-
金花茶(Camellia nitidissima Chi)属于山茶科、山茶属、金花茶组、金花茶系落叶小灌木植物,主要分布在中国南部和越南北部。金花茶是一种天然草药,2009年被卫生部批准为新食品来源[1],列为国家一级保护植物,被誉为“植物界的大熊猫”[2]。金花茶对于调节人体血脂、血糖、胆固醇,增强机体免疫力具有明显的效果,且能够抗菌消炎、清热解毒、利尿消肿、增强肝肾脏活力、防止血动脉硬化,抑制肿瘤生长等[3]。金花茶的叶和花中主要化学成分有黄酮、皂苷、多糖和茶多酚等[4]。虽然国内外学者对山茶属的其他种群的化学成分及其药理活性进行了比较深入的研究[5-8],但对于金花茶的单体化合物研究却鲜有报道。为了进一步探明金花茶的有效物质基础,笔者对金花茶花的化学成分进行分析,旨在为金花茶花的开发利用提供依据。
HTML
-
实验用的金花茶花于2016−06−16采自广西防城港,经广西“桂人堂”公司杨继住高级工程师鉴定确认。金花茶标本(JHCL-003)保存在中国广西防城港“桂人堂”公司。乙醇、二氯甲烷、乙酸乙酯、甲醇均为化学纯,购自南京双领化玻有限公司,高温低压蒸馏后使用;分析纯正丁醇购自天津科密欧化学试剂有限公司;色谱纯甲醇购自美国Tedia公司;100~200目、200~300目硅胶和GF 254型薄层层析板购自青岛海洋化工有限公司;葡聚糖Sephadex LH-20购自瑞典GE公司;反相硅胶C18购自日本YMC公司;反相硅胶板RP-18 F254购自德国默克公司;MCI(CHP 20P)材料购自日本三菱化学公司。
-
旋转蒸发仪(上海爱朗仪器有限公司),超纯水仪(南京易普易达科技发展有限公司),ZF-2型紫外灯(上海市安亭电子仪器厂),500 MHz核磁共振谱仪(德国Bruker),Agilent 1100 MSD液质联用仪(美国安捷伦),TSQ/Q/LC/MS光谱分析仪(Thermo Scientific),高效液相色谱Dionex Ultimate 3000(Thermo Scientific)。
-
取金花茶干花6 kg,粉碎后过40目筛,用95%的工业乙醇高温回流提取3次,时间分别为3,2,1 h。合并提取液,用旋转蒸发仪50 ℃低压回收工业乙醇,得金花茶花乙醇提取浸膏1 500 g。加6 L超纯水使其充分混悬后,加入等体积二氯甲烷萃取3次[9],40 ℃低压旋蒸去二氯甲烷后,得金花茶花二氯甲烷萃取部位52 g,剩余水相部分再加入等体积水饱和乙酸乙酯萃取3次,40 ℃低压回收乙酸乙酯后,得金花茶花乙酸乙酯萃取部位266.2 g。二氯甲烷相经过硅胶柱柱层析梯度洗脱,洗脱剂为二氯甲烷−甲醇,洗脱梯度为1∶0,49∶1,25∶1,15∶1,9∶1,5∶1,0∶1,依据薄层层析结果进行分析,合并浓缩洗脱剂得到4个组分A1~A4。组分A2经过硅胶层析处理后分为A2-1和A2-2 2段。采用硅胶柱、Sephadex LH-20凝胶柱、C18反相色谱柱、半制备高压液相色谱等色谱方法对金花茶花的化学成分进行分离纯化,同时运用1H-NMR,13C-NMR,ESI-MS等多种波谱技术鉴定化合物的结构。
1.1. 材料与试剂
1.2. 实验仪器
1.3. 提取与分离
-
A2-2经过Sephadex LH-20凝胶柱、C18色谱柱和HPLC制备柱处理分离后得到化合物2(3 mg)和化合物8(19 mg)。同样,乙酸乙酯相经过硅胶柱柱层析分离得到17个组分B1~B17。随后17个组分别分经过硅胶层析柱,Sephadex LH-20凝胶柱、C18色谱柱、MCI层析柱和HPLC制备柱处理分离后,从组分B3中分离得到化合物1(6.8 mg);从组分B10中分离得到化合物3(5.5 mg)、化合物4(55 mg);从组分12中分离得到化合物5(11.5 mg)、化合物6(18 mg)和化合物7(4.5 mg)。
-
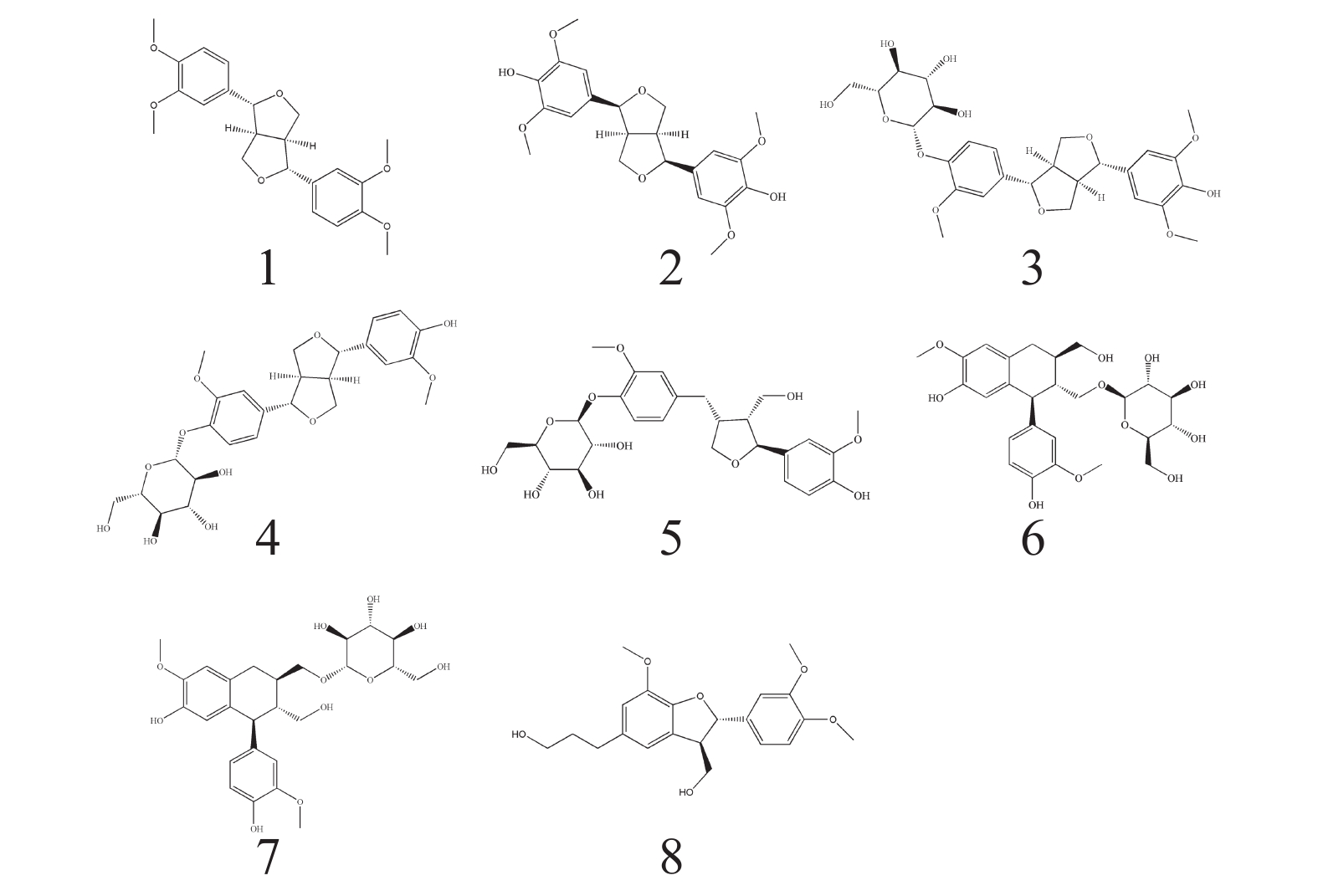
根据波谱数据,并结合参考文献[10-19] 的方法对化合物进行结构鉴定,得到了以下8个木脂素类化合物(图1)。
化合物1:白色无定形固体,ESI-MS,m/z 384.78 [M-H]−,分子式: C22H26O6。1H-NMR (500 MHz,CDCl3) δ: 6.87-6.95 (6H,m,H-2,H-2′,H-5,H-5′,H-6,H-6′),4.80 (2H,d,J=4.3 Hz,H-7,H-7′),4.31 (2H,dd,J=9.1,6.7 Hz,H-9b,H-9′b),3.94 (6H,s,4,4′-OCH3),3.91 (6H,s,3,3′-OCH3),3.91~3.93 (2H,H-9a,H-9′a),3.15 (2H,m,H-8,H-8′). 13C-NMR (125 MHz,CDCl3) δ: 150.8 (C-4,4′),150.3 (C-3,3′),135.2 (C-1,1′),119.8 (C-6,6′),112.7 (C-5,5′),110.9 (C-2,2′),87.4 (C-7,7′),73.3 (C-9,9′),57.6 (4,4′-OCH3),57.5 (3,3′-OCH3),55.8 (C-8,8′)。以上数据与文献[10]报道的数据对比基本一致,故鉴定化合物1为桉脂素。
化合物2:白色无定形固体,ESI-MS,m/z 417.09 [M-H]−,分子式为C22H26O8。1H-NMR (500 MHz,CDCl3) δ: 6.59 (4H,s,H-2,6,2′,6′),4.74 (2H,d,J=4.2 Hz,H-7,7′),4.30 (2H,m,H-9a,9′a),3.91 (2H,s,H-9b,9′b),3.91 (12H,s,H-OCH3),3.10 (2H,m,H-8,8′). 13C-NMR (125 MHz,CDCl3) δ: 147.2 (C-3,5,3′,5′),134.4 (C-4,4′),132.2 (C-1,1′),102.8 (C-2,6,2′,6′),86.1 (C-7,7′),71.8 (C-9,9′),56.4 (C-OCH3),54.4 (C-8,8′)。以上数据与文献[11]报道的数据对比基本一致,故鉴定化合物2为(+)-diasyringaresinol。
化合物3:白色无定形固体,ESI-MS,m/z 549.34 [M-H]−,分子式为C27H34O12。1H-NMR (500 MHz,CD3OD) δ: 7.16 (1H,d,J=8.4 Hz,H-5′),7.04 (1H,d,J=1.8 Hz,H-2′),6.93 (1H,dd,J=8.5,1.7 Hz,H-6′),6.66 (2H,s,H-2,6),5.34 (1H,m,H-1″),4.78 (1H,d,J=4.0 Hz,H-7′),4.72 (1H,d,J=4.3 Hz,H-7),4.26 (2H,m,H-9b,9′b),3.90 (3H,m,H-9a,9′a,6″a),3.87 (3H,s,3′-OCH3),3.85 (6H,s,3,5-OCH3),3.68 (1H,m,H-6b),3.38~3.49 (4H,m,H-2″,3″,4″,5″),3.14 (2H,m,H-8,8′). 13C-NMR (125 MHz,CD3OD) δ: 151.2 (C-3′),149.5 (C-3,5),147.7 (C-4′),137.8 (C-4),137.3 (C-1′),133.3 (C-1),120.0 (C-6′),118.3 (C-5′),111.9 (C-2′),104.8 (C-2,6),103.1 (C-1″),87.8 (C-7),87.2 (C-7′),78.4 (C-5″),78.0 (C-3″),75.1 (C-2″),73.0 (C-9′),72.9 (C-9),71.5 (C-4″),62.7 (C-6″),57.0 (3,5-OCH3),57.0 (3′-OCH3),55.7 (C-8),55.7 (C-8′)。以上数据与文献[12-13]报道的数据对比基本一致,故鉴定化合物3为(+)-isoeucommin A。
化合物4:白色无定形固体,ESI-MS,m/z 519.28 [M-H]−,分子式为C26H32O11。1H-NMR (500 MHz,CD3OD) δ: 7.15 (1H,d,J=8.3 Hz,H-5),7.02 (1H,d,J=2.4 Hz,H-2),6.94 (1H,m,H-2′),6.91 (1H,m,H-6),6.81 (1H,m,H-6′),6.77 (1H,d,J=8.1 Hz,H-5′),4.88 (1H,m,H-1″),4.74 (1H,d,J=2.9 Hz,H-7),4.69 (1H,m,H-7′),4.24 (2H,m,H-9a,9′a),3.86 (2H,m,H-9b,9′b),3.84 (3H,s,3-OCH3),3.84(3H,s,3′-OCH3),3.70 (1H,m,H-6″a),3.39-3.50 (5H,m,H-2″,3″,4″,5″,6″b),3.12 (2H,m,H-8,8′). NMR (125 MHz,CD3OD) δ: 151.0 (C-4′),149.2 (C-4),147.5 (C-3′),147.4 (C-3),137.5 (C-1),133.9 (C-1′),120.2 (C-6′),119.9 (C-6),118.0 (C-5),116.2 (C-5′),111.7 (C-2),111.1 (C-2′),102.9 (C-1″),87.5 (C-7′),87.1 (C-7),78.2 (C-5″),77.9 (C-3″),75.0 (C-2″),72.8 (C-9′),72.8 (C-9),71.4 (C-4″),62.6 (C-6″),56.9 (3-OCH3),56.6 (3′-OCH3),55.6 (C-8′),55.4 (C-8)。以上数据与文献[14]报道的数据对比基本一致,故鉴定化合物4为Pinoresinol 4-O-glucoside。
化合物5:白色无定形固体,ESI-MS,m/z 521.33 [M-H]−,分子式为C26H34O11。1H-NMR (500 MHz,CD3OD) δ: 7.14 (1H,d,J=8.3 Hz,H-5′),6.99 (1H,brs,H-2),6.89 (1H,d,J=8.3 Hz,H-5),6.79 (1H,d,J=8.0 Hz,H-2′),6.72 (1H,d,J=8.0 Hz,H-6),6.65 (1H,dd,J=8.0,1.4 Hz,H-6′),4.84 (1H,m,H-1″),4.83 (1H,d,J=6.3 Hz,H-7),4.01 (1H,m,H-9′a),3.88 (1H,m,H-6″a),3.86 (3H,s,3′-OCH3),3.83 (3H,s,3-OCH3),3.82 (1H,m,H-9a),3.75 (1H,dd,J=8.2,6.5 Hz,H-9′b),3.70 (1H,m,H-6″b),3.66 (1H,m,H-9b),3.51-3.39 (4H,m,H-2″,3″,4″,5″),2.93 (1H,dd,J=13.6,4.9 Hz,H-7′a),2.74 (1H,m,H-8′),2.53 (1H,dd,J=13.3,11.3 Hz,H-7′b),2.37 (1H,m,H-8). 13C-NMR (125 MHz,CD3OD) δ: 151.0 (C-3),149.2 (C-3′),147.4(C-4),146.0 (C-4′),139.7 (C-1),133.6 (C-1′),122.3 (C-6′),119.7(C-6),118.2 (C-5′),118.1 (C-5),116.4 (C-2),113.4 (C-2′),103.0 (C-1″),84.0 (C-7),78.3 (C-3″),78.0 (C-5″),75.0 (C-2″),73.8 (C-9′),71.5 (C-4″),62.6 (C-6″),60.7 (C-9),56.9 (3′-OCH3),56.6 (3-OCH3),54.3 (C-8),43.9 (C-8′). 33.8 (C-7′)。以上数据与文献[15-16]报道的数据对比基本一致,故鉴定化合物5为7S,8R,8′R-(-)-lariciresinol-4′-O-D-glucopyranoside。
化合物6:白色无定形固体,ESI-MS,m/z 521.30 [M-H]−,分子式为C26H34O11。1H-NMR (500 MHz,CD3OD) δ: 6.79 (1H,brs,H-2),6.75 (1H,d,J=8.1 Hz,H-5),6.65 (1H,s,H-2′),6.63 (1H,m,H-6),6.18 (1H,s,H-5′),4.12 (1H,d,J=7.8 Hz,H-1″),4.07 (1H,m,H-8),4.07 (1H,m,H-9a),3.81 (3H,s,3-OCH3),3.84 (1H,m,H-6″a),3.80 (3H,s,3′-OCH3),3.76 (2H,m,H-9′),3.66 (1H,m,H-6″b),3.64 (1H,m,H-5″),3.60 (1H,m,H-2″),3.35 (2H,m,H-3″,4″),3.22 (1H,m,H-9b),2.86 (2H,m,H-7′),2.03 (1H,m,H-8′),1.88 (1H,m,H-7). 13C-NMR (125 MHz,CD3OD) δ: 149.1 (C-3′),147.3 (C-3),146.0 (C-4′),145.3 (C-4),138.8 (C-6′),134.6 (C-1),129.3 (C-1′),123.3 (C-6),117.6 (C-5′),116.3 (C-5),114.6 (C-2),112.6 (C-2′),105.4 (C-1″),78.3 (C-3″),78.1 (C-5″),75.4 (C-2″),71.9 (C-4″),69.7 (C-9),65.4 (C-9′),63.0 (C-6″),56.7 (C-3-OCH3),56.6 (C-3′-OCH3),48.1 (C-8),46.1 (C-7),39.8 (C-8′),34.0 (C-7′)。以上数据与文献[17-18]报道的数据对比基本一致,故鉴定化合物6为(+)-Isolariciresinol 9-O-β-D-glucopyranoside。
化合物7:白色无定形固体,ESI-MS,m/z 521.28 [M-H]−,分子式为C26H34O11。1H-NMR (500 MHz,CD3OD) δ: 6.75 (1H,d,J=8.0 Hz,H-5),6.69 (1H,d,J=1.6 Hz,H-2),6.65 (1H,s,H-2′),6.64 (1H,m,H-6),6.18 (1H,s,H-5′),4.05 (1H,d,J=7.8 Hz,H-1″),3.84 (1H,m,H-6″a),3.80 (3H,s,3-OCH3),3.78 (3H,s,3′-OCH3),3.75 (1H,m,H-8),3.71 (2H,m,H-9′),3.69 (1H,m,H-9a),3.66 (1H,m,H-6″b),3.63 (1H,m,H-5″),3.60 (1H,m,H-2″),3.29 (2H,m,H-3″,4″),3.17 (1H,m,H-9b),2.85 (1H,m,H-8′),2.75 (2H,m,H-7′),1.97 (1H,m,H-7). 13C-NMR (125 MHz,CD3OD) δ: 149.1 (C-3′),147.4 (C-3),146.2 (C-4′),145.4 (C-4),138.9 (C-6′),133.9 (C-1),129.4 (C-1′),123.6 (C-6),117.5 (C-5′),116.1 (C-5),114.1 (C-2),112.5 (C-2′),104.0 (C-1″),78.4 (C-3″),78.0 (C-5″),75.2 (C-2″),71.9 (C-4″),70.9 (C-9′),65.6 (C-9),62.9 (C-6″),56.6 (C-3-OCH3),56.5 (C-3′-OCH3),48.7 (C-8),45.5 (C-7),41.3 (C-8′),33.8 (C-7′)。以上数据与文献[17-18]报道的数据对比基本一致,故鉴定化合物7为(+)-Isolariciresinol 9′-O-β-D-glucopyranoside。
化合物8:棕色无定形固体,ESI-MS,m/z 373.13 [M-H]−,分子式为C21H26O6。1H-NMR (500 MHz,CD3OD) δ: 6.95 (1H,d,J=1.6 Hz,H-2),6.83 (1H,dd,J=8.2,1.6 Hz,H-6),6.78 (1H,d,J=8.1 Hz,H-5),6.73 (2H,s,H-2′,H-6),5.50 (1H,d,J=4.9 Hz,H-7),3.85 (3H,s,3′-OCH3),3.83 (1H,m,H-9a),3.81 (6H,s,3-OCH3,4-O-CH3),3.77 (1H,m,H-9b),3.58 (2H,m,H-9′),3.49 (1H,m,H-8),2.64 (2H,t,J=7.5 Hz,H-7′),1.83 (2H,t,J=8.0 Hz,H-8′). 13C-NMR (125 MHz,CD3OD) δ: 147.7 (C-3),146.2 (C-4),146.1 (C-4′),143.8 (C-3′),135.5 (C-1′),133.5 (C-1),128.5 (C-5′),118.3 (C-6),116.6 (C-6′),114.8 (C-2′),112.8 (C-5),109.2 (C-2),87.6 (C-7),63.6 (C-9),60.9 (C-9′),55.4 (3′-OCH3),55.1 (3-OCH3),55.0 (4-OCH3),54.0 (C-8),34.4 (C-8′),31.5 (C-7′)。以上数据与文献[19]报道的数据对比基本一致,故鉴定化合物8为3′,4-O-dimethylcedrusin。
2.1. 金花茶花的化学成分分离纯化结果
2.2. 8个化合物的结构鉴定结果
-
由于金花茶的花比叶子更加稀有和珍贵,取材不易,国内外研究多集中在金花茶的叶子上,金花茶花的化学成分组成尚未完成清楚[20]。木脂素是植物雌激素的重要类别,广泛分布于高等植物界[21]。木脂素具有许多令人感兴趣的生物学特性,使其成为药物化学领域可利用的新型候选药物和主要结构支架的重要来源。其药理活性主要包括抗肿瘤、抗病毒、抗菌、抗氧化、抗炎、保肝、血小板活化因子拮抗活性和中枢神经系统保护作用等[22-27]。研究表明,化合物1具有支气管扩张作用[28],化合物2能够抑制酪氨酸酶的活性[29],化合物3,4,6,7具有抗氧化活性[30-32],化合物5能够抑制NO的生成[33],化合物8对内皮细胞有保护作用[19]。本实验采用多种色谱技术,从金花茶的花中共分离得到8个木脂素类化合物,且该8个化合物均为首次从金花茶花中分离得到。本实验结果丰富了金花茶中化学成分的类型,为木脂素类化合物的进一步研究提供了依据和理论基础,对金花茶花进行下一步化学成分及构效关系研究提供了物质基础。







 DownLoad:
DownLoad: