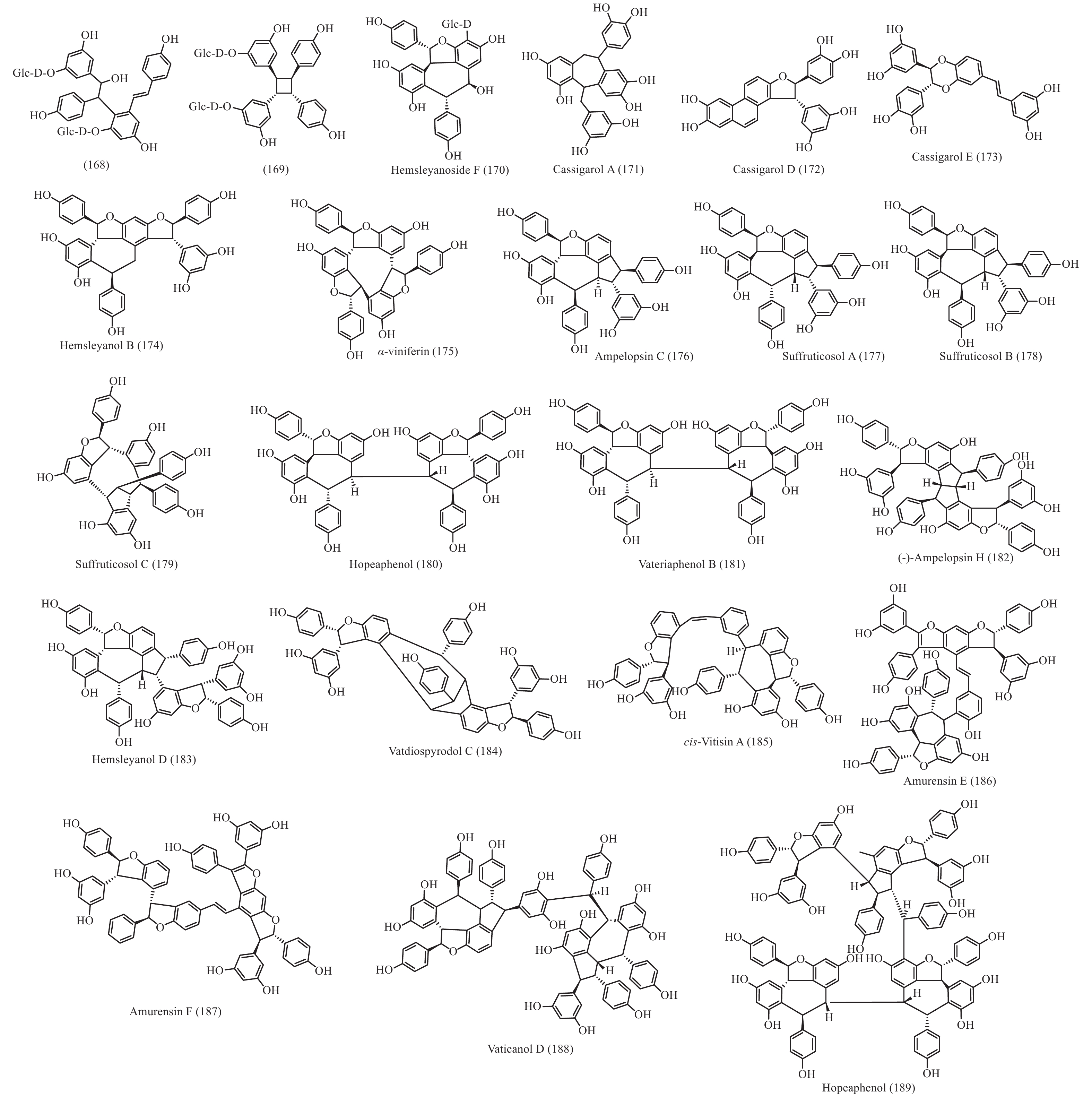
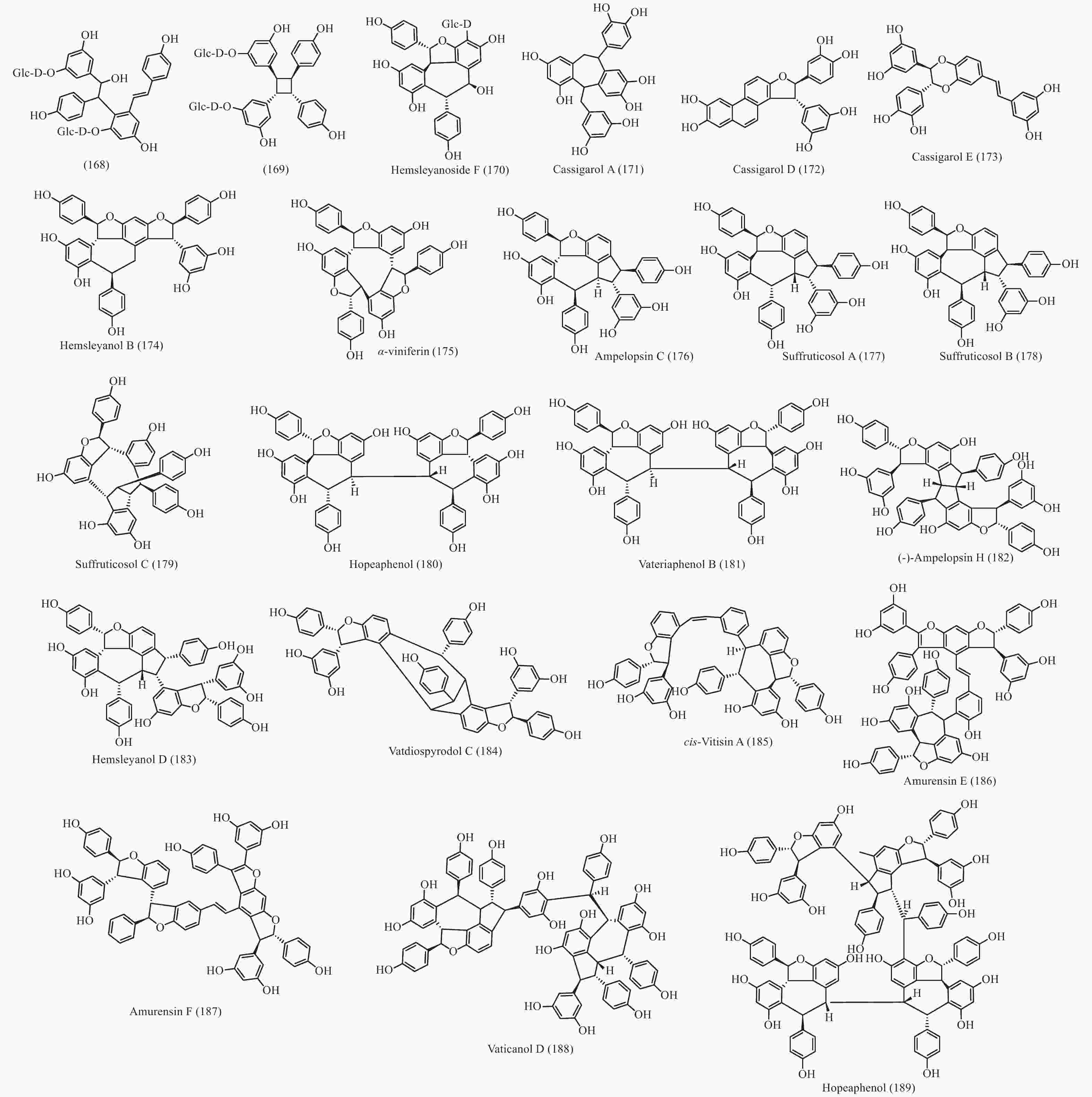
在被子植物中菧类成分主要分布于龙脑香科、葡萄科、莎草科和豆科等4个科中,使君子科、鸢尾科、卫矛科、芍药科、蓼科和桑科植物中也有少量分布 (部分结构见图9)。简单的菧类成分如丹叶大黄素(rhapontigenin 14)、异丹叶大黄素(isorhapontigenin 15) 和白藜芦醇(resveratrol 26)及其糖苷分布同样广泛,结构相对复杂一点的lehmbachol D (49)也有分布[89];二聚菧类同样发现于被子植物的有:射干素B (shegansu B 65)发现与鸢尾科植物射干Belamcanda chinensis[90]和葡萄素[(-)-ε-viniferin 74]发现于葡萄科植物Vitis vinifera[91],另外被子植物中还发现了一些不一样骨架类型的化合物,如发现于龙脑香科植物Shorea hemsleyana的 hemsleyanoside F (170)[92]、发现于蓼科植物Polygonum cuspidatum 的168 和 169 (图9)[93]和豆科植物Cassia garrettiana 的cassigarol A (171)、cassigarol D (172)和 cassigarol E (173)[94-96]等;三聚菧类成分也有相同的化合物在被子植物有发现,如cis-ampelopsin E (156)和ampelopsin E (157)发现于葡萄科植物Ampelopsis brevipedunculata var. hancei[97],ampelopsin E (157)和malaysianol A (155)还发现于龙脑香科植物Dryobalanops aromatica[98],另外被子植物中有不同于买麻藤的其他骨架类型的三聚菧类如:从龙脑香科植物S. hemsleyana 得到的hemsleyanol B (174)[99]、从莎草科植物Carex humilis 中分离得到的α-viniferin (175)[100]、来自葡萄科植物Vitis coignetiae 的ampelopsin C (176)[101]和芍药科植物中的suffruticosol A-C (177-179)[102]; 四聚菧类成分在被子植物也被大量分离到,早在20世纪50年代就从龙脑香科植物Hopea odorata 和 Nalanocarpus hemii 分离到了四聚菧类化合物hopeaphenol (180)[103]、龙脑香科植物Vateria indica 也分离到了hopeaphenol的异构体vateriaphenol B (181)[104]、龙脑香科植物S. hemsleyana 分离到的(-)-ampelopsin H (182)[97]、龙脑香科植物S. gibbosa的
hemsleyanol D (183)[105]、龙脑香科植物Vatica diospyroids中分离到的vatdiospyrodol C (184)[106]和葡萄科植物Vitis coignetiae分离到cis-vitisin A(185)[101];并从葡萄科植物Vitis amurensis 中分离到了五聚体菧类 amurensis E(186)和F(187)[107];甚至从龙脑香科植物Vatica rassak 和Vateria indica中分别分离到六聚体菧类vaticanol D (188)[108]和八聚体菧类 vateriaphenol A (189)[104]。这极大地扩展和丰富了菧类化合物的结构类型化学多样性。 通过对买麻藤目报道的菧类成分进行分析,通过结构相似性和生源关系进行关联,获得了一个推测的菧类化合物的生源图,见图10。结合裸子植物的菧类成分和被子植物的菧类成分,可以看出,从裸子植物经过买麻藤目向被子植物进化的过程中,菧类化合物的合成起源于裸子植物,并在买麻藤目得以发展,形成了数量众多的二聚体、三聚体和四聚体,涉及的骨架类型多样化;到了被子植物有一些类群进化出可以相同功能的其他类型次生代谢物,将菧类逐步替代,或留有痕迹,但是菧类在一些类群如葡萄科和龙脑香科等植物中得以进一步的发展和壮大,形成了数量更多结构更复杂的三聚体、四聚体、五聚体、六聚体、甚至八聚体,是生物合成菧类成分的性状得到了极好的继承和巨大的发展。这也恰恰说明了买麻藤目是在裸子植物向被子植物进化的过渡类群,并且买麻藤目应该更靠近被子植物。
2.1. 买麻藤目的苄基异喹啉类生物碱
2.2. 买麻藤目的菧类stibenes
-
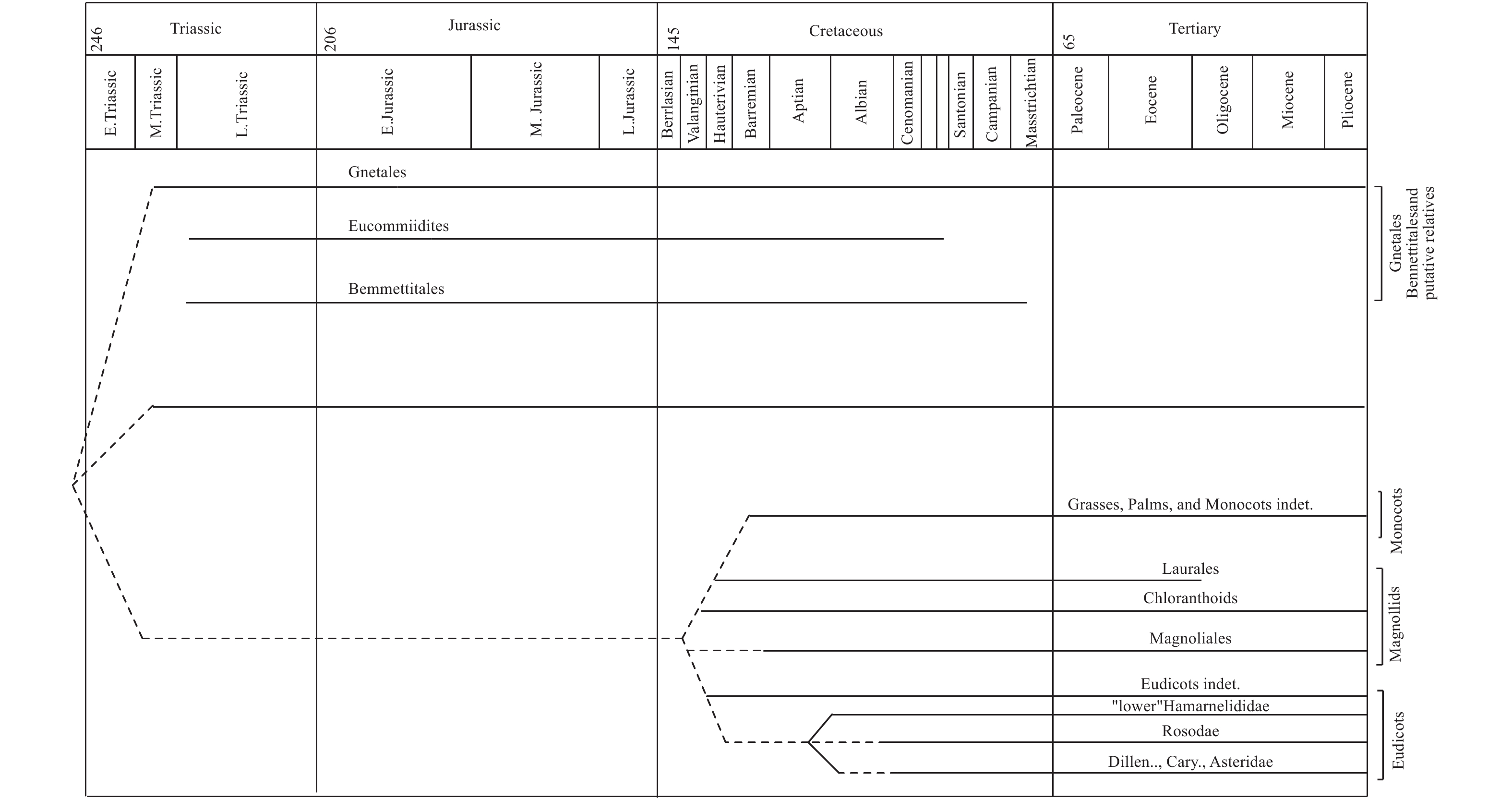
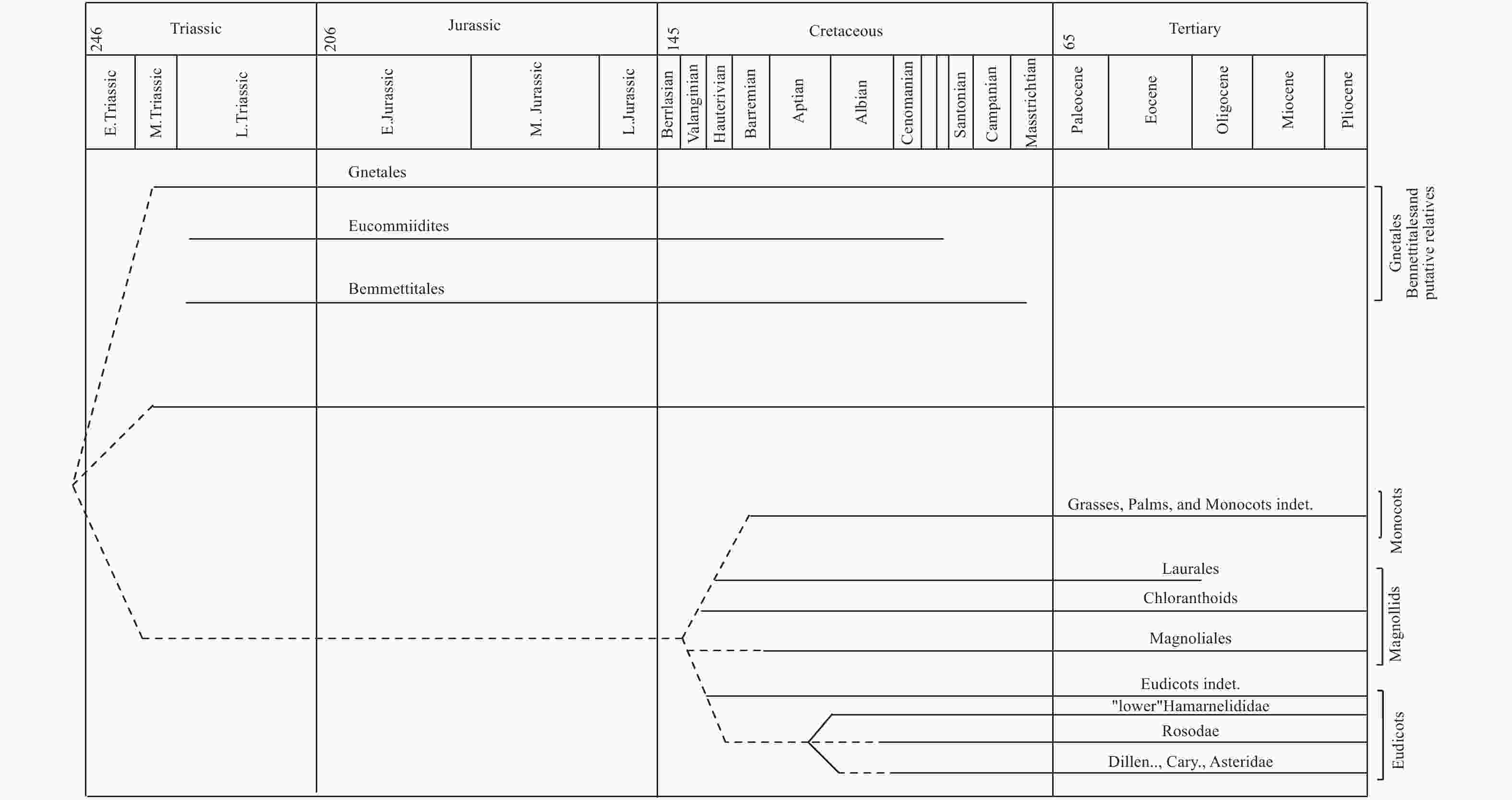
化石作为研究被子植物起源的直接证据,一直受到大量植物学家的关注,结合不同时期发表的化石证据,总结出买麻藤目到被子植物的化石分布图[1]。其中可以看出应该存在1个被子植物的早期类群,即被子植物的单系共同祖先,与买麻藤目和本内苏铁目是姊妹群,共同存在于三叠纪到侏罗纪时期,其中买麻藤目的部分类群存活下来成为了现存的买麻藤目植物,而本内苏铁目的植物则在白垩纪晚期灭绝,而早期的被子植物也到了白垩纪以后,伴随着昆虫、鸟类和哺乳类动物开始大量协同发展和演化,所以本内苏铁化石的发现和解剖结构的研究也证实了其作为连接买麻藤目和被子植物的关键纽带[109]。因此,有学者结合现代被子植物花的解剖、早期和中期白垩纪被子植物化石中的花药结构和花粉结构,认为被子植物起源于买麻藤目,本内苏铁目应该是两者的中间过渡类群的观点(图11)。
-
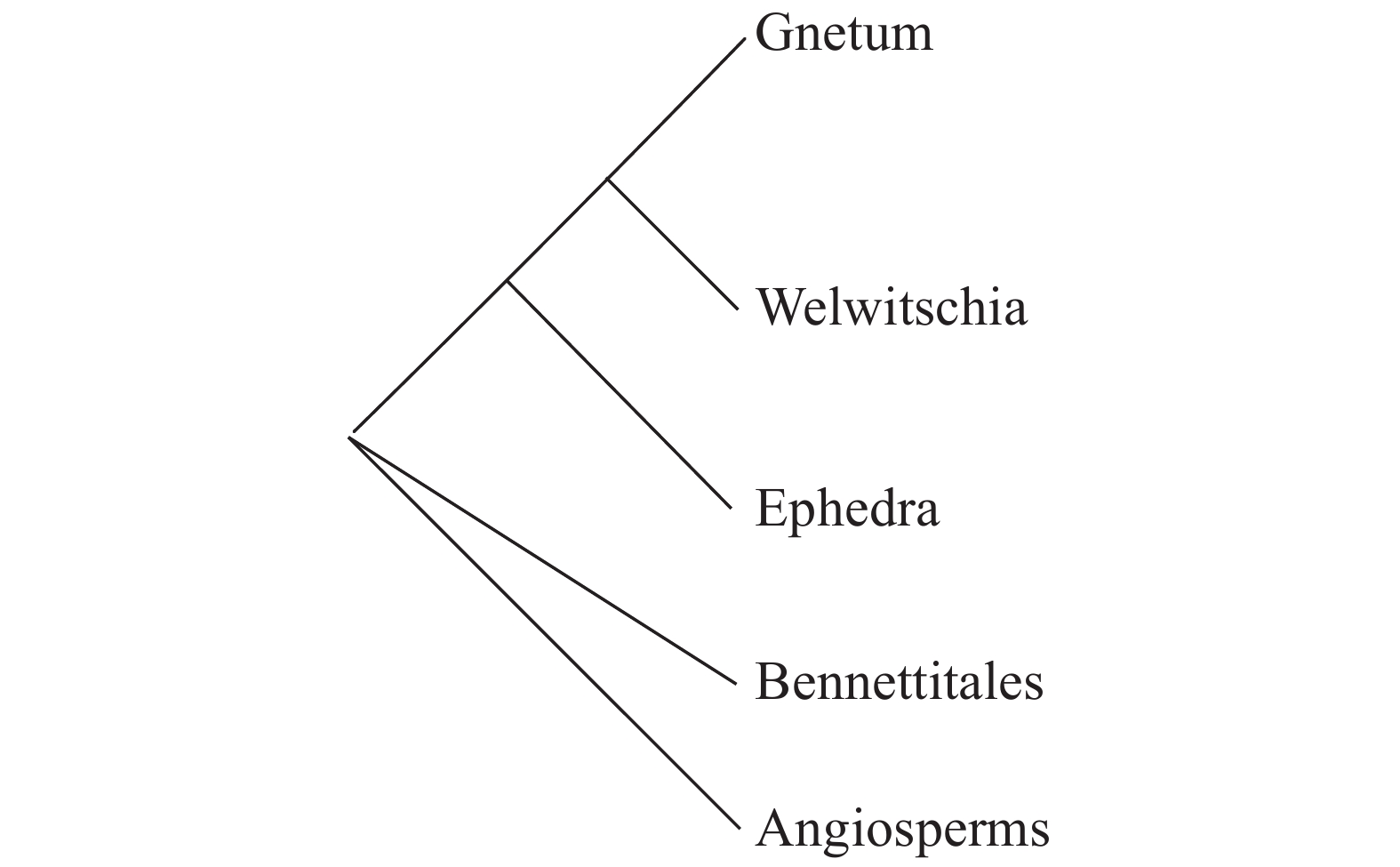
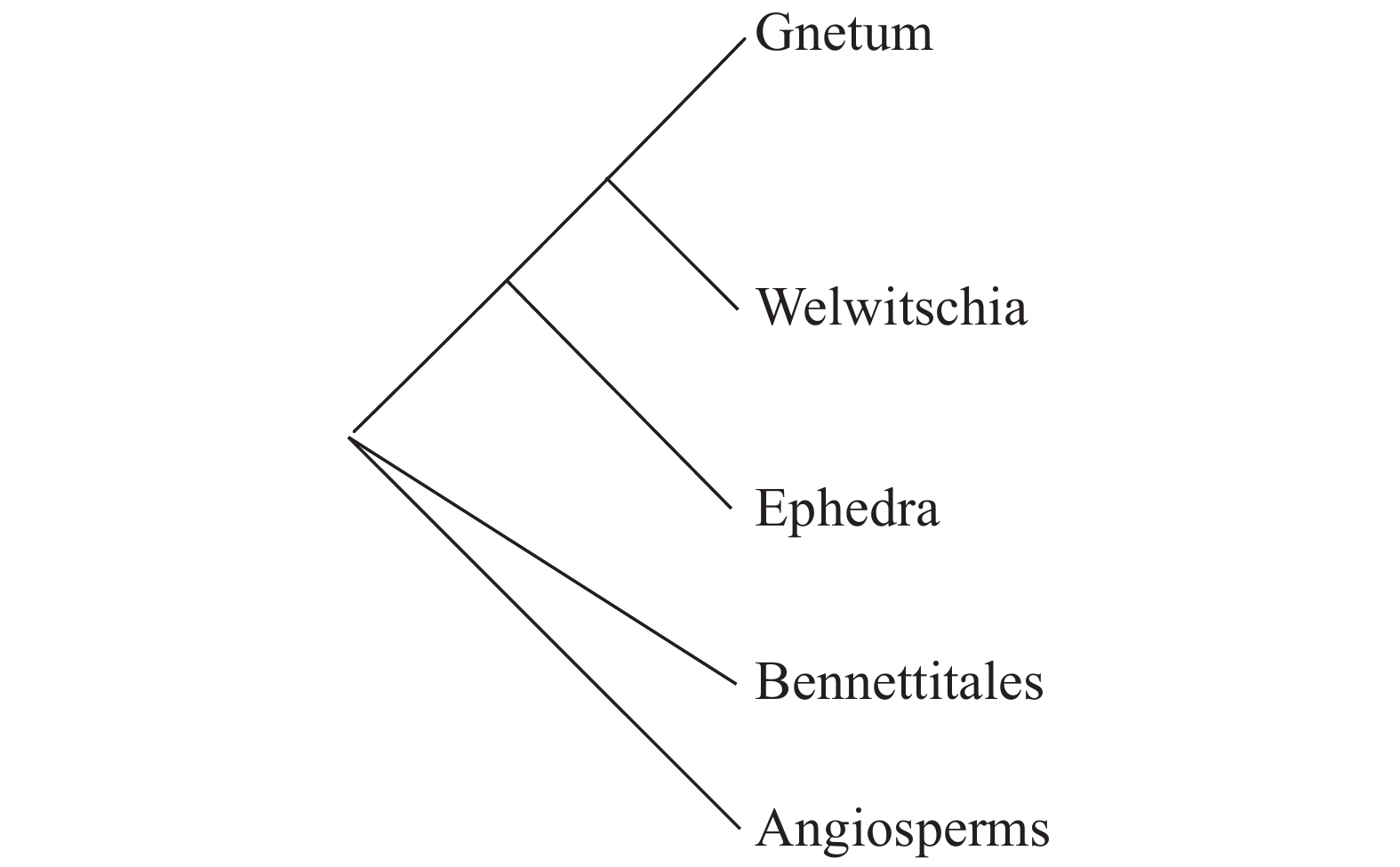
基于现代分子生物学的植物系统学研究,多数研究对象主要针对叶绿体DNA和核糖体DNA的系统学研究。目前研究的热点多是集中于被子植物内部的不同科属类群之间的关系,且根据最新的分子系统学证据,推出了APG II系统,且在不断更新当中,其系统接受被子植物为单系的观点,认为其基部类群为无油樟科,仅存一种无油樟(Amborella trichopoda),又称互叶梅,其他类群如睡莲科Nymphaeales、木兰藤科Austrobaileyaceae、Trimeniaceae、八角科Illiciaceae、五味子科Schisandraceae、蜡梅目Calycanthales和金粟兰科Chloranthaceae木兰目Magnoliales,为相对基部的类群[11]。但是对于被子植物与裸子植物和买麻藤目的关系,一直存在争议。Doyle J A等[110]基于形态学和核糖体RNA的数据分析发现在形态学上支持买麻藤目作为被子植物姊妹群的观点,同样得到了核糖体RNA数据的验证。这一结论在通过研究质体rbcL的核苷酸序列,分析了种子植物系统发育,也验证了这一观点[111]。但是Qiu Y L[2]通过对线粒体、质体和核基因组的5个基因的DNA序列进行分析,建立了系统树(图 12),发现买麻藤目并非被子植物的姊妹群,而是与裸子植物特别是银杏纲比较接近。也有研究现在分子生物学在植物系统学应用方法学研究的学者认为,基于质体或核的单个基因或者少数基因序列的分析,仍然存在一定的局限性[112]。最近报道了买麻藤属植物Gnetum montanum的接近4.5G的全基因组测序组装,同时结合转录组数据,对现今发表的17种代表性陆地植物进行了全基因组比较及相关分析,认为买麻藤的基因组特征显著区别于其他已报道的种子植物(针叶树、银杏、被子植物),在某些特定的特征上如内含子、重复序列进化模式和现存最古老被子植物无油樟“相似”。另外买麻藤在种子植物保守功能基因集合呈现出非常古老的状态(现存种子植物共享)[113]。
-
综合以上的观点,无论是经典的植物分类学、化学分类学、古植物学都能较好的支持买麻藤目作为被子植物姊妹群的观点,甚至可以认为被子植物是起源于买麻藤目的。经典植物分类学展示了被子植物从买麻藤目演化到被子植物的形态特征痕迹;化学分类学观点也很好地显示了苄基异喹啉生物碱和菧类成分在种子植物从裸子植物−买麻藤目−到被子植物的演化过程中通过与昆虫等动物进行化学信息的交流协同进化的作用;同样古植物学研究中虽然买麻藤目向被子植物演化的重要过渡环节标本还未找到,但是对于买麻藤目−本内苏铁目−被子植物的演化过程已然可见;由于用单个基因或少数几个基因来定位买麻藤目的系统学地位现代分子生物学证据,仍然显得单薄,目前从买麻藤植物的基因组测序及这些序列的分析研究已经初步看到了种子植物从裸子植物−买麻藤目−到被子植物的演化脉络,因此,还有待买麻藤目其他植物的全基因组测序,以进一步解开被子植物起源的谜团。
致谢:中国科学院院士、植物资源与植物化学家周俊先生长期关注被子植物进化,在本文的撰写前期进行了大量资料的收集,并在文章的撰写和修改过程中起了非常重要的作用,在本文投稿后周先生去世,谨以本文向周俊先生致敬。中国科学院昆明植物所牛洋博士提供丽江麻黄Ephedra likiangensis Florin 照片,一并致谢!








 DownLoad:
DownLoad: