-
芋螺毒素(conotoxins)是由热带海洋肉食性软体动物芋螺(Conus)分泌的一类活性多肽,主要用于捕食猎物和防御天敌,可特异性作用于配体门控离子通道(包括烟碱型乙酰胆碱受体、5−羟色胺、γ−氨基丁酸等)和电压门控离子通道(包括钠离子、钙离子、钾离子等通道)、G蛋白耦联受体和神经递质转运体等诸多疾病的关键靶点[1-6]。由于其分子量小、结构多样、针对靶点特异性强、活性显著等特点,现已经成为二硫键氧化折叠、多肽−受体相互作用、以及新药开发的研究热点[7-9]。芋螺毒素对神经痛、成瘾、癫痫、精神病、癌症等多种疑难杂症的治疗有极好的开发应用前景[6, 10-11],已成为当今神经科学研究和新药研发的热点。国际上已有10种芋螺毒素进入过临床研究的不同阶段[12],其中来源于幻芋螺(Conus magus)的含3对二硫键的ω−芋螺毒素MVIIA(齐考诺肽,ziconotide,Prialt,Elan Pharmceuticals)于2004年经FDA批准用于严重慢性疼痛的治疗[13-15]。芋螺毒素通常由10~40个氨基酸组成,富含半胱氨酸,可形成2~4对分子内二硫键[16]。二硫键是芋螺毒素发挥生物活性的结构基础[17],对稳定其高级结构、增强其作用活性、提高其对靶受体的选择性等具有重要意义[18]。二硫键连接方式不同(形成二硫键构造异构体),直接影响芋螺毒素的三维结构,继而导致其活性以及选择性的差异[18-20]。芋螺毒素的化学合成过程中,二硫键氧化折叠过程是其中的关键步骤,同时也是难点所在[19, 21]。当含有2对、3对、4对二硫键的芋螺毒素进行氧化折叠时,理论上应分别存在3,15,105种二硫键连接方式的构造异构体。目前,芋螺毒素二硫键的氧化折叠多采用化学方法,其中包括一步氧化法和定点合成法[21]。相比于定点合成法,一步氧化法经济成本较低、操作简便、且不使用有毒有害化学试剂,还可快速获得在化学结构上更稳定的二硫键异构体,减少对二硫键异构体的探索步骤,提高合成效率,以便进行后续的相关活性研究。全世界约有700种芋螺,遍布世界各个暖海海域[22]。我国有超过100种芋螺,南海分布的芋螺超过60种,是我国迈进“海洋强国”、振兴“蓝色医药产业”的重要战略资源,极具研究开发价值。疣缟芋螺(Conus lividus)为南海产食虫芋螺,迄今为止,近百种芋螺毒素成熟肽序列从疣缟芋螺中被发现[23],其中,LvIA不仅是α3β2 nAChR的强阻断剂(IC50为8.7 nmol·L−1),还能显著区分α3β2与α6/α3β2β4 nAChRs,具有较好的研究潜力[24]。芋螺毒素Lv68为本课题组从疣缟芋螺cDNA中克隆得到的新型M超家族芋螺毒素,氨基酸序列为CCLWTCNPYCFPCCY,具有典型的III型半胱氨酸框架(-CC-C-C-CC-),含6个半胱氨酸,氧化折叠后可形成三对分子内二硫键。M超家族芋螺毒素通常可作用于电压门控钠离子通道(μ-conotoxins)、电压门控钾离子通道(κM-conotoxins)和烟碱型乙酰胆碱受体(ψ-conotoxins)[17, 19, 25-26]。芋螺毒素Lv68具有III型半胱氨酸框架,与其同类的M超家族芋螺毒素的作用靶点大多集中于电压门控钠离子通道,其中仅有3个ψ-conotoxins作用于肌肉型乙酰胆碱受体[25, 27],未见有作用于神经型乙酰胆碱受体的该类芋螺毒素报道。通过序列比对,发现芋螺毒素Lv68与芋螺毒素Asi3a,Pr3a,Pu3.4等具有较高的序列相似性。LEBBE[28]等对芋螺毒素Asi3a作用于电压门控钠离子通道的7个亚型、电压门控钾离子通道的6个亚型,以及乙酰胆碱受体的4个亚型(α1β1δε,α7,α4β4,α3β4 nAChRs)进行了活性测试,均无阻断作用。JIMENEZ[29]等发现Pr3a在金鱼实验中可致使金鱼瘫痪。说明此类芋螺毒素的活性靶点尚不明确。笔者对含半胱氨酸框架III的M超家族新型芋螺毒素Lv68的一步氧化折叠及其产物活性进行研究,旨在为以烟碱型乙酰胆碱受体为靶点的先导药物分子的发现奠定基础,同时为该类其他芋螺毒素的氧化折叠方法、生物活性研究以及深入开发利用提供参考和借鉴。
HTML
-
Fmoc−氨基酸[Fmoc-Asn(Trt)-OH,Fmoc-Cys(Trt)-OH,Fmoc-Leu-OH,Fmoc-Phe-OH,Fmoc-Pro-OH,Fmoc-Thr(tBu)-OH,Fmoc-Trp(Boc)-OH,Fmoc-Tyr(tBu)-OH];Wang树脂、N,N−二环己基碳二亚酰胺(DCC)、N,N−二甲基甲酰胺(DMF)、1羟基苯并三唑(HOBt)、三羟甲基氨基甲烷(Tris)、二甲基亚砜(DMSO),均购自吉尔生化上海有限公司;色谱级乙腈(Acetonitrile,ACN,美国Tedia公司);色谱级三氟乙酸(Trifluoroacetic acid,TFA,美国Tedia公司);分析型C18柱(Vydac,5 μm,4.6 mm×250 mm)和制备型C18柱(Vydac,10 μm,22 mm × 250 mm),均购于美国Grace公司。其他常用试剂均为国产分析纯,均购自广州化学试剂公司。缓冲液ND96溶液组成:96.0 mmol·L−1 NaCl,2.0 mmol·L−1 KCl,1.8 mmol·L−1 CaCl2,1.0 mmol·L−1 MgCl2,5 mmol·L−1 Hepes,pH 7.1~7.5。流动相A为ddH2O含0.1% TFA,流动相B为90% 乙腈含0.1% TFA。
-
全自动多肽合成系统(ABI433,Applied Biosystems);超纯水系统(PURELAB Ultra,ELGA);制备型高效液相色谱仪(2535 Separations Module,Waters);分析型高效液相色谱仪(ACQUITY UPLC H-Class Bio,Waters);液相色谱−质谱联用仪(Acquity H Class-Xevo TQD,Waters);电子天平(Xs365M-sc,Precisa);磁力搅拌器(RET Basic C,IKA);超微量分光光度计(NanoDrop 2000,ThermoFisher);离心浓缩仪(RVC 2-25 CDplus,北京五洲东方科技发展有限公司);AXON CLAMP 900A双电极电压钳(美国Molecular Devices公司);P-97微电极拉制仪(美国Sutter公司);SZX-ILLD2-200体视显微镜(日本Olympus公司);Nanoliter 2000显微注射仪(美国Sutter公司)。
-
芋螺毒素Lv68线性肽(CCLWTCNPYCFPCCY)采用Fmoc固相合成法[30]合成,切割后的线性肽的所有氨基酸均不含有侧链保护基,6个半胱氨酸的巯基均处于还原状态,该线性肽的理论分子量为1 819.19 Da。固相合成得到的Lv68线性肽粗肽采用制备型HPLC进行纯化,分离条件为流动相B由25%至50%,30 min线性梯度洗脱,柱温箱40 ℃,流速10 mL·min−1,检测波长214 nm。收集主峰,将纯化后的线性肽进行UPLC-MS分析鉴定。UPLC-MS线性洗脱梯度条件:柱温箱40 ℃,90%~50% 流动相 A,10%~50% 流动相 B,7 min线性梯度洗脱,流速0.5 mL·min−1,质谱参数Source Voltages:Capiliary 0.5 kV,Cone 30 V;Source Temperalutes:Desolvation Temp. 600 ℃;Source Gas Flow:Desolvation 800 L·Hr−1,Cone 50 L·Hr−1。经离心干燥后冻存,用于后续的氧化折叠反应。
-
选择浓度均为0.1 mol·L−1的2种缓冲液( Tris-HCl和 NH4HCO3)、3种氧化还原对(GSH/GSSG 0.5 mmol·L−1/0.5 mmol·L−1,GSH/GSSG 1 mmol·L−1/0.5 mmol·L−1,GSH/GSSG 1 mmol·L−1/1 mmol·L−1)、3种线性肽浓度(10 ,20 ,50 μmol·L−1)和反应时间(10 min,0.5 ,1 ,2 ,3 ,4 ,6 h)作为一步法氧化折叠反应条件的组合方案(表1),并各加入1 mmol·L−1 EDTA。将纯化后的线性肽溶于少量60%乙腈水溶液,转移至含缓冲液和氧化还原对的反应体系中,采用选定的线性肽浓度,磁力搅拌速度500 r·min−1,室温条件下反应。在适宜的反应时间时取反应溶液20 μL,在所取反应溶液样品中加入甲酸,使甲酸终浓度为8%从而终止氧化反应。以UPLC-MS分析不同取样时间的反应溶液中产物色谱峰的变化,动态监测其反应进程,根据分子量区分氧化产物和副产物,根据峰面积大小确定主异构体产物。UPLC线性洗脱梯度条件为:柱温箱40 ℃,95%~50% 流动相A,5%~50% 流动相B,7 min线性梯度洗脱,流速0.5 mL·min−1,紫外吸收检测波长为214 nm。通过动态监测反应进程,分别考察缓冲液、氧化剂、线性肽浓度以及反应时间对主异构体产物产率的影响,以液相图中折叠肽峰面积计算折叠肽的产率,筛选出产率最高的一步氧化反应条件。
序号
Code缓冲液
Buffer线性肽浓度
Linear peptide
concentration氧化还原对
Redox pairs1 0.1 mol·L−1 Tris-HCl, 1 mmol·L−1 EDTA 20 μmol·L−1 GSH/GSSG 0.5 mmol·L−1/0.5 mmol·L−1 2 0.1 mol·L−1 Tris-HCl, 1 mmol·L−1 EDTA 20 μmol·L−1 GSH/GSSG 1 mmol·L−1/0.5 mmol·L−1 3 0.1 mol·L−1 Tris-HCl, 1 mmol·L−1 EDTA 20 μmol·L−1 GSH/GSSG 1 mmol·L−1/1 mmol·L−1 4 0.1 mol·L−1 NH4HCO3, 1 mmol·L−1 EDTA 20 μmol·L−1 GSH/GSSG 0.5 mmol·L−1/0.5 mmol·L−1 5 0.1 mol·L−1 NH4HCO3, 1 mmol·L−1 EDTA 20 μmol·L−1 GSH/GSSG 1 mmol·L−1/0.5 mmol·L−1 6 0.1 mol·L−1 NH4HCO3, 1 mmol·L−1 EDTA 20 μmol·L−1 GSH/GSSG 1 mmol·L−1/1 mmol·L−1 7 0.1 mol·L−1 Tris-HCl, 1 mmol·L−1 EDTA 10 μmol·L−1 GSH/GSSG 1 mmol·L−1/1 mmol·L−1 8 0.1 mol·L−1 Tris-HCl, 1 mmol·L−1 EDTA 50 μmol·L−1 GSH/GSSG 1 mmol·L−1/1 mmol·L−1 Table 1. Optimization scheme of oxidative folding reaction of conotoxin Lv68
-
取20 mg芋螺毒素Lv68线性肽,采用筛选的最优氧化折叠条件合成Lv68折叠肽。将折叠反应终溶液中的主异构体产物通过制备型HPLC进行分离纯化,纯化后的主异构体由UPLC分析其纯度,并用UPLC-MS进行相对分子量检测,用以判断是否为氧化折叠产物。将氧化折叠产物通过超微量分光光度计NanoDrop进行定量,计算产率,分装(每管含有50 nmol折叠产物),离心干燥后,于−20 ℃贮存待用。
-
取纯化后的氧化折叠主异构体产物(约50 nmol)溶解于适量ND96溶液中,涡旋使其充分溶解,浓度为10−4 mol·L−1。以该浓度作为母液,10倍梯度稀释,获得一系列浓度的肽溶液。利用各种不同亚型的烟碱型乙酰胆碱受体(nAChRs)模型,对芋螺毒素Lv68主异构体折叠肽的活性进行筛选。将不同的nAChRs亚基(α,β等亚基)的cRNA按一定比例分别注入爪蟾卵母细胞中,分别静待3~5 d表达各种亚型的nAChRs(α3β4,α6β4,α4β2,α9α10,α1β1γδ,α1β1δε),利用双电极电压膜片钳系统,钳制膜电压在−70 mV, 灌流速度为2 mL·min−1,通过记录受体产生的电流值变化,反映受体离子通道开闭情况。
-
数据处理和分析采用统计软件GraphPad Prism6。活性测试的数据分析及剂量反应曲线绘制,采用Clampfit 10.2软件进行处理。
1.1. 实验材料
1.2. 实验仪器
1.3. 线性肽的合成及纯化
1.4. 一步法氧化折叠及其条件优化
1.5. 氧化折叠产物的分离纯化
1.6. 氧化折叠产物活性测试
1.7. 数据统计分析
-
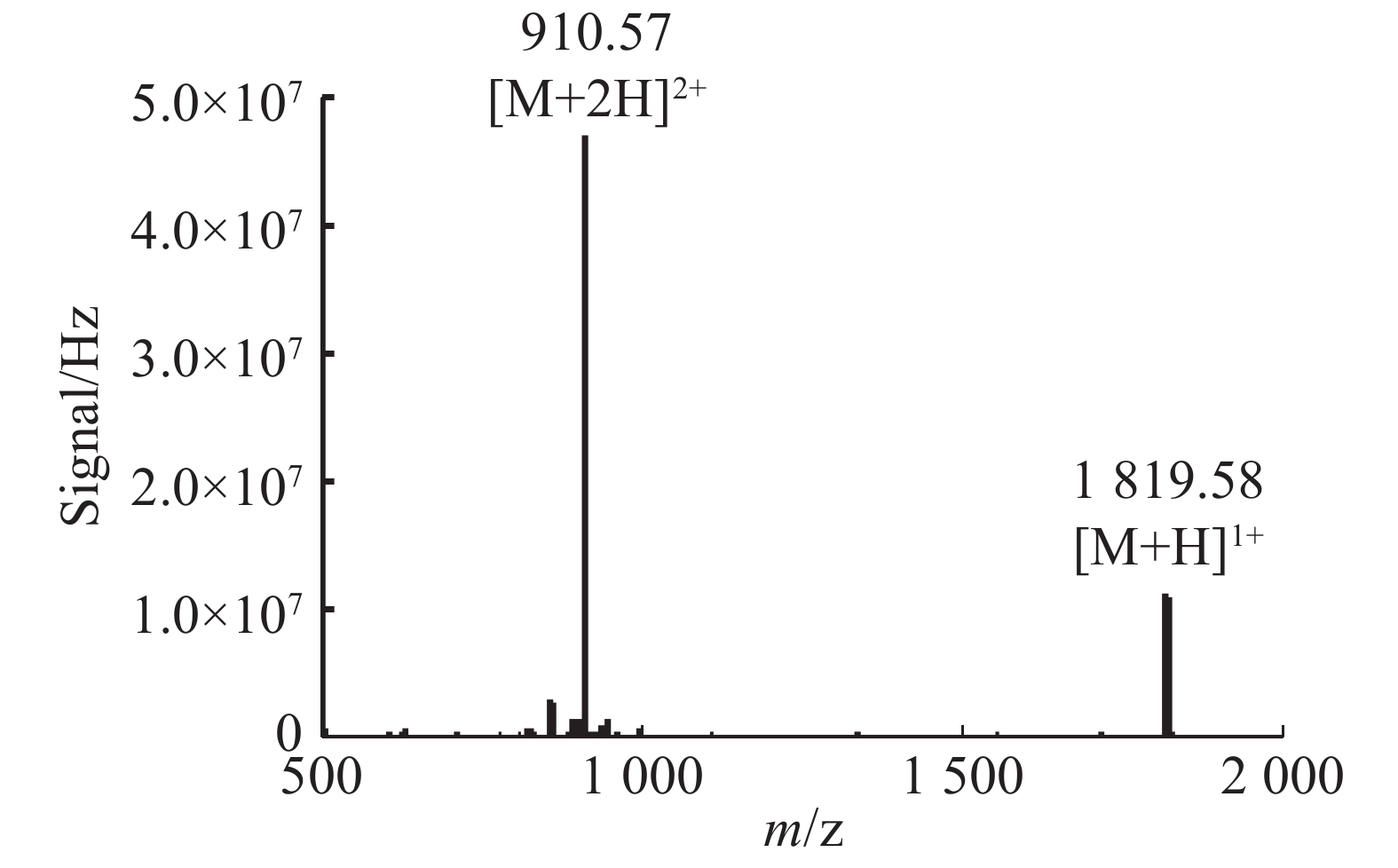
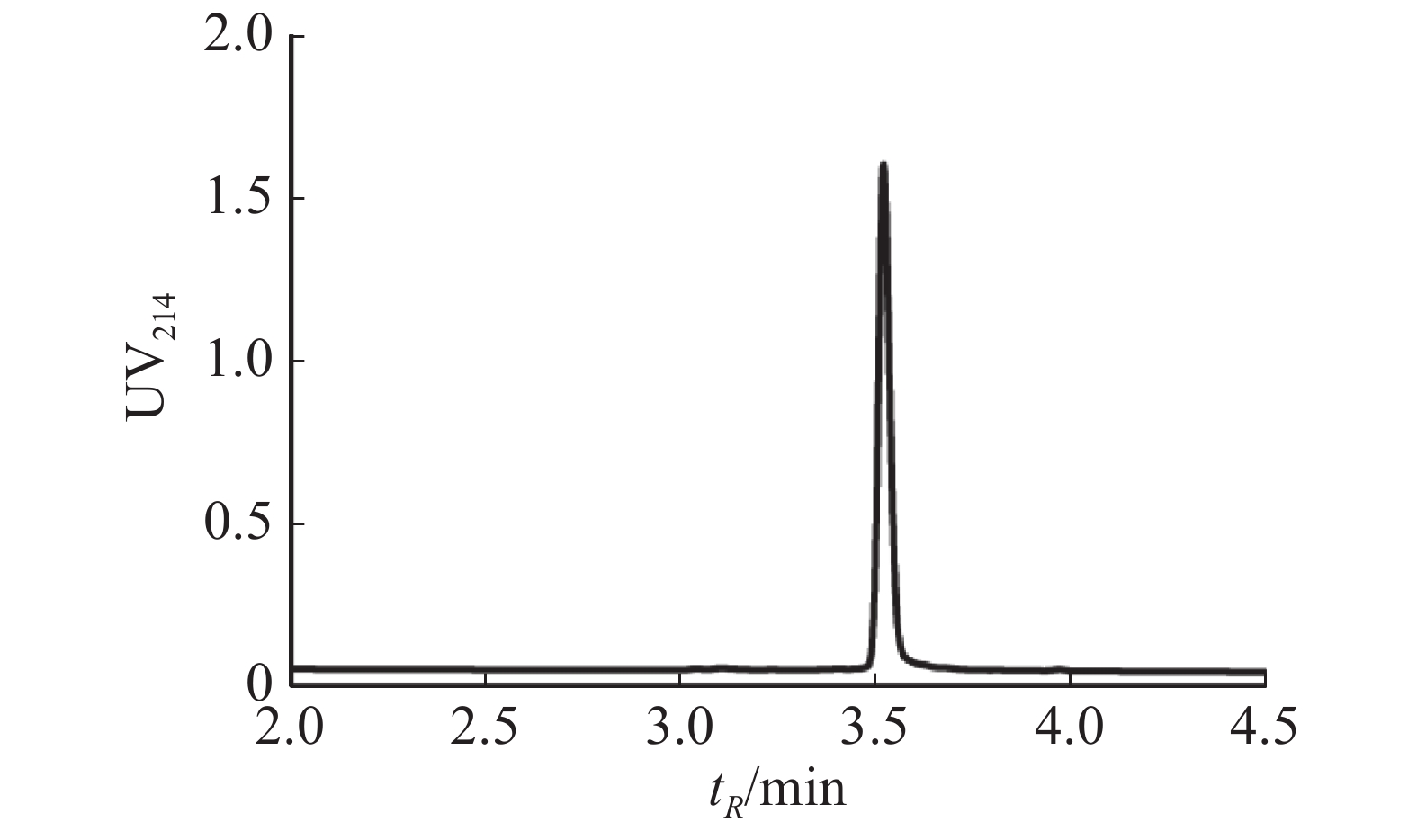
通过固相合成法合成Lv68线性肽粗肽,采用制备型HPLC纯化除杂,UPLC分析其纯度可达95%以上(图1),通过UPLC-MS对其相对分子量进行鉴定(图2),其实验值为1 819.58 Da,与理论值一致。
-
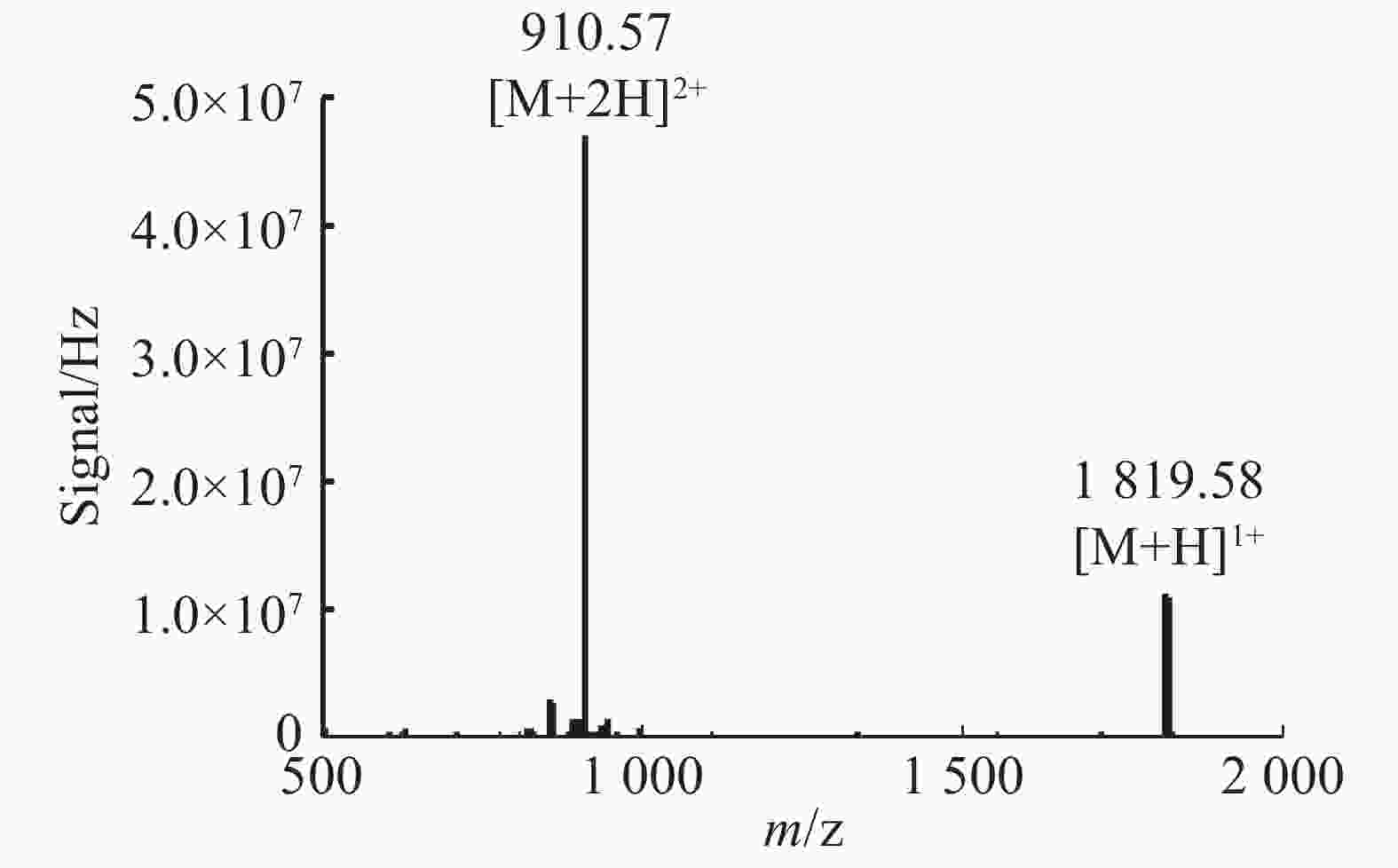
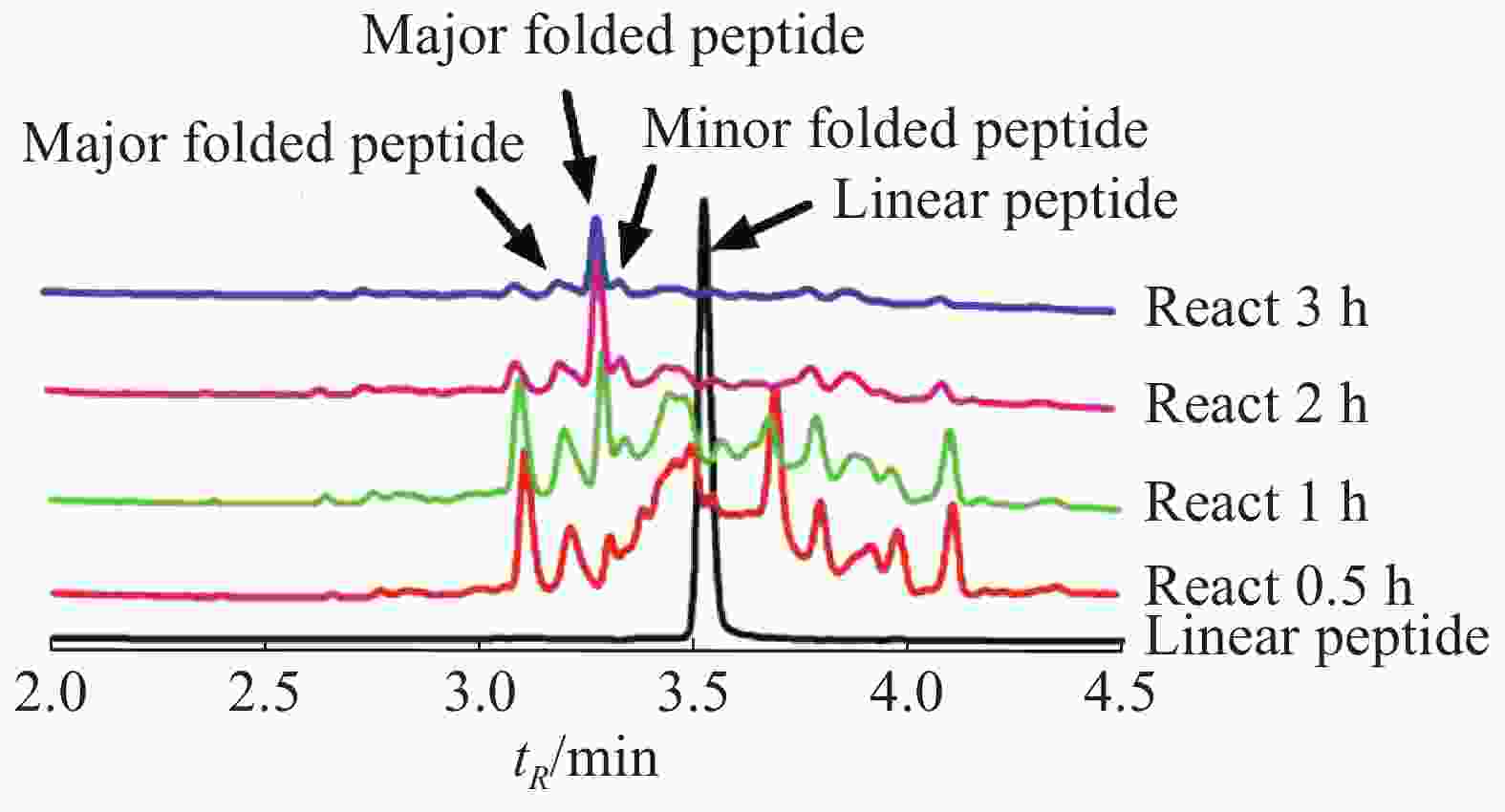
Lv68线性肽经氧化折叠后通过UPLC对产物进行分析(图3),将主异构体产物分离纯化后,经质谱鉴定其相对分子量为1 813.55 Da(图4),与线性肽相对分子量相差6.03 Da,即脱去了6个氢原子,证明其形成了3对二硫键的折叠肽。
通过一步法氧化折叠反应体系的条件优化,考察缓冲液、氧化剂、线性肽浓度、反应时间对芋螺毒素Lv68主异构体产物产率的影响,发现缓冲液和氧化剂的选择对其氧化折叠产率具有重要影响,而10~50 μmol·L−1的线性肽浓度范围对产率的影响不大。综合实验结果,确定Lv68的一步氧化折叠最优条件为0.1 mol·L−1 Tris-HCl,1 mmol·L−1 EDTA,GSH/GSSG 1 mmol·L−1/1 mmol·L−1、线性肽浓度50 μmol·L−1,反应时间2 h。
-
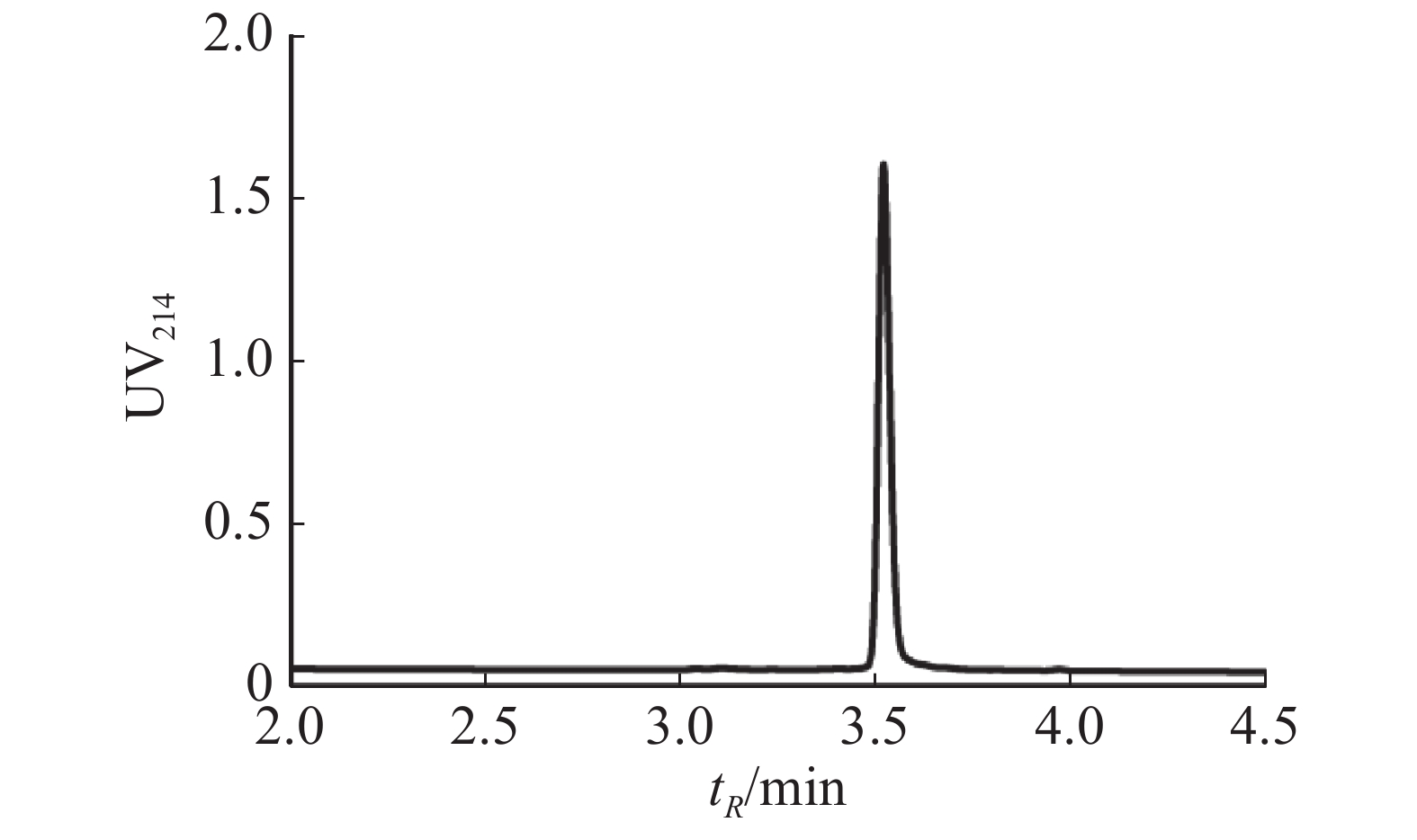
取20 mg Lv68线性肽,采用该最优反应条件合成Lv68折叠肽,以8%甲酸酸化淬灭反应后,经制备型HPLC分离纯化,并通过UPLC-MS进行纯度和相对分子量鉴定,最终获得芋螺毒素Lv68氧化折叠产物约2 000 nmol,计算产率为18.14 %,纯度达95%以上(图5),可用于电生理活性测试。
-
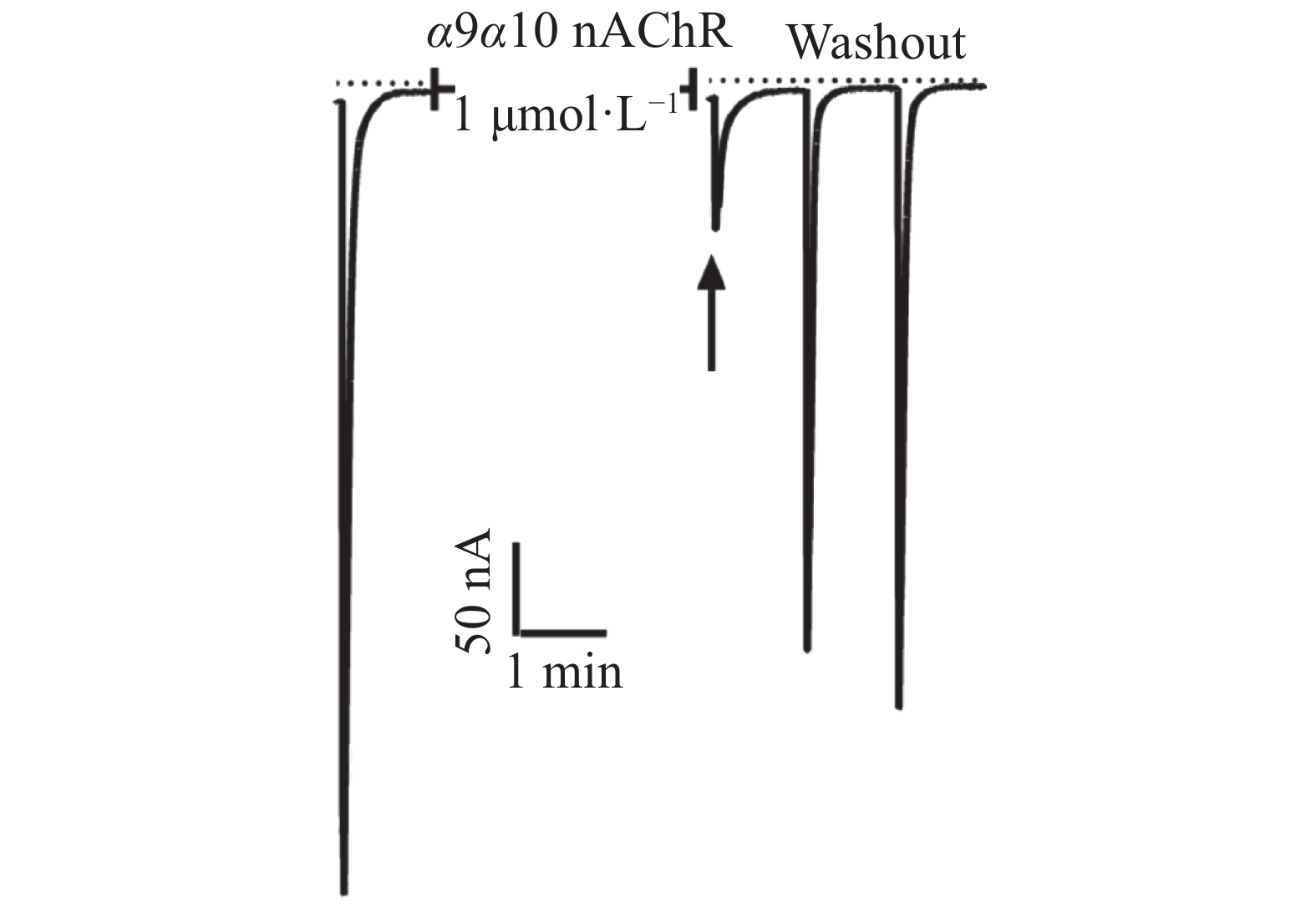
芋螺毒素Lv68氧化折叠主产物对多种亚型的烟碱型乙酰胆碱受体的电生理活性测试结果表明,在终浓度为1 μmol·L−1下,Lv68对α3β4,α6β4,α4β2,α1β1γδ,α1β1δε乙酰胆碱受体均无阻断活性,对α9α10乙酰胆碱受体具有一定的阻断活性,1 μmol·L−1可阻断约80%(图6),其IC50小于1 μmol·L−1。
2.1. 线性肽的合成与纯化
2.2. 一步法氧化折叠及其条件优化
2.3. 氧化折叠产物的分离纯化及定量干燥
2.4. 氧化折叠产物活性测试
-
芋螺毒素Lv68含6个半胱氨酸,可形成3对二硫键,理论上有15种二硫键异构体,本研究采用一步氧化法对线性Lv68进行氧化折叠,但由于其自身的结构性质,通过一步氧化法得到的二硫键异构体比例悬殊,主产物占有明显优势。一步氧化法具有操作步骤简单、合成效率高、折叠产物稳定、产率较高等优点,适用于芋螺毒素Lv68的大量合成。芋螺毒素Lv68的大量合成利于开展Lv68的结构与功能等相关的研究。本研究结果证明,芋螺毒素Lv68对α9α10 nAChR具有一定阻断活性。文献[31]报道α9α10 nAChR与神经痛治疗靶点密切相关。α9α10 nAChR强阻断剂(例如:芋螺毒素Vc1.1[32],RgIA[33],GeXIVA[34])具有显著的镇痛活性。癌症、创伤等神经病理性疼痛是中枢或外周神经系统损伤或功能异常导致的疼痛综合征[35],且这类神经病变很难自我修复,给患者带来极大的痛苦[36]。临床常用的作用于阿片受体的镇痛药物(如吗啡)对神经痛镇痛效果差、副作用多、易成瘾、存在剂量耐受效应。因此,新型高效镇痛药的研发需求迫在眉睫,新型神经痛治疗药物的候选分子亟待研究开发。Vc1.1曾作为镇痛药进入临床II期研究阶段(后因种属差异问题终止)[37],GeXIVA[38]正作为治疗神经痛的镇痛药处于临床前研究阶段,说明了α9α10 nAChR阻断剂作为镇痛开发的前景良好。芋螺毒素Lv68对α9α10 nAChR具有阻断作用,不仅拓宽了含半胱氨酸框架III的M超家族芋螺毒素的作用靶点范围,对该类芋螺毒素的进一步研究具有重要意义,而且也提示Lv68可作为以α9α10 nAChR为作用靶点的镇痛药物先导分子,尚有待进行深入研究。本实验结果可为其他含半胱氨酸模式III的M超家族Ψ−芋螺毒素的氧化折叠反应、生物活性研究以及深入开发利用提供重要的参考和借鉴。







 DownLoad:
DownLoad: