-
农膜通常为0.002~0.200 mm的薄膜塑料,因具有良好的增温保墒、促进种子发芽和控制杂草生长等作用被广泛使用。近年,我国每年农膜使用量约260万t,每年残留在耕地土壤中的农膜约30~50万t[1]。残留农膜在人为或自然因素驱动下进入生态系统,通常作物收割后破残农膜留在耕作层[2],致使土壤通透性受到影响、耕地质量下降[3]、农事操作受阻、作物水分利用效率降低[4-5]、作物减产,品质降低[3]。农膜颗粒具有一定的孔隙结构与比表面积,对土壤中重金属具有一定的富集能力[6-7],且不同粒径农膜对重金属的富集能力不同。农膜颗粒富集的重金属等污染物通过食物链等方式进入到其他环境介质,可加速污染物的迁移与转化[8]。残留在自然环境中的农膜颗粒在紫外线、臭氧、酸雨等作用下逐渐老化[9],致使农膜的表面结构和官能团发生改变。老化不仅影响农膜颗粒对重金属等污染物的吸附行为和机制[10],还会进一步与其他污染物发生相互作用,引发复合污染,造成潜在的环境风险[11-12]。因此,探究农膜及其老化颗粒对重金属的富集行为与评价其环境风险和污染物的生物地球化学循环具有重要的意义[13]。本研究以镉(Cd)为目标污染物,选取3种粒径[4目(4.75 mm)、10目(2.0 mm)、30目(0.6 mm)]的农膜分别作为载体,在农膜浓度、初始pH、吸附时间、Cd2+初始浓度及农膜老化等因素上分析农膜颗粒对Cd2+吸附的影响,结合扫描电镜(SEM)、傅里叶变换红外光谱(FT-IR)及吸附模型拟合等解析农膜颗粒及老化方式对Cd2+的吸附特性,以期为农膜的合理利用与管控提供参考。
-
农膜:海南广泛使用的0.08 mm的难降解黑色PE膜,利用自制低温农膜破碎装置制成颗粒,然后依次过4目、10目、30目粒径筛网筛分,分别获得4.75 ,2.0 ,0.6 mm农膜颗粒。去离子水清洗3遍,40 ℃条件烘干至恒重,储存于玻璃罐中备用。农膜颗粒老化参照赵楚云等[14]的方法,将溶液(1 mol·L−1 HNO3,1 mol·L−1 H2SO4,30% H2O2)分别与不同粒径农膜按50∶1(v/w)比例混合;混合样品在60 ℃,160 r·min−1振荡摇匀老化24 h。随后用筛网分离,去离子水冲洗农膜颗粒至滤液中性为止,40 ℃烘箱烘干至恒重,备用。
用Cd(NO3)2·4H2O (AR) 配制1 000 mg·L−1的镉母液,利用母液稀释配置每一批次实验的工作液。将一定浓度的镉溶液20 mL移至装有农膜的50 mL离心管,于25 ℃,160 r·min−1条件下进行振荡,用0.45 μm滤膜过滤,原子吸收光谱仪测定滤液中镉的质量浓度。每组实验平行3次。
Cd2+浓度利用原子吸收光谱仪(PinAAcle 900T)测定;农膜颗粒表面利用扫描电镜 (SEM) (S-3000N,HITACHI, 日本)成像;农膜颗粒表面官能团通过傅里叶变换红外光谱(FT-IR) (Nicolet 5700, Thermo Fisher, MA, 美国)分析[15]。
-
取母液配置成2 mg·L−1的Cd2+溶液,并用NaOH/HNO3溶液调节pH值为5.0,向分装好Cd2+溶液的离心管中分别投加0,0.5,1.0,2.0,4.0,6.0 g·L−1的农膜颗粒,并在25 ℃,160 r·min−1条件下摇匀振荡24 h,过滤测定滤液中Cd2+的浓度。
-
取母液配置成2 mg·L−1的Cd2+溶液,分装,用NaOH/HNO3溶液调节工作液初始pH值分别为1.0,3.0,5.0,7.0,9.0,农膜颗粒投加量为1.0 g·L−1,然后按1.2.1的方法进行吸附、过滤及测试分析。
-
取母液配置成2 mg·L−1的Cd2+溶液,并用NaOH/HNO3溶液调节pH值为5.0,每个处理的农膜颗粒浓度1.0 g·L−1,分别在0.5,1 ,3 ,6,12,18,24 h和2 ,3 d时取样,同时设置不投加农膜的对照组。过滤与测试分析方法同1.2.1。
-
取Cd2+母液分别配置成0.5,1,2,4,6,10 mg·L−1的Cd2+工作溶液,分装后投加1 g·L−1的农膜颗粒,吸附、过滤与测试分析方法同1.2.1,同时设置不投加农膜的对照组。
-
取母液配置成2 mg·L−1的Cd2+溶液,并用NaOH/HNO3溶液调节pH值为5.0,向分装好Cd2+溶液的离心管中分别投加6个浓度(0,0.5,1.0,2.0,4.0,6.0 g·L−1)的3种老化农膜颗粒(HNO3老化农膜颗粒、H2SO4老化农膜颗粒、H2O2老化农膜颗粒),于25 ℃、160 r·min−1条件摇匀振荡24 h,过滤测定滤液中Cd2+的浓度。
-
将老化前后的农膜颗粒经恒温干燥后,均匀粘在含导电胶的样品台上,喷金处理使用扫描电镜进行扫描成像,比对老化前后及不同老化处理表面的微观结构差异;同时,对老化前后农膜颗粒以及不同老化方式颗粒采用KBr压片法进行FT-IR光波谱分析(波数为4 000~400 cm−1)。
-
农膜对溶液中Cd2+的平衡吸附容量qe由方程式(1)计算得到。农膜对溶液中Cd2+的吸附率η由方程式(2)计算得到。吸附等温模型拟合分别采用Langmuir吸附模型[16]方程式(3)和Freundlich吸附模型[17]方程式(4)的非线性公式进行曲线拟合。吸附动力学模型拟合分别采用准一级动力学方程式(5)和准二级动力学方程式(6)的非线性公式进行曲线拟合[16-18]。
式中:qe为吸附平衡时农膜对重金属离子的单位吸附容量/(μg·g−1);Co为溶液的初始浓度/(mg·L−1);Ce吸附平衡时的浓度/(mg·L−1);V为溶液的体积,L;m为农膜的投加量/g。
式中:η为吸附平衡时农膜对重金属离子的吸附率/%;Co为溶液的初始浓度/(mg·L−1);Ce分别和吸附平衡时的浓度/(mg·L−1)。
式中:qe为吸附平衡时的单位吸附容量/(μg·g−1);Ce为溶液平衡浓度/(mg·L−1);qm为单分子吸附时的饱和吸附容量/(μg·g−1);b为Langmuir亲和力常数/(L·μg−1);k为Freundlich平衡吸附常数;n代表吸附剂表面的异质性。
式中:qe为吸附平衡时的单位吸附容量/(μg·g−1); qt为各时刻的单位吸附容量/(μg·g−1); t为吸附时间/h; k1为准一级吸附速率常数/h−1; k2为准二级吸附速率常数/(g·μg−1·h−1)。
-
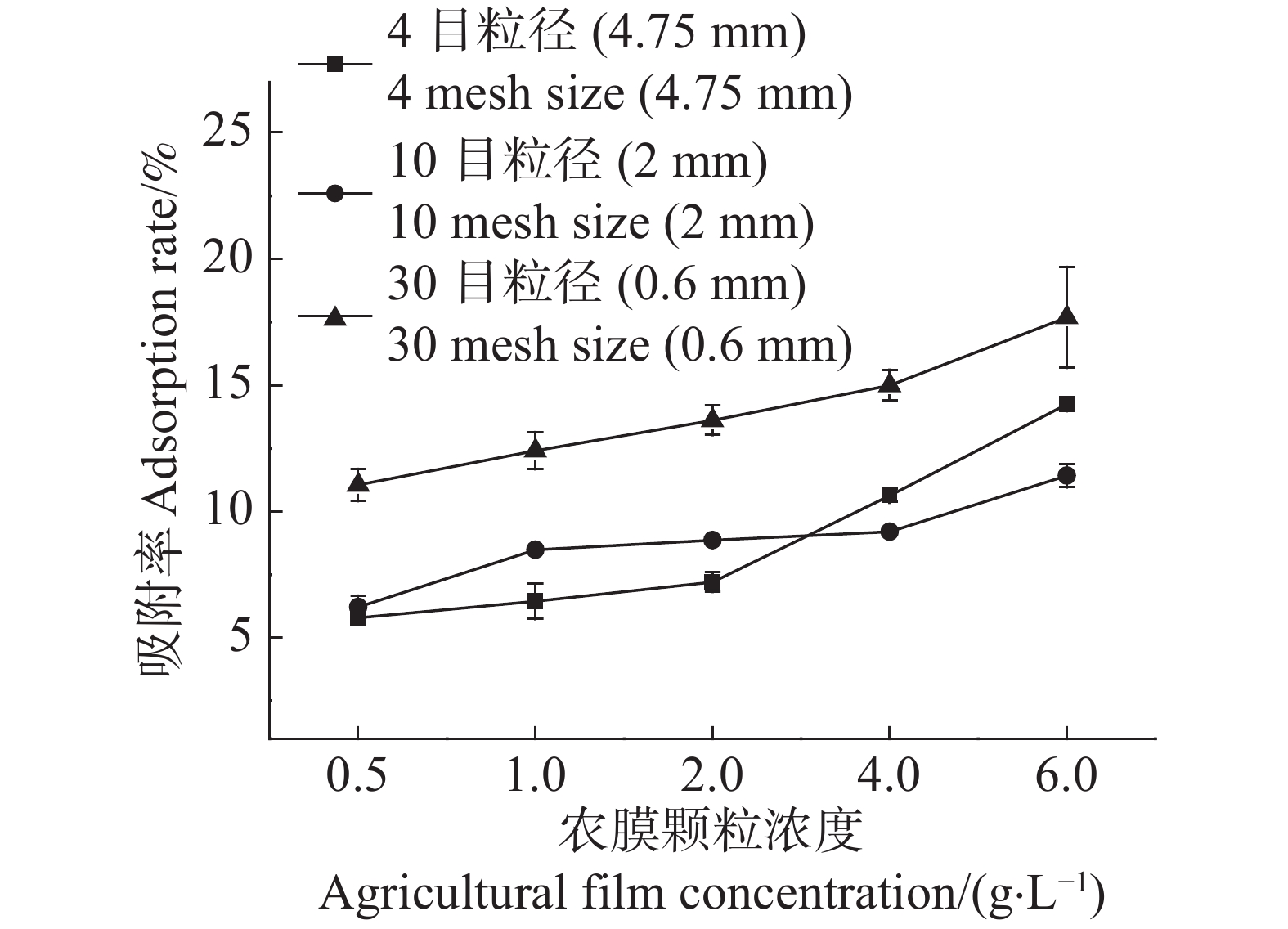
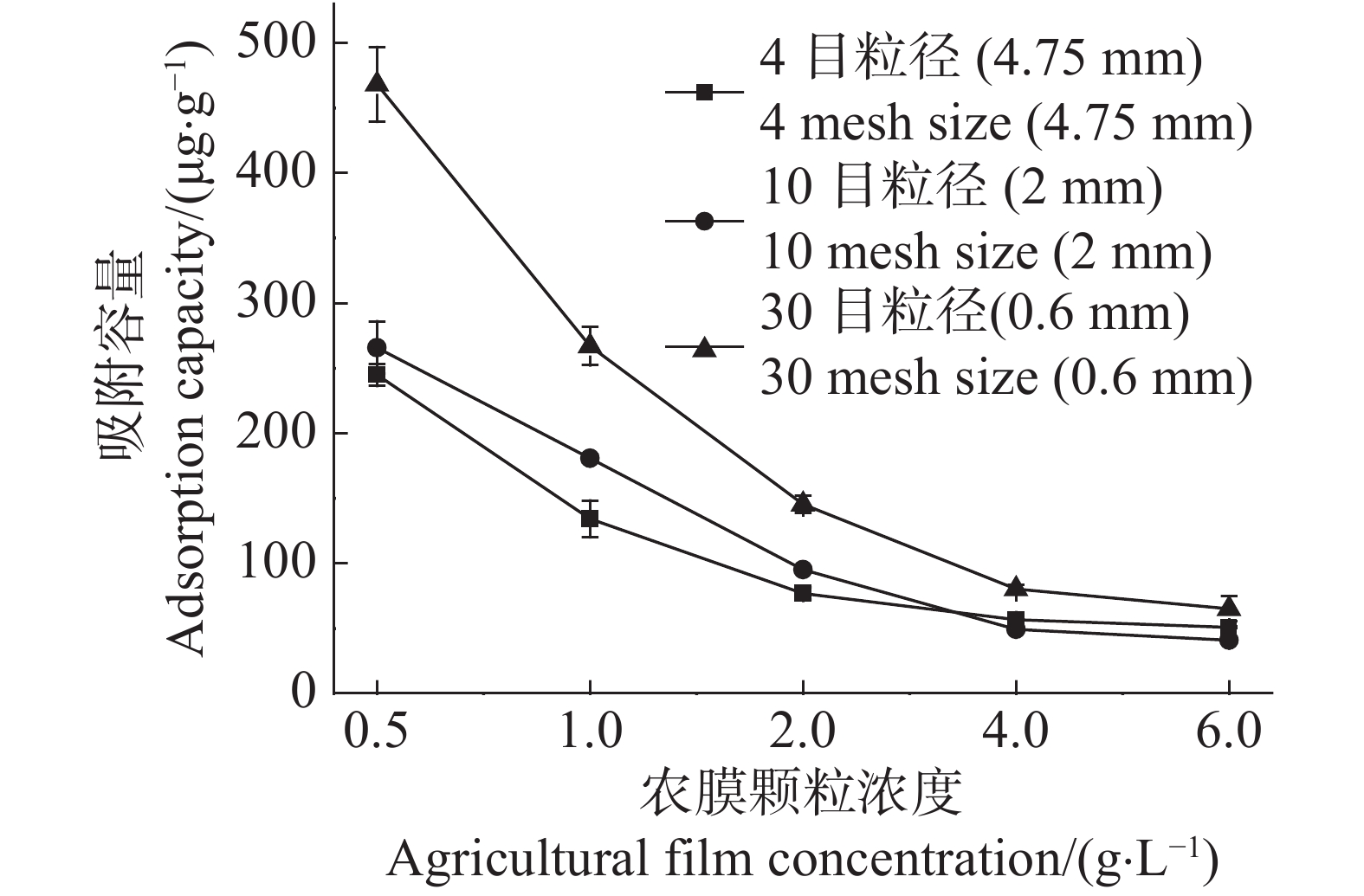
随着农膜颗粒浓度由0.5 g·L−1增至6.0 g·L−1,4目、10目、30目3种粒径农膜对Cd2+的吸附容量分别下降了79.18%,84.63%,86.11%(图1)。这表明在Cd2+浓度一定的情况下,随着农膜颗粒浓度的增加,其对Cd2+的吸附容量随之降低。30目粒径农膜对Cd2+的吸附容量最大,以农膜浓度0.5 g·L−1为例,30目粒径的吸附容量是10目和4目的1.76倍和1.91倍,说明随农膜粒径的减小吸附容量增加(图1)。
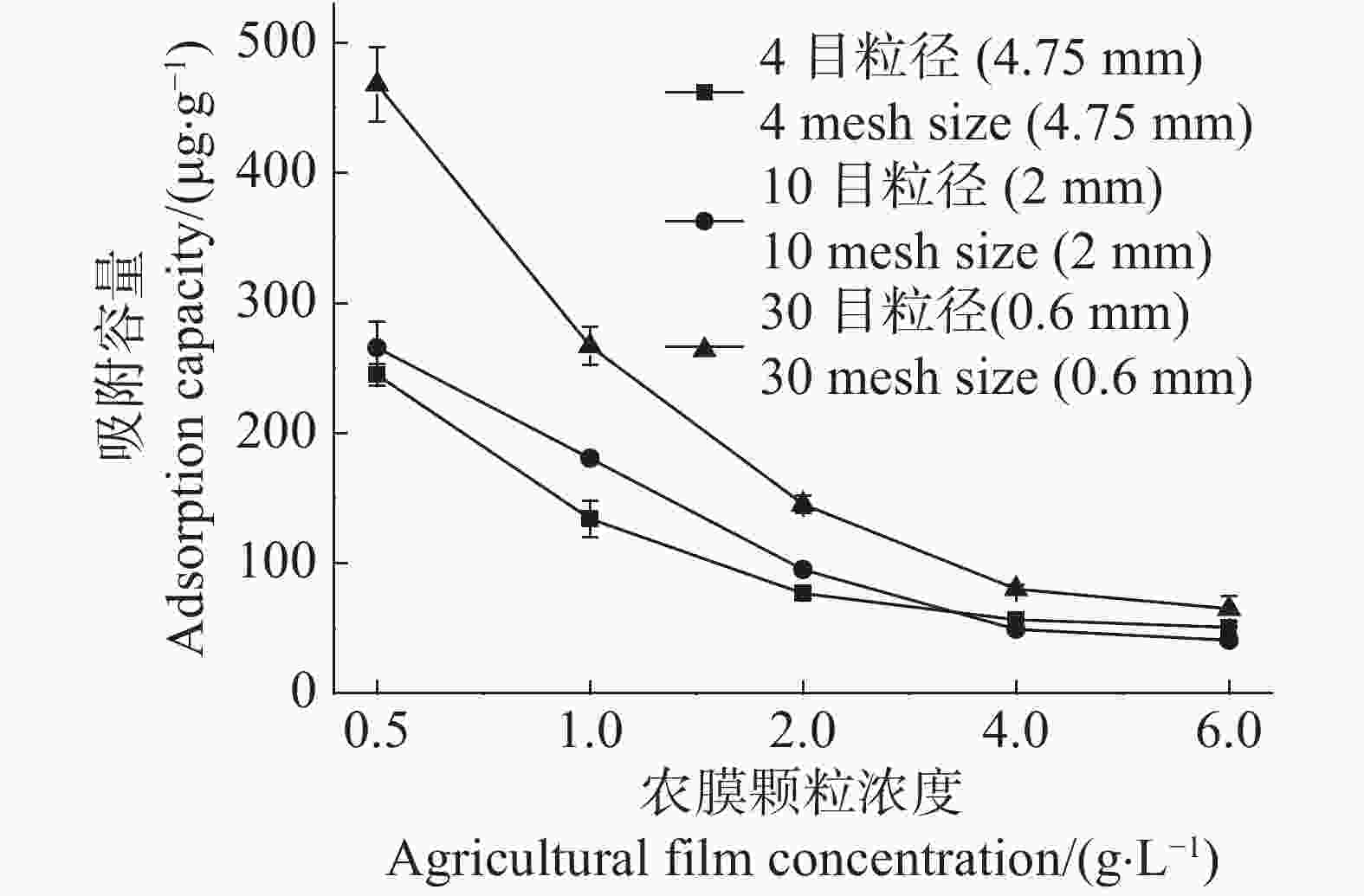
Figure 1. Effects of agricultural film concentration on the Cd2+ ad-sorption capacity of agricultural films of three different pariticle sizes
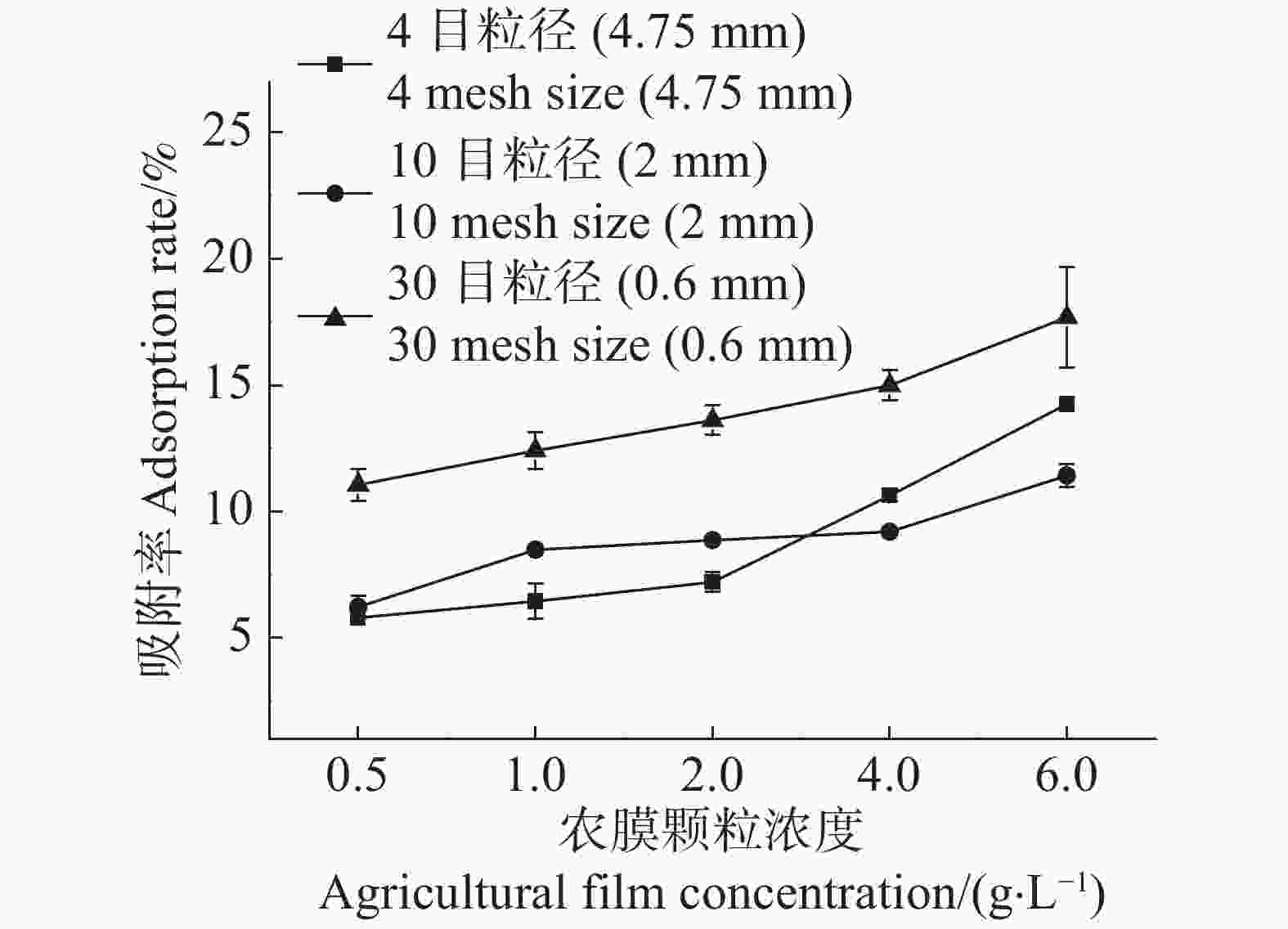
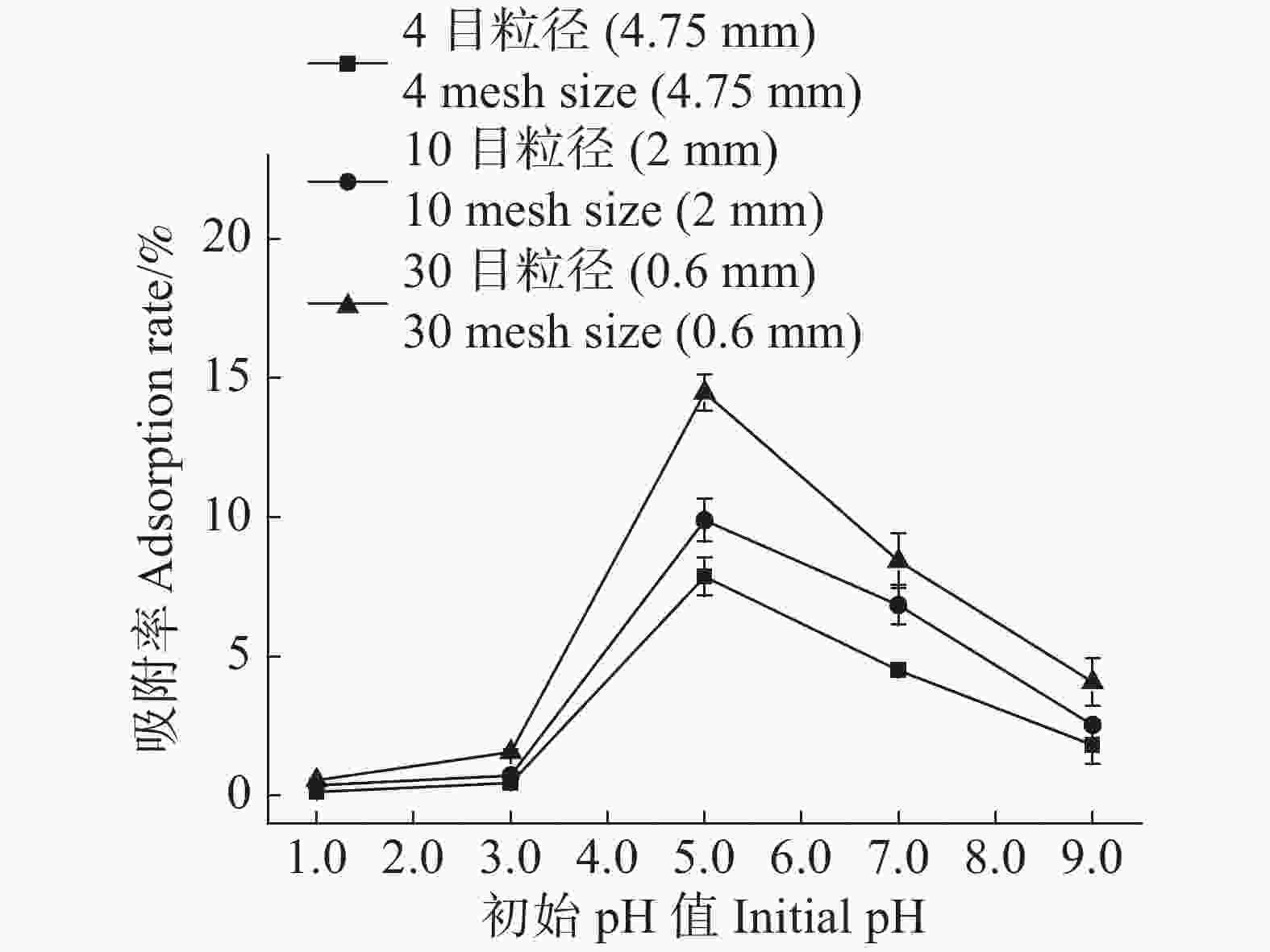
随着农膜颗粒浓度的增加,3种粒径农膜对Cd2+的吸附去除率逐渐提高,而其吸附容量逐渐降低(图1,2)。当农膜浓度为1.0 g·L−1时,4目、10目、30目粒径农膜的吸附率依次为6.44%,8.48%,12.41%;吸附容量依次为134.38 ,180.77 ,267.09 μg·g−1,此时Cd2+的吸附率与农膜的吸附容量均达到相对较高的水平,故选取最佳浓度1.0 g·L−1进行下一步的实验。
-
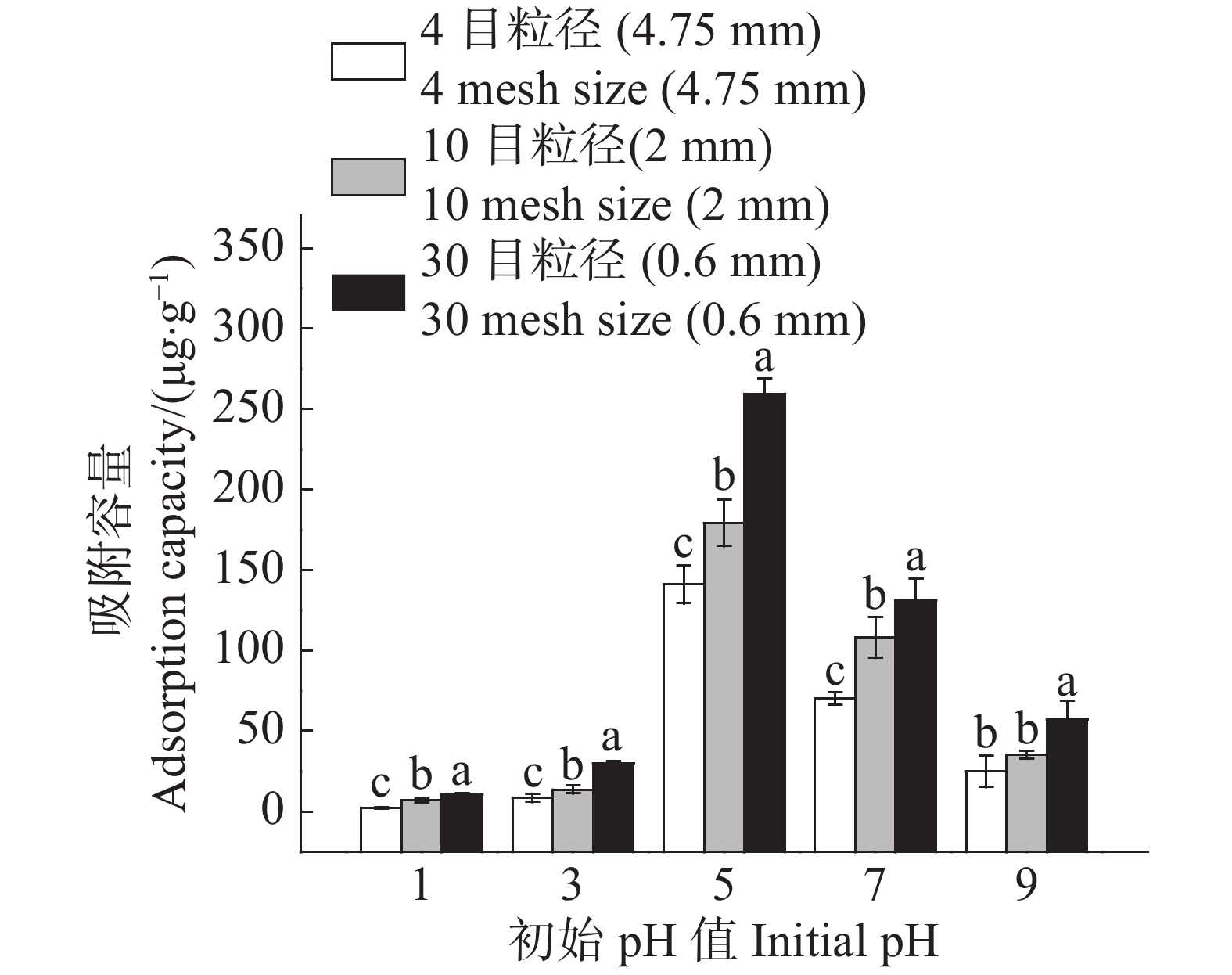
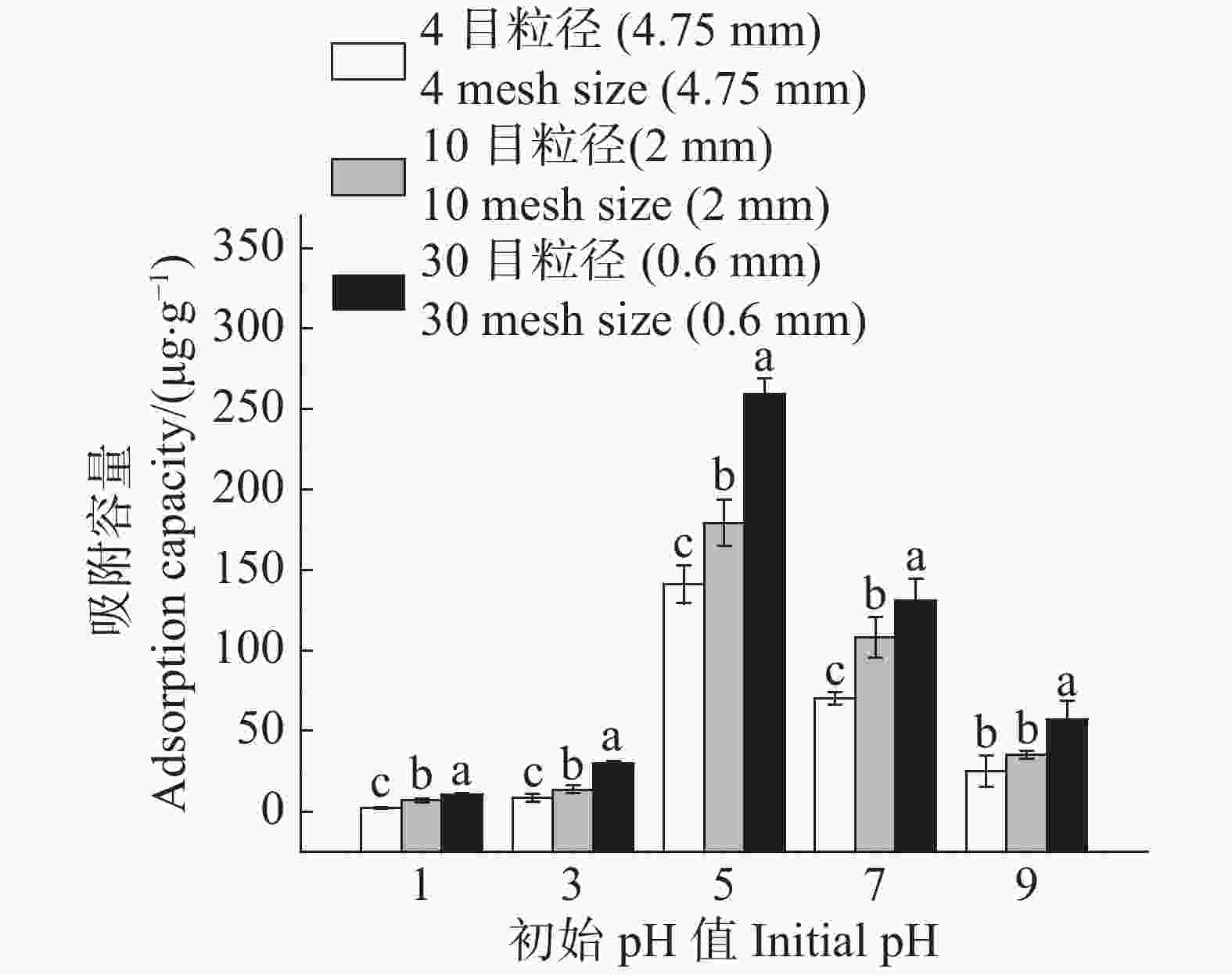
溶液初始pH对农膜颗粒对Cd2+的吸附容量与吸附率的变化趋势呈抛物线状(图3,4);在pH值为5时,农膜吸附容量最高;4目、10目、30目粒径农膜的吸附容量分别为141.04 ,179.23 ,259.45 μg·g−1(图3),当溶液pH小于5时,农膜颗粒对Cd2+的吸附容量随着溶液初始pH的升高而增大;当溶液pH大于5时,农膜颗粒对Cd2+的吸附去除率及吸附容量会减少。故控制pH值为5进行后续相关研究。
-
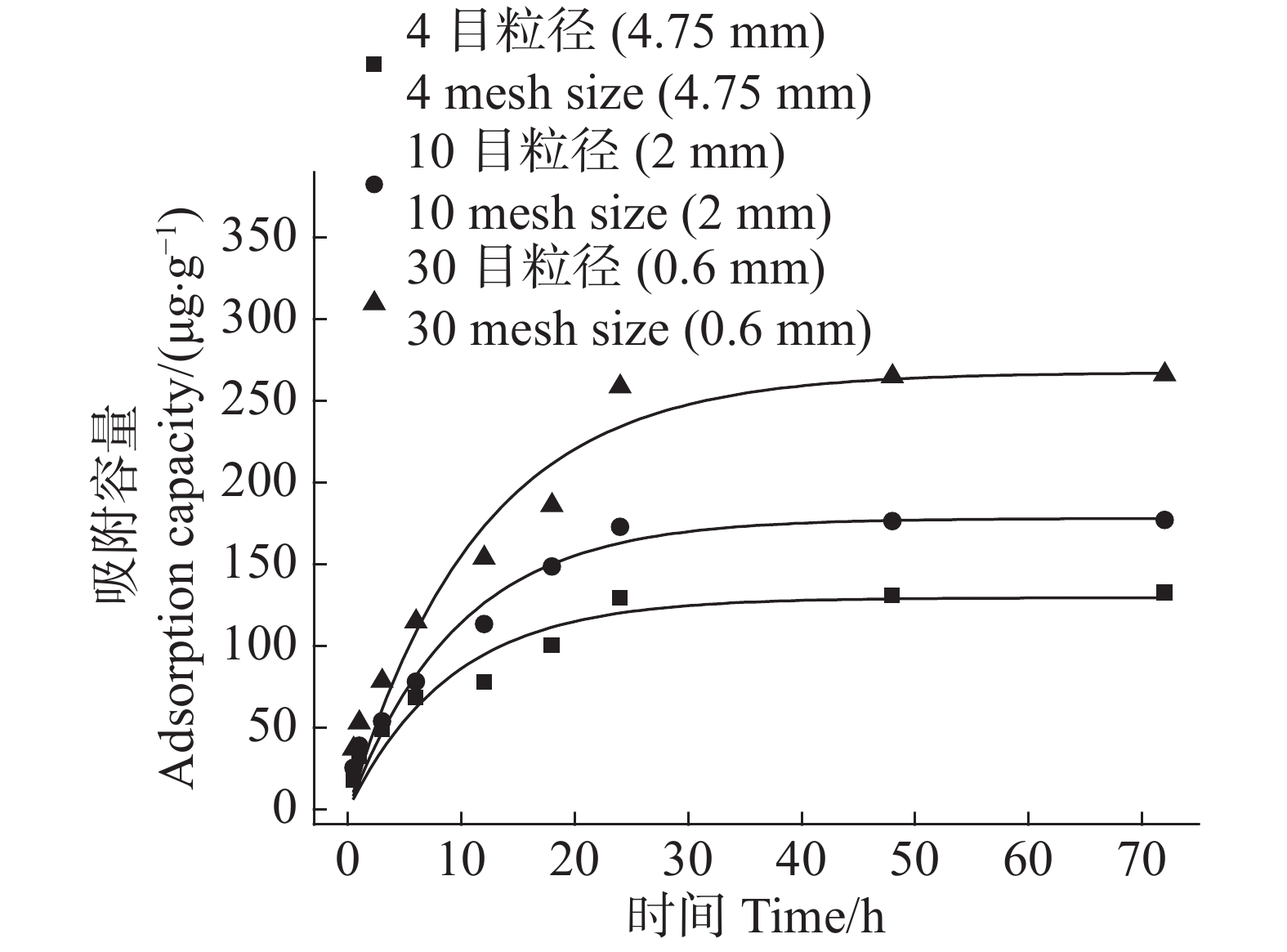
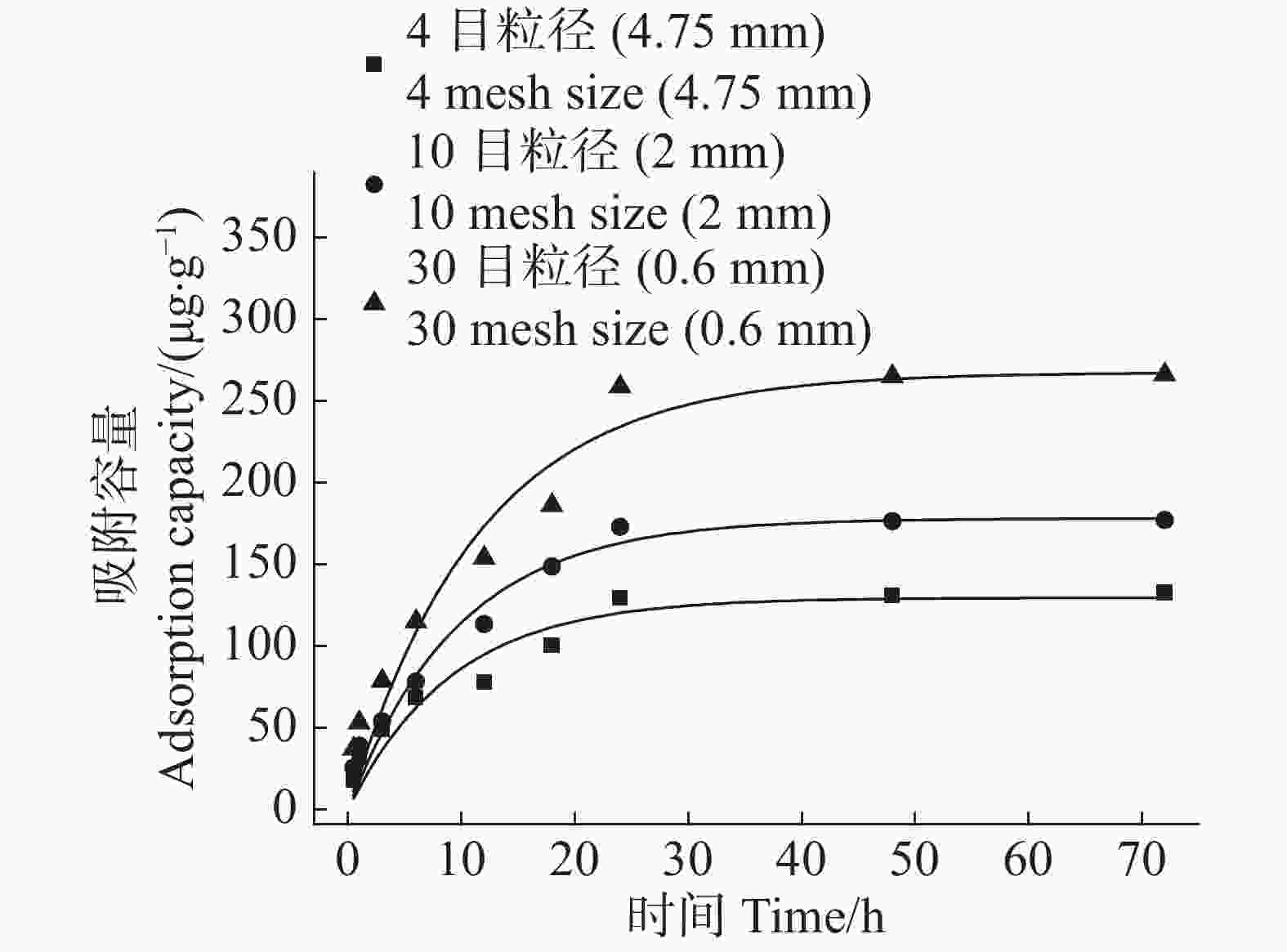
农膜颗粒对Cd2+的吸附容量在吸附时间0~10 h呈快速增长,在吸附时间10~20 h转为缓慢增长,在吸附时间20~30 h趋于平缓,吸附速率与农膜颗粒粒径成反比(图5)。由于吸附开始时农膜颗粒表面吸附位点充足,故前期吸附速率较快,随着吸附反应的进行,吸附位点被逐渐占满,吸附容量增长变缓,直至吸附平衡;30目粒径农膜因其比表面积最大,提供的吸附位点较多所致,所以吸附速率最大。
采用准一级动力学模型和准二级动力学模型对3种粒径农膜吸附Cd2+进行拟合,结果显示:3种粒径农膜准二级动力学模型的R2值(0.943~0.960)高于准一级动力学模型(0.913~0.960),即准二级动力学模型可以将3种粒径农膜对Cd2+的吸附过程进行很好的描述,拟合结果显示,4目、10目、30目粒径农膜的平衡吸附容量分别为146.15,205.19,311.12 μg·g−1(表1)。
农膜粒径
Agricultural film
particle size准一级动力学吸附模型 Pseudo-first-order 准二级动力学吸附模型 Pseudo-second-order qe/ (μg·g−1) k1/h−1 R2 qe/ (μg·g−1) k2·102/(g·μg−1·h−1) R2 4目(4.75 mm) 129.67 0.110 0.913 146.15 0.104 0.943 10目(2 mm) 178.29 0.103 0.960 205.19 0.063 0.960 30目(0.6 mm) 267.59 0.087 0.939 311.12 0.034 0.947 Table 1. Fitting parameters of cadmium adsorption by agricultural films with three different particle sizes based on Pseudo-first-order and Pseudo-second-order kinetic models
-
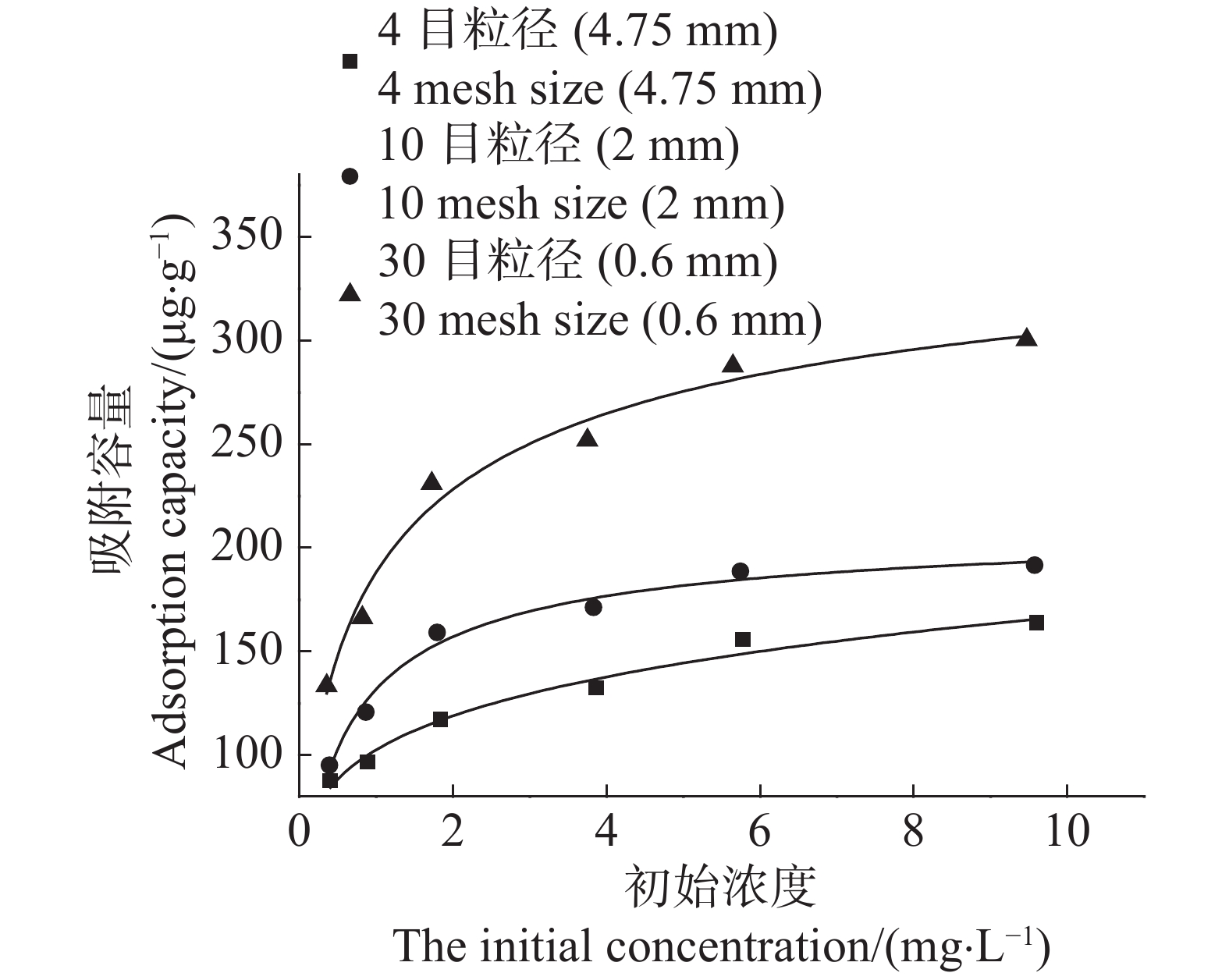
当Cd2+的初始浓度较低时,3种粒径农膜对Cd2+的吸附容量随溶液初始浓度的增高而增加,当Cd2+溶液初始浓度大于2 mg·L−1时,随着初始浓度的增高,3种粒径农膜对Cd2+的吸附容量增加的趋势逐渐变缓(图6)。这是因为在Cd2+溶液初始浓度较低条件下,农膜颗粒为Cd2+提供了充足的吸附位点,而当Cd2+溶液初始浓度较高时,农膜为Cd2+提供的吸附位点不足以满足Cd2+的吸附需求,但由于高浓度Cd2+溶液对吸附具有较高的推动力,从而使得Cd2+的平衡吸附容量有所增长,但未能呈现出线形增长趋势。
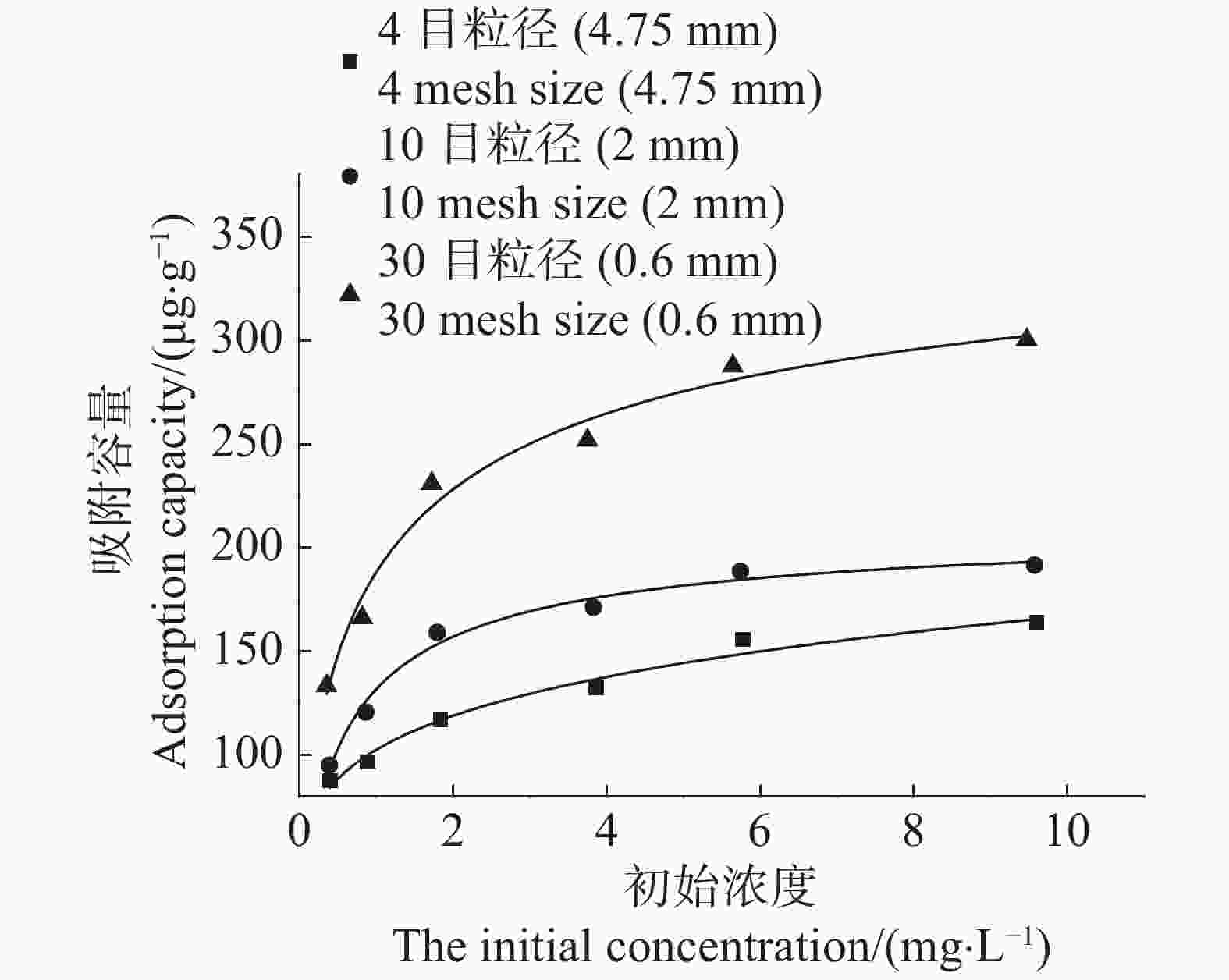
Figure 6. Effects of agricultural films on the adsorption of Cd2+ under different initial cadmium concentrations
采用Langmuir和Freundlich模型对3种粒径农膜吸附Cd2+的等温吸附过程进行拟合,其中使用Langmuir模型的拟合结果(R2值在0.968~0.977之间)优于Freundlich模型的拟合结果(R2值在0.914~0.955之间)(表2)。Langmuir模型拟合结果表明,3种粒径农膜对Cd2+的吸附容量的大小顺序为:30目>10目>4目(图5)。
农膜粒径
Agricultural film particle sizeLangmuir 模型 Langmuir model Freundlich 模型 Freundlich model qm/(μg·g−1) b/(L·μg−1) R2 1/n K/(μg·g−1) R2 4目(4.75 mm) 163.64 2.232 0.968 0.21 102.56 0.955 10目(2 mm) 213.26 1.618 0.977 0.20 127.89 0.914 30目(0.6 mm) 377.60 0.994 0.971 0.24 184.24 0.946 Table 2. Fitting parameters of cadmium adsorption by agricultural films with three particle sizes based on Langmuir and Freundlich models
-
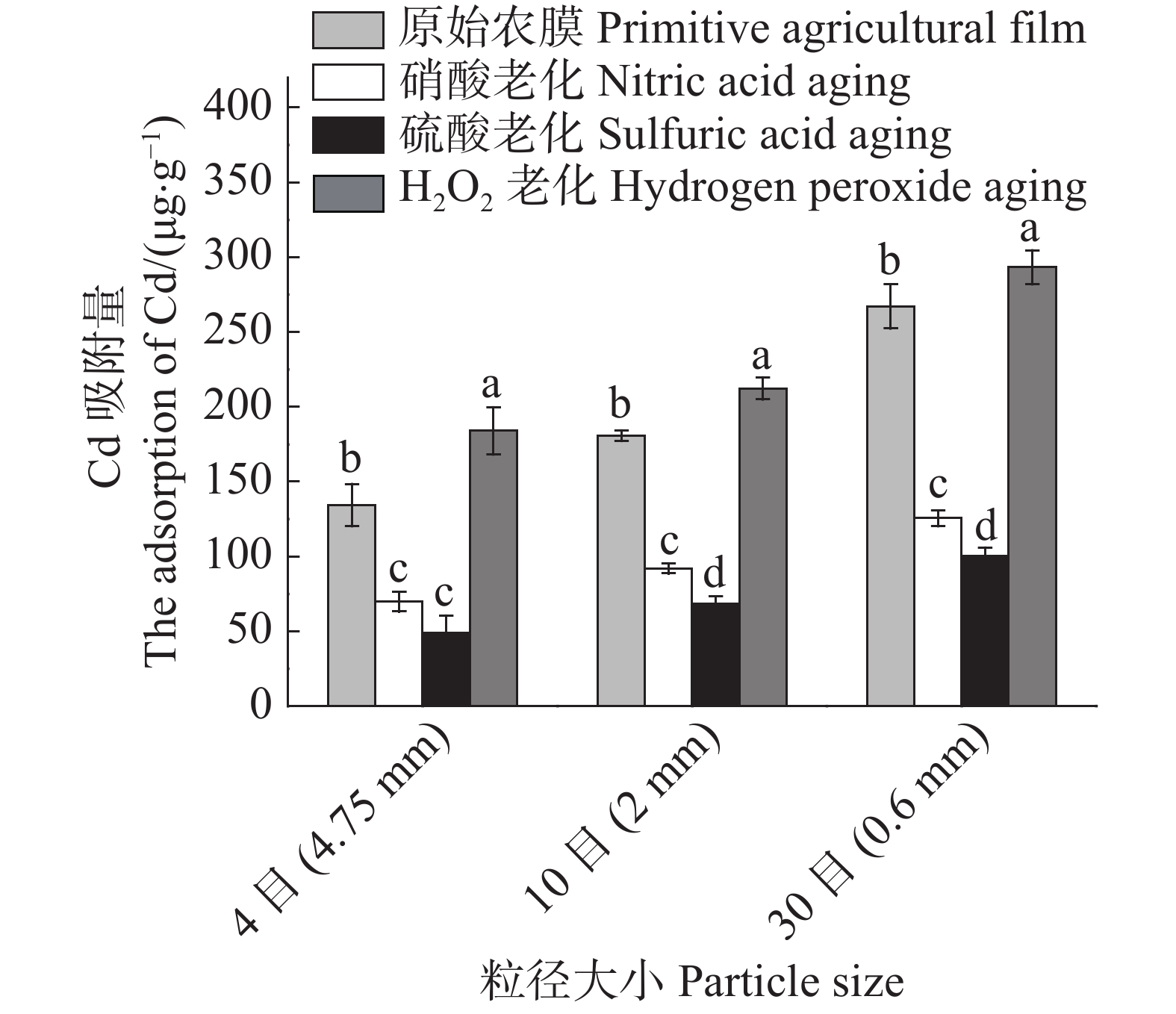
3种农膜颗粒老化后对Cd2+的吸附影响结果(图7)表明:同一老化方式,3种粒径农膜Cd2+吸附容量大小与原农膜颗粒的趋势相同,即粒径越小吸附容量越大;同一粒径,过氧化氢老化颗粒农膜吸附容量较原颗粒农膜有所增加,硝酸老化颗粒农膜和硫酸老化颗粒农膜吸附容量比原颗粒农膜有所降低,且硫酸老化颗粒农膜吸附容量减少最大。
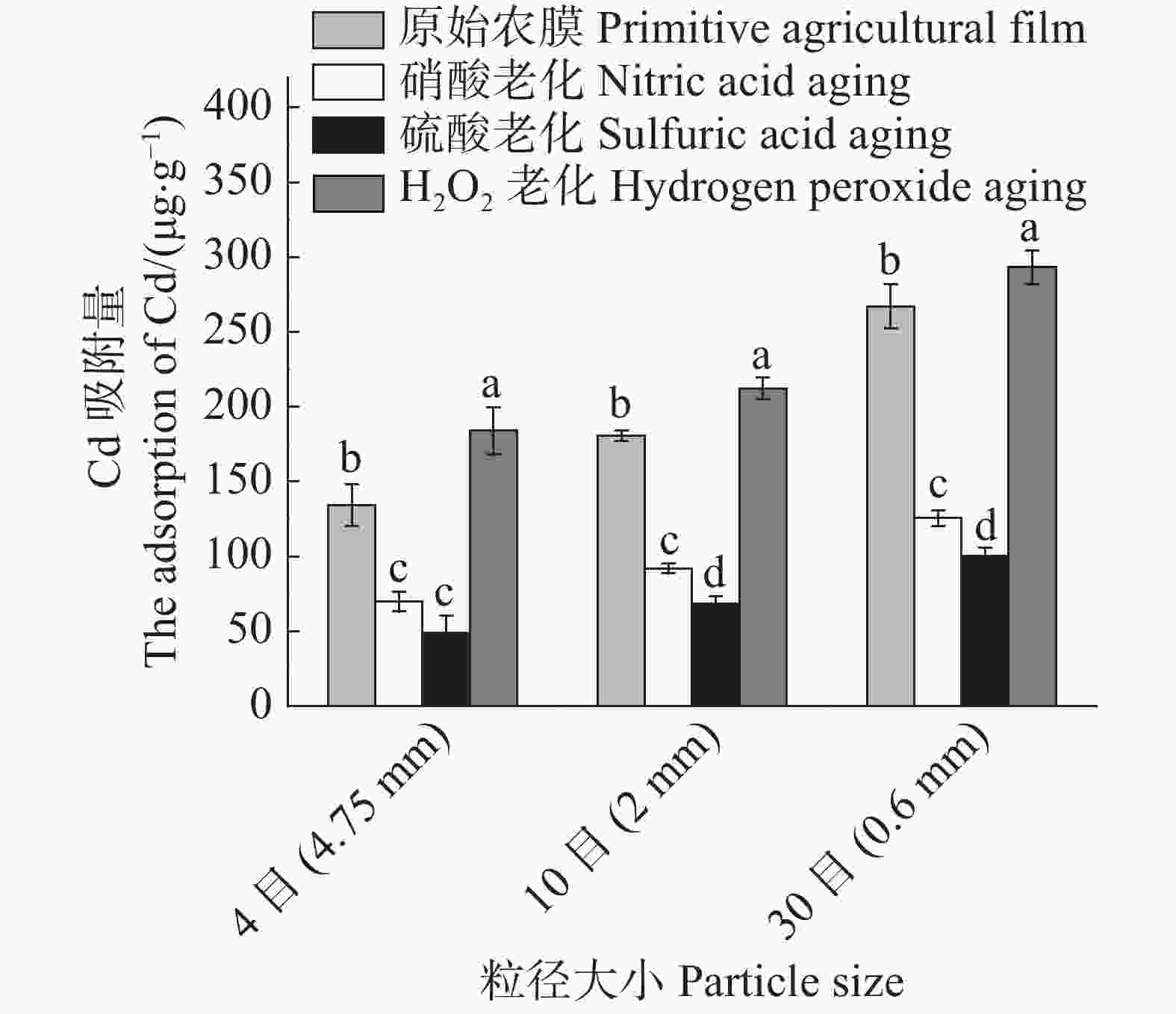
Figure 7. Comparison of cadmium adsorption by agricultural films with different particle sizes before and after aging
3种粒径农膜老化前后对Cd2+的吸附去除率差异显著,其中过氧化氢老化后相比原农膜吸附显著增加;4目、10目、30目3种粒径农膜老化后吸附容量分别增加了36.85%,17.44%,9.78%,说明粒径越小,褶皱程度对Cd2+吸附的影响越小;硝酸老化后相比原农膜吸附容量显著减少,其4目、10目、30目3种粒径吸附容量分别减少了48.14%,49.15%,53.02%,说明粒径越小,H+的竞争吸附作用对Cd2+吸附的影响越大(表3)。硝酸与硫酸老化后的4目粒径农膜对Cd2+的吸附差异不显著,这是因为4目粒径农膜提供的吸附位点过少,H+对Cd2+的竞争吸附影响较弱,故吸附差异不明显。
农膜粒径
Agricultural film
particle size老化前 Before aging 老化后 After aging 原始农膜 硝酸老化 硫酸老化 H2O2老化 q/(μg·g−1) q/(μg·g−1) q/(μg·g−1) q/(μg·g−1) 4目(4.75 mm) 134.38b 69.69c 49.04c 183.91a 10目(2 mm) 180.77b 91.93c 68.39d 212.29a 30目(0.6 mm) 267.09b 125.49c 100.26d 293.21a 注:同一列数据中标以不同字母表示在P=0.05的水平上差异显著。
Note: Values with different lowercase letters in the same column are significantly different at the 0.05 probability level .Table 3. Significant difference analysis of agricultural films with three particle sizes in adsorption of Cd2+ before and after aging
-
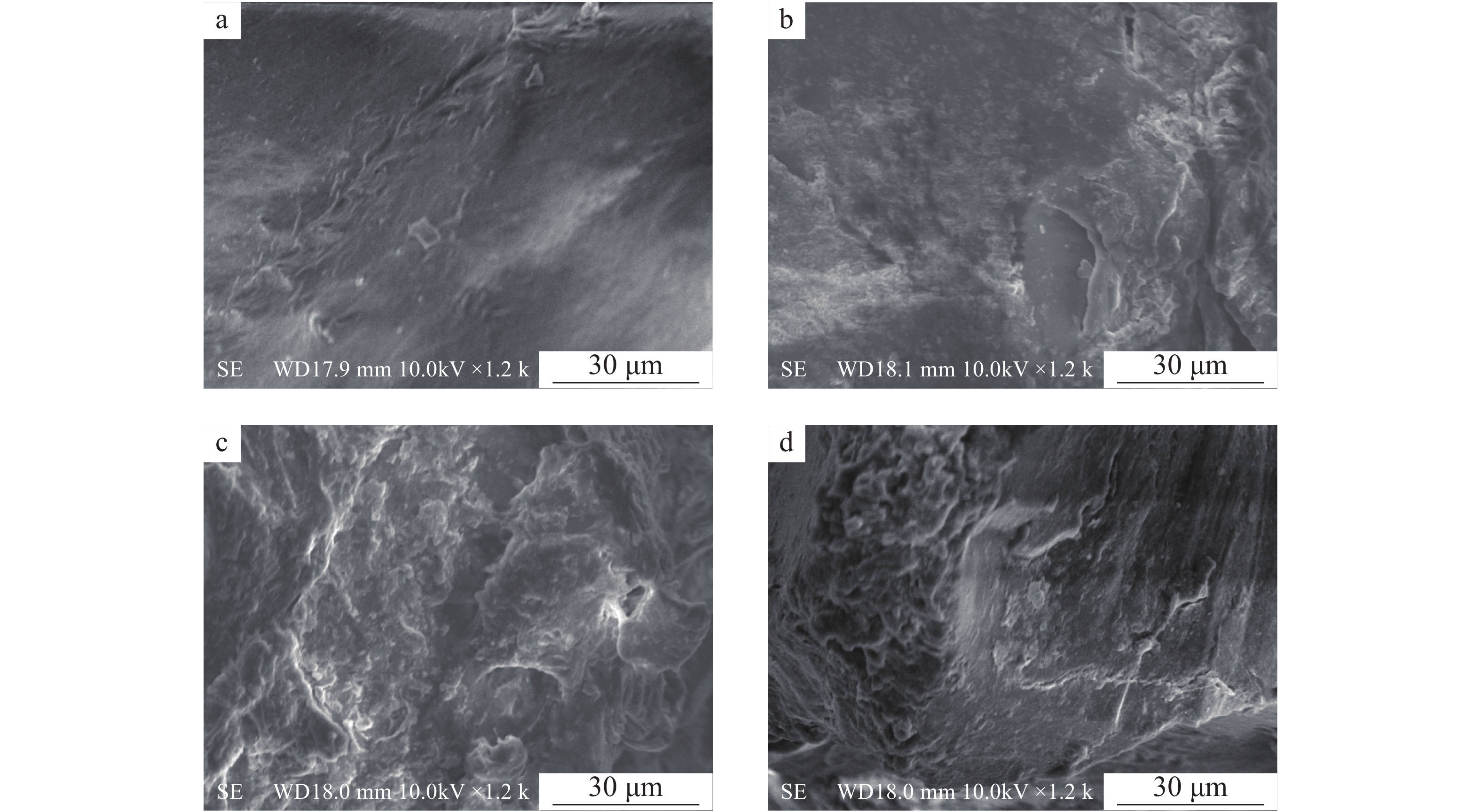
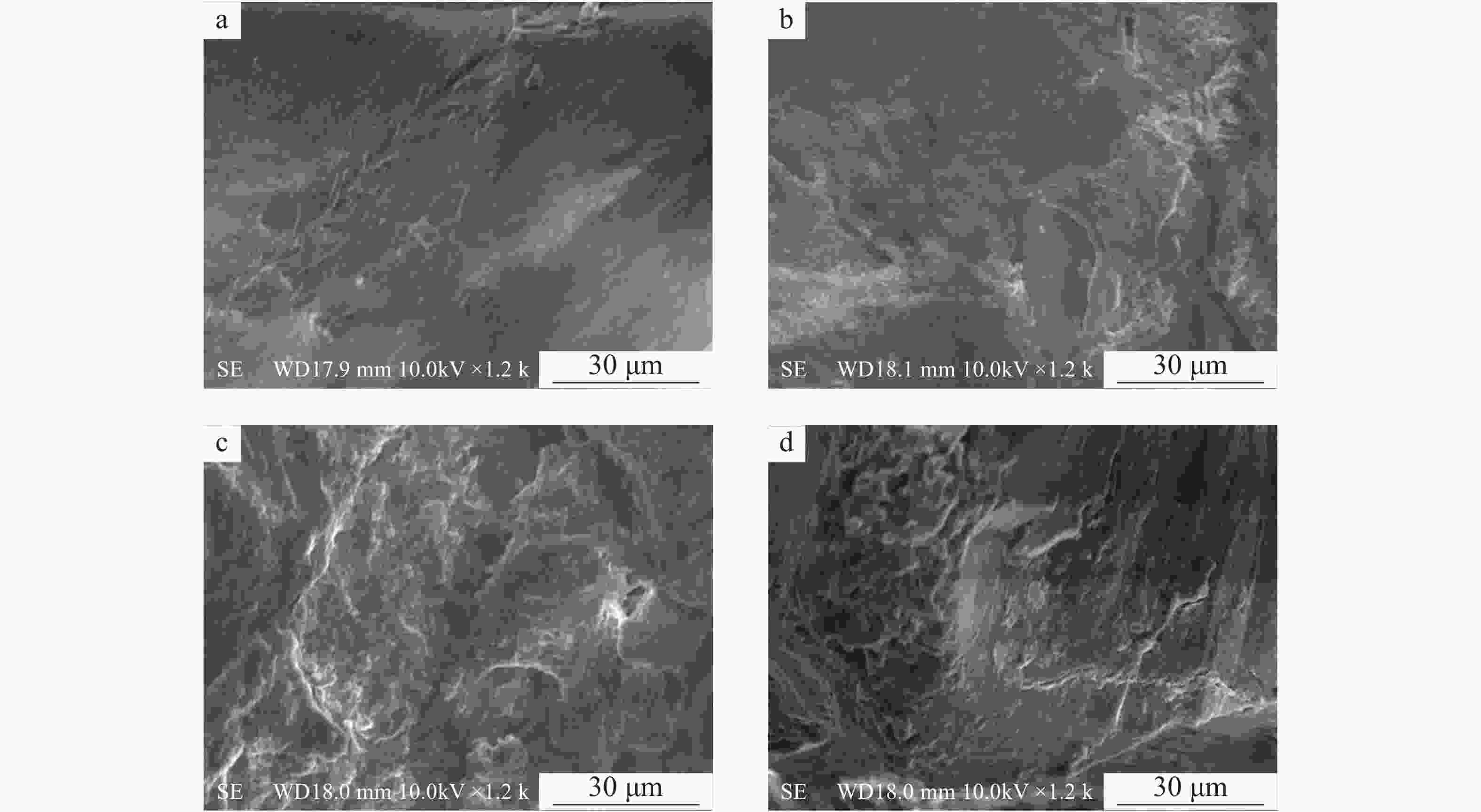
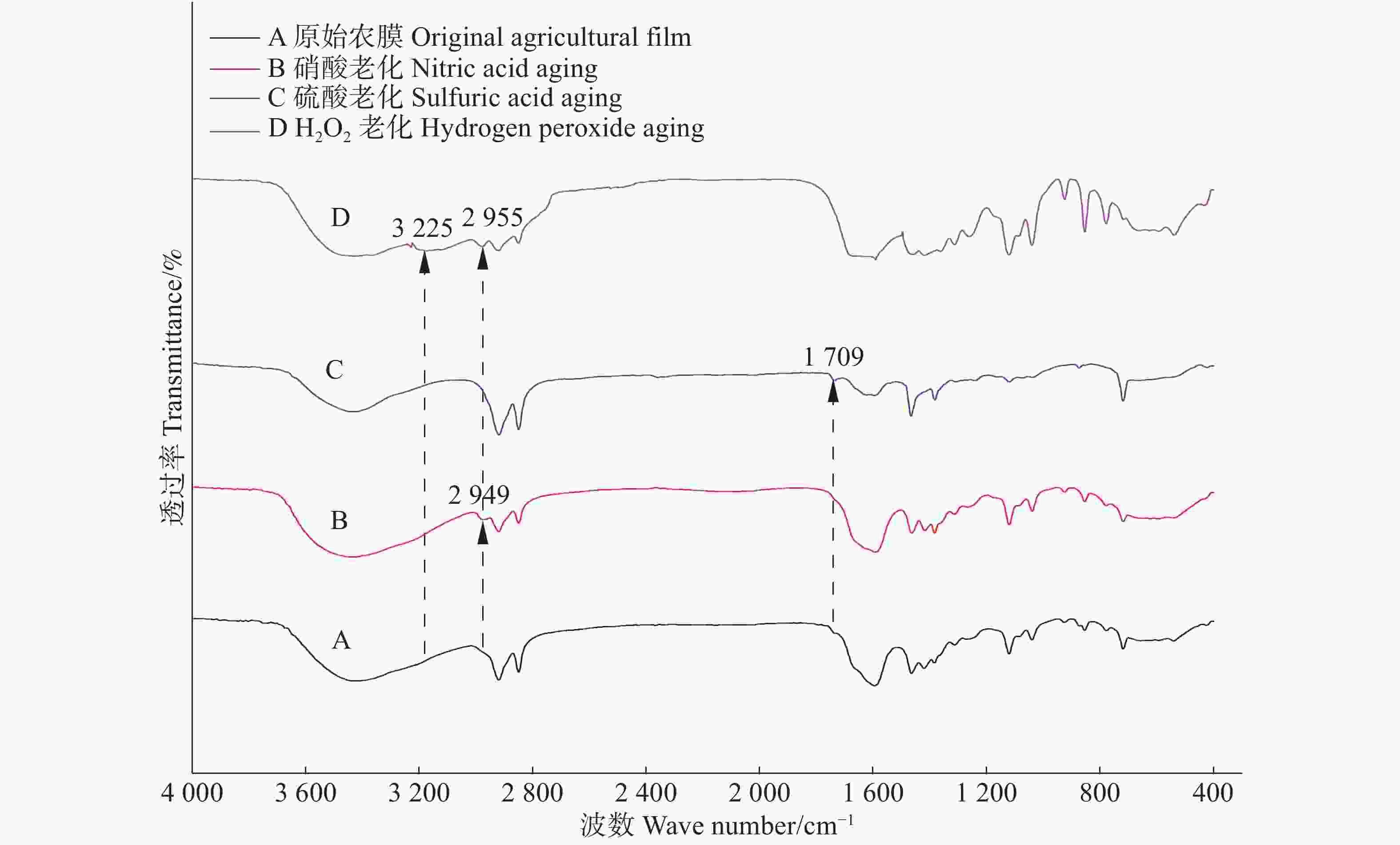
老化后农膜颗粒表面的粗糙程度明显增加,不同老化方式,农膜表面褶皱程度有所差异。过氧化氢老化对农膜颗粒表面的腐蚀程度最重,其表面出现大范围残屑及凸起的小球状气泡;硝酸老化与硫酸老化相比,硝酸老化对农膜表面有着相对较严重的褶皱(图8),推测为硝酸的氧化性较强于硫酸所致。
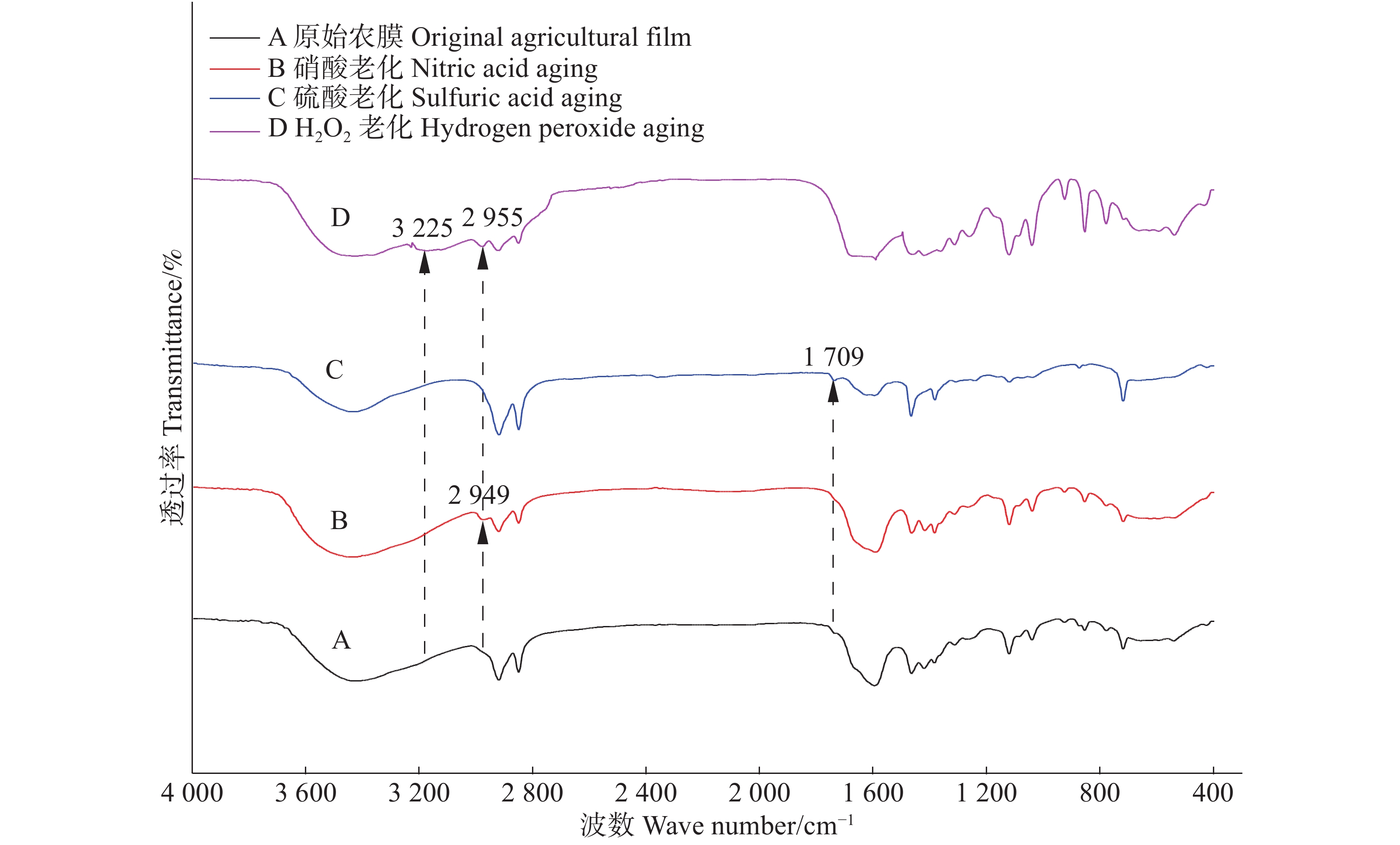
老化前后农膜颗粒的傅立叶变换红外光谱图解析,根据FT-IR自带及萨特勒(Sadtler)谱图数据库比对查证,在基团频率区大体相同,只在过氧化氢老化农膜颗粒中观测到3225 cm−1处增加了-NH-基团的伸缩振动;指纹区不同波数范围内产生新的特征峰明显(图9);此外,过氧化氢老化与硝酸老化农膜颗粒的光谱分别在波数2955 cm−1和2949 cm−1处出现了原始农膜颗粒中没有的特征峰,查证核实该处为-OH和-CHO伸缩峰。有意思的是硫酸老化农膜颗粒指纹区波数区特征峰不明显,仅在1709 cm−1处出现了一个新特征峰,查证核实该处为-C=O伸缩峰。指纹区过氧化氢老化于758 cm−1和644 cm−1处出现两个特征峰,查证核实该处分别为苯环邻二酚基基团-OH以及卤素类伸缩振动峰,这也同时支持了过氧化氢老化农膜颗粒对Cd2+吸附较强的机理。
-
本实验结果表明,农膜颗粒对Cd2+的吸附能力与粒径大小成反比。对于相同Cd2+浓度下吸附剂投加量对单位吸附容量的影响,有研究表明,随着投加量的增加,吸附剂对Cd2+的吸附容量逐渐下降[19-20],本研究结果与文献[19-20]所得结论一致。在溶质一定的情况下,随着吸附剂的增加,尽管有着众多的吸附位点,但溶质不足,导致实际吸附容量降低。随着农膜粒径的减小,其对Cd2+的吸附容量上升,这是因为粒径减小导致比表面积增大,进而为Cd2+提供了更多的吸附位点[21-23]。当溶液初始pH为1时,此时溶液中H+含量较多,致使H+与Cd2+产生强烈的吸附位点竞争作用,因此,农膜颗粒对Cd2+吸附容量较低;当初始pH增加至5时溶液中H+含量逐渐降低,致使H+对Cd2+吸附位点的竞争作用有所下降,故吸附容量逐渐升高。周瑛等[24]在探讨微塑料吸附水中痕量砷、铅的研究中也得到相同的结论,由此说明H+对吸附位点的竞争作用是导致不同pH条件下农膜颗粒对Cd2+吸附容量变化的原因。
农膜颗粒对Cd2+的吸附模型拟合吸附动力学结果更符合准二级动力学方程,基于准二级动力学模型假设,3种粒径农膜对Cd2+的吸附行为非单一因素决定,而是多因素共同作用的结果,包括范德华引力、疏水分配、静电作用、化学键等均可能对吸附速率产生影响[25-27],而等温吸附过程更符合Langmuir模型,因此,农膜颗粒对Cd2+是单层吸附过程[28]。郑文俊等[29]在探究PS微塑料对水体中铅的吸附机理其结果准二级动力学模型和Langmuir吸附等温模型能够有效描述PS颗粒对铅吸附过程。本研究结果与文献[25-29]所得结论一致。
-
本实验结果表明,硝酸老化农膜颗粒较原农膜颗粒吸附容量减少了50.10%,过氧化氢老化农膜颗粒较原农膜颗粒吸附容量增加了21.36%。有学者研究表明,老化使农膜颗粒的性能发生改变,不同老化方式对农膜颗粒影响不同,过氧化氢的氧化致使农膜表面发生褶皱甚至腐蚀,农膜的比表面积增大,吸附位点增多,有助于对Cd2+的吸附[30-31]。酸对农膜老化过程中,推测农膜表面吸附了大量的H+,导致农膜表面的吸附位点减少[14],吸附容量相比原农膜颗粒有所下降。同等浓度下,硫酸要比硝酸提供更多的H+,农膜表面的吸附位点更少,吸附容量下降的更多。老化后农膜颗粒表面含氧基团数量的增多,如过氧化氢老化,出现了新的-OH和-CHO管能团;推测老化是表面氧化的结果[32]。本研究结果与文献[10,33]的研究结果一致。
Adsorption of Cadmium by Agricultural Films with Different Particle Sizes and Aging Methods
DOI: 10.15886/j.cnki.rdswxb.2020.03.007
- Received Date: 2020-03-14
- Rev Recd Date: 2020-06-01
- Available Online: 2020-09-24
- Publish Date: 2020-09-24
-
Key words:
- agricultural film /
- different particle sizes /
- aging /
- cadmium /
- adsorption /
- Langmuir model
Abstract: The wide application of agricultural film makes the problem of residual pollution of agricultural film impossible to be ignored, and the impact of agricultural film particles on the migration and transformation of pollutants in the soil environment is rarely studied. Agricultural film with three different particle sizes (4 mesh, 10 mesh, 30 mesh)were used to study the effects of agricultural film concentration, initial pH, adsorption time, initial Cd2+ concentration and aging methods on Cd2+ adsorption, and Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FT-IR) and adsorption model fitting were used to explore the Cd2+ adsorption characteristics of three different particle sizes of agricultural films under different aging methods. The results showed that the adsorption capacity of agricultural film particles for Cd2+ was inversely proportional to the particle size.The pseudo-second-order kinetic model and Langmuir isotherm adsorption model better fitted the adsorption process of Cd2+ by the agricultural films. The agricultural film particles treated with nitric acid aging reduced its adsorption capacity of Cd2+ by 50.10% and the hydrogen peroxide aging treatment increased its adsorption capacity of Cd2+ by 21.36% as against the control(agricultural film particles without aging). These results can provide a reference for exploration of the migration process and mechanism of Cd2+ in soil through agricultural film particles and the prevention and control of residual agricultural film.
| Citation: | XUE Rixin, ZHANG Haixiang, LI Tinghang, LI Changheng, LIU Yin, WANG Qingqing, GUO Genmao. Adsorption of Cadmium by Agricultural Films with Different Particle Sizes and Aging Methods[J]. Journal of Tropical Biology, 2020, 11(3): 301-309. doi: 10.15886/j.cnki.rdswxb.2020.03.007 |






 DownLoad:
DownLoad: