-
埃及伊蚊(Aedes aegypti)是虫媒病的主要传播媒介之一,雌蚊通过吸血传播登革热病毒、基孔肯亚病毒、寨卡病毒和黄热病病毒等多种病毒[1-2],严重危害人类的健康。2015年CDC报告指出,蚊虫在全球范围内造成了近一亿虫媒病病例,有效的蚊虫防控有利于公共卫生与健康。目前蚊虫防控主要包括环境防控、物理防控、化学防控和生物防控等方法,其中又主要依赖化学防控[3]。化学防控具有高效、简单、广谱等优点,常见的有拟除虫菊酯类、氨基甲酸酯类、有机磷、有机氯[4]、伊维菌素[5]和多杀菌素[6-7]等,但随着杀虫剂的大量及不合理使用,导致蚊虫对杀虫剂产生严重的抗性[8-11],探索新型杀虫剂对蚊虫防控具有重要意义。天维菌素(Tenvermectin,TVM)是一种新型十六元大环内酯类杀虫剂,是从基因工程菌链霉菌MHJ1011的发酵液中分离纯化的代谢产物。通过对阿维菌素产生菌(S. avermitilis)与米尔贝霉素(S. hygroscopicus)产生菌进行基因工程改造,从其代谢物中分离得到TVM A和TVM B两种有效成分[12]。有研究表明,天维菌素对小菜蛾(Plutella xylostella)、粘棉铃虫(Plutella xylostella)、松材线虫(Bursaphelenchus xylophilus)、朱砂叶螨(Tetranychus cinnabarinus)、柑橘红蜘蛛(Panonychus citri)和二斑叶螨(Tetranychus urticae)均有较高的杀虫活性[13-14]。本实验室的研究结果证明了大环内酯类抗寄生虫药伊维菌素、多杀菌素等对埃及伊蚊有非常好的杀灭作用[15];本实验室的前期实验结果表明,天维菌素对埃及伊蚊也具有一定的杀灭作用,但尚不清楚其作用机制。本研究以埃及伊蚊敏感株为实验材料,使用天维菌素处理埃及伊蚊幼虫,利用转录组测序并结合生物信息学分析,在转录组水平上比较埃及伊蚊幼虫在给药前后的基因表达差异,并利用Real-time Quantitative PCR(rt-qPCR)对差异表达基因进行验证。本研究在转录组水平上探究天维菌素对埃及伊蚊幼虫的作用机制,旨在为深入研究埃及伊蚊对天维菌素的抗性机制奠定基础。
HTML
-
供试埃及伊蚊幼虫由海南大学生命科学与药学院热带动物医学与媒介生物学实验室提供。将20 g·L−1天维菌素用二甲基亚砜(DMSO)梯度稀释为200 mg·L−1稀释液,然后将1 mL天维菌素稀释液加入至99 mL的过夜去氯水里使天维菌素处理液的终浓度为2 mg·L−1(T),同时在对照组(Control)中加入1 mL二甲基亚砜,每处理组设3次重复。将20只3龄末4龄初的埃及伊蚊幼虫放入盛有天维菌素处理液或对照组处理液的杯中,处理24 h后统计并移除死亡幼虫,收集存活蚊虫用于下游转录组分析。
-
采用Trizol法[16]提取药物处理组和对照组幼虫的总RNA,用1%的琼脂糖凝胶电泳检测,并用微量核酸检测仪测RNA浓度和纯度。利用Nanodrop及Bioanalyzer进行RNA质量验证。
-
用带有Oligo(dT)的磁珠富集有polyA尾巴的mRNA,然用DNA探针杂交rRNA,去除rRNA纯化后即得到所需RNA。以获得的片段化RNA为模板,用随机的N6引物进行反转录得到cDNA,将cDNA末端补平,5′端磷酸化,3′段连接接头后进行PCR,PCR产物热变性成单链,再用一段桥式引物将单链DNA环化得到单链环状DNA文库,测序由华大基因有限公司完成。获得的转录组数据利用生物信息学方法进行数据过滤,基因组比对,差异表达分析及基因注释等。
-
选取6个差异表达基因(Cuticle protein 19,Tetraspanin-11-like,Glycine-rich cell wall structural,Cuticle protein,Glutathione S-transferase-1,Carboxylesterase 2),利用Primer 5软件设计定量PCR引物(表1),以RPS17为内参基因,每个样品设3个重复,利用2−ΔΔCt法计算基因表达量。
基因Gene 正向引物Forward primer 方向引物Reverse primer Cuticle protein 19 GGGGATGTCGTTAAGGGAG TTGTGGTGGTCGGATTTG Tet raspanin-11-like TGGACCGTAGTGGATAAGAA GTCGCAATCAGCACATAGA Glycine-rich cell wall structural CGATAGGTGGACAGGGAC AAACTGTGGACCGAAAGG Cuticle protein TCTTGTAGCTGCTCCACTCA TTCCTGCTGGGACTTCTG Glutathione S-transferase-1 AAGCCGAAGAGCACAAGA GACTCCACCAGGTAGACCA Carboxylesterase 2 GCAAAGCGATGAACATAA TACTGGTTGAACGGGACT Ribosomal protein S17 AAGAAGTGGCCATCATTCCA GGTCTCCGGGTCGACTTC Table 1. Primers for fluorescence quantitative PCR
1.1. 埃及伊蚊幼虫的药物处理实验
1.2. 埃及伊蚊幼虫总RNA的提取
1.3. 埃及伊蚊幼虫转录组测序及分析
1.4. 差异表达基因的实时荧光定量PCR验证
-
对埃及伊蚊幼虫对照组(Control 1-3)和处理组(T1-3)的RNA样本进行转录组测序,样品比对基因组的平均比对率为90.89%,比对基因集的平均比对率为87.88%;样品比对基因组和比对基因集分别得到14 748,14 679,14 667个Unigene和14 354,14 402,14 356个Unigene,这表明埃及伊蚊幼虫表达的基因数量大约为14 500个左右。从各个样品基因表达分布图(图1)可见,对照组和处理组的极低表达水平和中高表达水平基因数均接近,较低表达水平基因数则偏高,且处理组和对照组的组内3个重复之间的重复度较好。
-
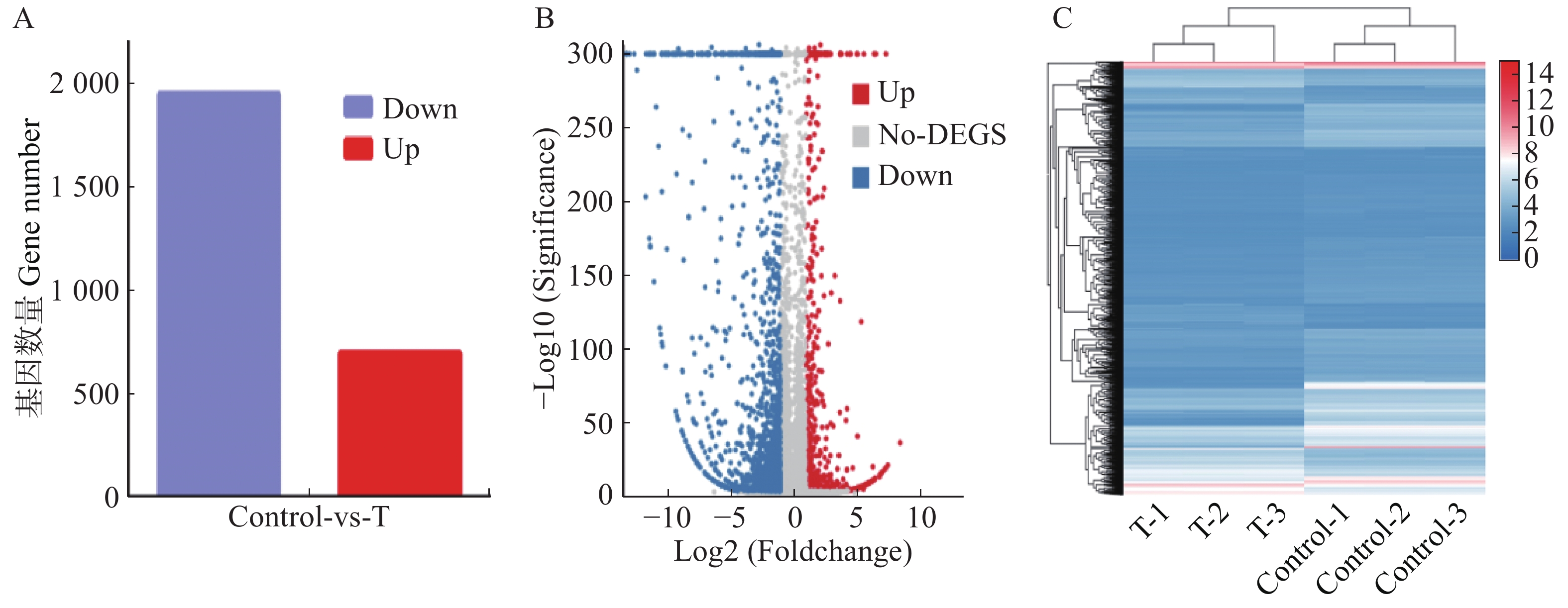
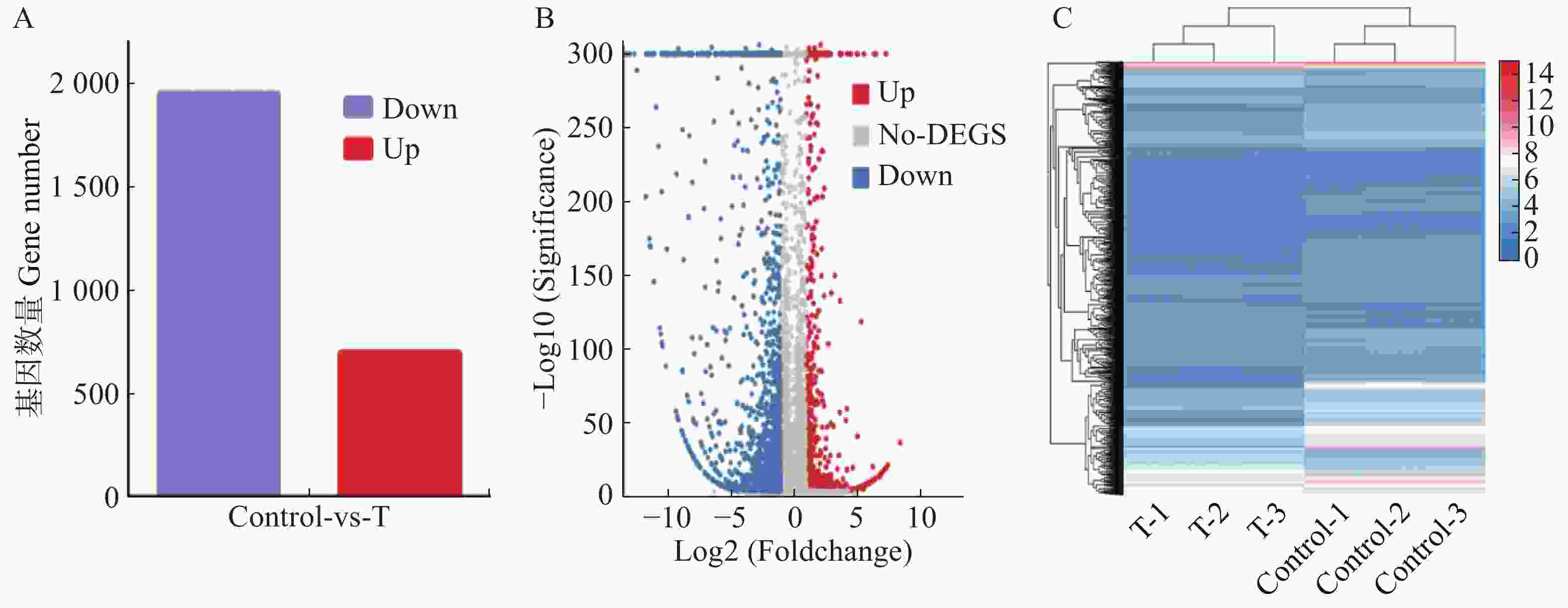
比较药物处理组与对照组的基因表达量,当基因差异倍数(Fold Change)为2倍及以上并且P≤0.05时,即log2(FoldChange) ≤ −1或log2(FoldChange) ≥ 1的基因,筛选为显著差异表达基因。通过差异表达基因数量的分析共筛选出2 647个表达差异显著的基因,其中上调表达的基因有697个,下调表达的基因有1 950个(图2-A)。差异基因表达量的火山图(图2-B)表明,在差异表达的基因中,上调表达的基因93.54% 主要分布在log2(FoldChange)绝对值1~5之间;下调表达的基因98.31% 分布在log2 (FoldChange)绝对值1~10之间,极少部分(1.69%)分布在log2 (Fold Change)绝对值大于10倍的范围。差异基因表达量聚类热图(图2-C)可见,药物处理组与对照组基因总的表达趋势或表达变化趋势存在差异,但是药物处理组的组内3个样品表达趋势或表达变化趋势相似,对照组的组内3个样品表达趋势或表达变化趋势相似,表明组内样本的重复性好,数据的可信度较高。
-
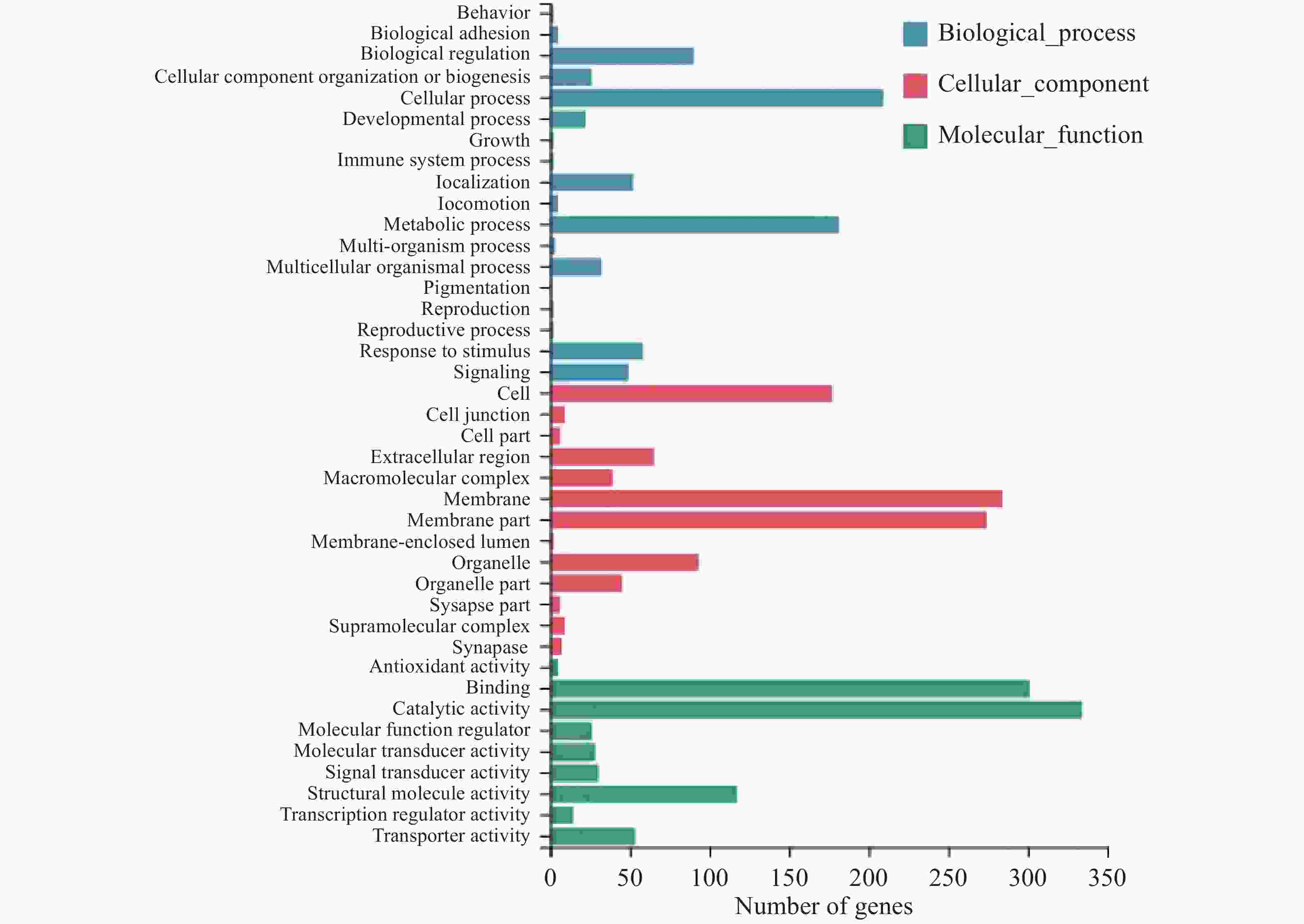
对差异表达基因进行GO(Gene Ontology)富集分析,共得到40个显著富集的GO功能条目,分为分子功能(Molecular function)、细胞组分(Cellular component)和生物过程(Biological process)三大功能类(图3)。在分子功能类别里,有686个差异表达基因,其中结合(Binding)、催化活性(Catalytic activity)、结构分子活性(Structural molecule activity)和转运活性(Transporter activity)中包含的差异基因最多;细胞组分类别中,差异表达基因数量为463个,主要富集在细胞(Cell)、膜(Membrane)、细胞器(Organelle);富集到生物过程中的差异表达基因有323个,其中细胞过程(Cellular process)、生物调节(Biological regulation)和代谢过程(Metabolic process)富集水平最高。
-
对埃及伊蚊幼虫差异基因进行KEGG pathway生物通路注释分类及富集分析,将基因参与的KEGG代谢通路分为6个分支:细胞过程(Cellular Processes)、环境信息处理(Environmental Information Processing)、遗传信息处理(Genetic Information Processing)、人类疾病(Human Disease)(仅限动物)、代谢(Metabolism)、有机系统(Organismal Systems)。根据KEGG pathway注释分类,对数据进行富集分析,由差异基因 KEGG Pathway 富集气泡图(图4)可见,差异基因主要富集在嘧啶代谢(Pyrimidine metabolism)、嘌呤代谢(Purine metabolism)、蛋白质的消化与吸收(Protein digestion and absorption)、Toll和Imd信号通路(Toll and Imd signaling pathway)、细胞色素P450对异生物素的代谢(Metabolism of xenobiotics by cytochrome P450)、谷胱甘肽代谢(Glutathione metabolism)、药物代谢−其他酶(Drug metabolism−other enzymes)等通路,主要富集在代谢、免疫应答、生物合成以及消化与吸收等过程。
-
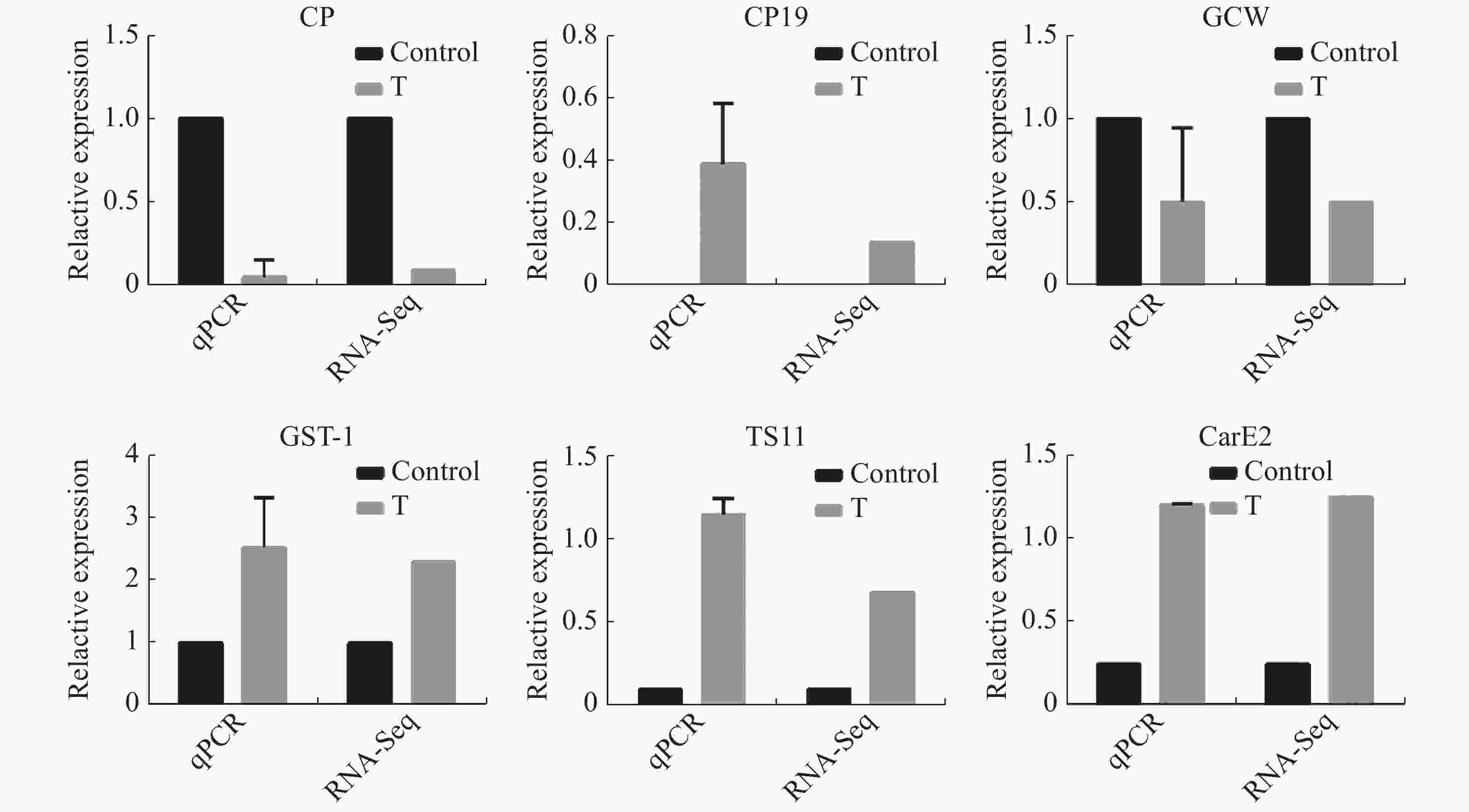
为验证RNA-Seq数据的准确性,从表达差异基因中随机挑选6个基因,其中3个是表达上调基因GST-1,TS11和CarE2,另外3个是表达下调基因CP,CP19和GCW。结果(图5)表明,6个基因的相对表达量与转录组测序结果变化趋势一样。
2.1. 埃及伊蚊幼虫转录组测序分析
2.2. 埃及伊蚊幼虫差异表达基因的筛选
2.3. 埃及伊蚊幼虫差异表达基因的GO功能富集分析
2.4. 埃及伊蚊幼虫差异表达基因KEGG pathway富集分析
2.5. 埃及伊蚊幼虫差异表达基因的实时荧光定量PCR验证
-
RNA测序(RNA-Seq)是一种高通量测序技术,已成为全转录组基因表达分析的首选工具,RNA-Seq最典型的应用是比较组之间差异表达基因的鉴定,此技术目前被广泛应用于生命科学领域[17-20]。本研究通过埃及伊蚊幼虫药物处理前后的比较转录组测序分析,从测序结果中筛选出2 647个显著性差异表达的基因,并对这些异基因进行GO功能富集分析和KEGG pathway 富集分析。GO功能富集分析表明,分子功能、细胞组分和生物过程中的差异表达基因数均较多,且主要集中在代谢过程、结合、催化活性、膜,这些功能又与药物的吸收、运输、代谢有关。结果表明,埃及伊蚊幼虫机体受到药物处理作出一系列反应,推测其可能与药物代谢抗性有关,这在后续实验中还有待进一步探究。
通过对差异基因KEGG通路分析,信号通路主要集中于嘌呤嘧啶代谢、谷胱甘肽代谢、细胞色素P450对异生物素的代谢等。细胞色素P450对于外源性和内源性成分的解毒或激活作用至关重要[21],在其必需的电子供体NADPH细胞色素P450还原酶或细胞色素b5的作用下氧化。P450基因转录上调,导致P450蛋白水平和P450活性升高,可增强昆虫体内杀虫剂的代谢解毒作用,导致抗药性的发展[22]。谷胱甘肽转移酶(glutathione S-transferase,GST)是一个大型多功能酶家族,主要参与内源性和外源性化合物的解毒[23]。GST抗药性的主要机制是谷胱甘肽偶联反应,GST催化还原谷胱甘肽与外源化合物结合,增加了它的水溶性,促进了它从细胞中排泄[24-25]。本研究发现GST和细胞色素P450基因表达量在处理前后差异显著,且KEGG分析,注释到细胞色素P450对异生物素的代谢和谷胱甘肽代谢,表明埃及伊蚊幼虫对天维菌素的代谢得到加强。本研究通过RNA-Seq,筛选出埃及伊蚊经天维菌素处理前后表达差异的基因,为进一步探究埃及伊蚊中天维菌素的毒理机制以及埃及伊蚊潜在的抗药性相关基因奠定了基础。









 DownLoad:
DownLoad: