-
遗传转化技术在作物性状改良上发挥了重要作用,改良的优异性状包括产量、品质、生物和非生物胁迫抗性等方面。其中,基于CRISPR/Cas9的基因编辑技术因具有操作简便、编辑效率高、支持多靶点编辑、编辑形式多样等优势,成为作物遗传改良的新兴技术,已在许多作物性状改良上发挥了重要作用[1-5],但是该方法严重依赖于作物的再生和转化体系[6]。对于遗传转化体系较为成熟的作物,如小麦(Triticum aestivum)[7]、水稻(Oryza sativa) [8]、番茄(Solanum lycopersicum)[9]等,基因编辑技术应用相对容易实现。然而,对于大多数蔬菜作物而言,遗传转化体系并不成熟,这也成为限制CRISPR/Cas9基因编辑技术在蔬菜作物上应用的关键瓶颈问题。因此,建立高效的遗传转化体系是实现蔬菜基因编辑的关键环节,亟待实现技术突破。大多数蔬菜作物离体培养时,从外植体中诱导分化形成再生芽过程非常困难,受基因型、外植体状态、培养基类型、生长调节剂类型及含量、培养环境等诸多因素影响 [10]。植物分生组织的再生是由几个主要的发育调节因子(Developmental Regulators,DRs),如WUS(WUSCHEL)、STM(SHOOT MERISTEMLESS)、MP(MONOPTEROS)和GROWTH-REGULATING FACTORs(GRFs)决定的,通过在体细胞中特定表达DRs可直接诱导分生组织再生[11-14]。有研究表明,利用AtGRF5可以将西瓜(Citrullus lanatus)的转化效率提高到25%左右,与传统载体相比效率增加了40倍[15]。 除离体培养利用DR提高遗传转化效率外,还可以直接在植株上与含有DR的农杆菌进行共培养直接再生,从而实现遗传转化,被称为Fast-Tracc(fast-treated agrobacterium coculture)。该方法的优点一方面体现在不用通过优化外源激素配方来诱导再生芽的生成,避免了从愈伤组织经脱分化、再分化形成再生芽的阶段,为解决遗传转化顽拗型蔬菜作物的转基因或者基因编辑技术难题提供了新思路。另一方面,该方法不需要经过组织培养就可获得转基因植株,与组培方法相比,用时可缩短2~3个月,大大提高了工作效率。已有研究表明,利用Fast-Tracc方法在烟草( Nicotiana tabacum)上的转化效率为6%~10% [16],金鱼草(Antirrhinum majus)上转化效率为3.8%~8.8%,番茄上转化效率为3.3%~13.3% [17]。苦瓜(Momordica charantia)属于遗传转化顽拗型蔬菜,遗传转化非常困难,目前还未发现利用Fast-Tracc方法的苦瓜遗传转化成功的相关报道。本研究利用含有不同DR基因的双元载体,以农杆菌GV3101为介导,直接侵染不同基因型苦瓜幼苗下胚轴,诱导切口直接再生成芽,获得转基因阳性植株。本研究结果能为后续利用非组培方式对遗传转化顽拗型蔬菜作物进行基因编辑提供新的解决思路。
-
供试的苦瓜材料(品种)为‘K122’(白皮,雌性弱,长势强旺,分枝多)和‘K103’(白皮,强雌性,长势弱,分枝少)为本课题组保存的高代自交系。载体pW502和pW503由北京大学现代农业研究院张华伟研究员惠赠,其中,pW502 含有的DR类型为AtGRF5, pW503 含有的DR类型为TaGRF4-OsGIF1[15],并含有红色荧光基因(DsRed)作为标记基因。GV3101菌株购自南京诺维赞生物公司。
-
将苦瓜种子浸种8 h后,置于28 ℃下催芽,选取发芽整齐一致的种子播种于50孔穴盘内,放置于温室(昼温28 ℃/夜温18 ℃;光周期:光照16 h/黑暗8 h) 中培养。待幼苗长至两叶一心期,进行实验。每次接种幼苗数为50株,重复3次。
-
将含有pW502或pW503的GV3101菌株接入5 mL LB液体培养基(含50 µg·mL−1 的Kan,20 µg·mL−1 的 Rif抗生素)中振荡培养(28 ℃,200 r·min−1 ), 过夜培养后按照1%的比例转接菌液至50 mL LB液体培养基中(含50 µg·mL−1 Kan,20 µg·mL−1 Rif抗生素, 20 µmol·L−1 乙酰丁香酮),继续培养使农杆菌浓度达到OD600 = 0.4~0.6,室温离心(5 000 r·min−1,10 min)收集菌体,用MS液体培养基(含10 mmol·L−1 MgCl2,150 µmol·L−1 乙酰丁香酮),重新悬浮菌体,将农杆菌浓度调至OD600 = 0.4或0.8后,28 ℃ 静置2 h后,进行注射。
-
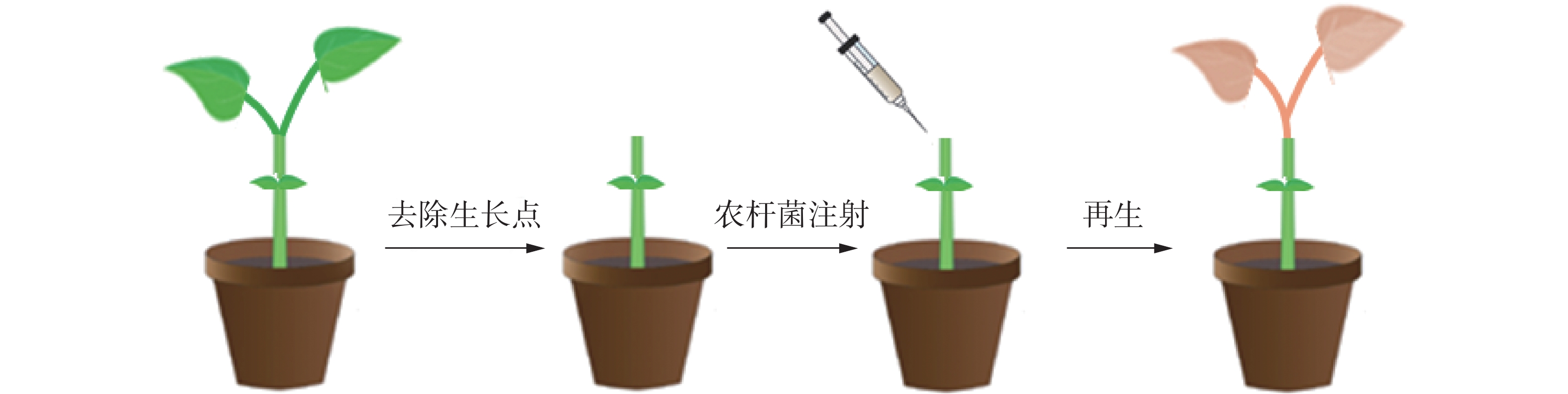
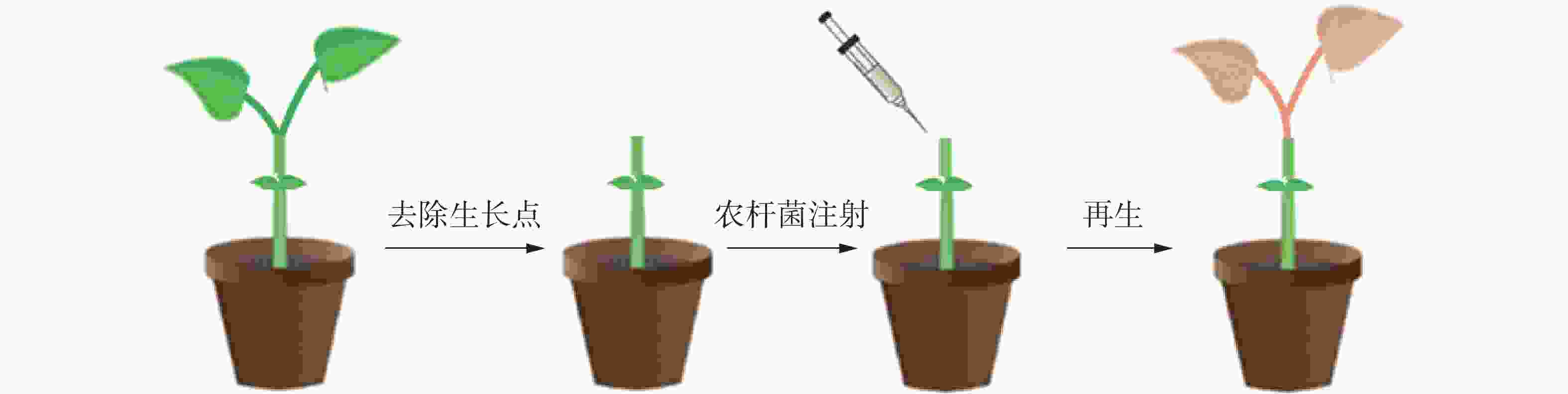
在苦瓜幼苗长至两叶一心期时,将幼苗顶端分生组织用新刀片切去,仅留有子叶(图1),将农杆菌悬浮液用微量注射器注射至幼苗伤口处,注射的量以不溢出幼苗伤口为准;将蘸有悬浮液的脱脂棉球置于伤口处保湿,黑暗条件下25 ℃放置2 d后,光周期为16 h(光照)/8 h(黑暗)条件下培养继续培养,培养时温度为25 ℃,直至植株再生。当切口处出现再生现象时,用手持式荧光仪观察是否有红色荧光信号,将有荧光信号的植株持续培养至叶片展开,进行分子检测确定阳性植株。
-
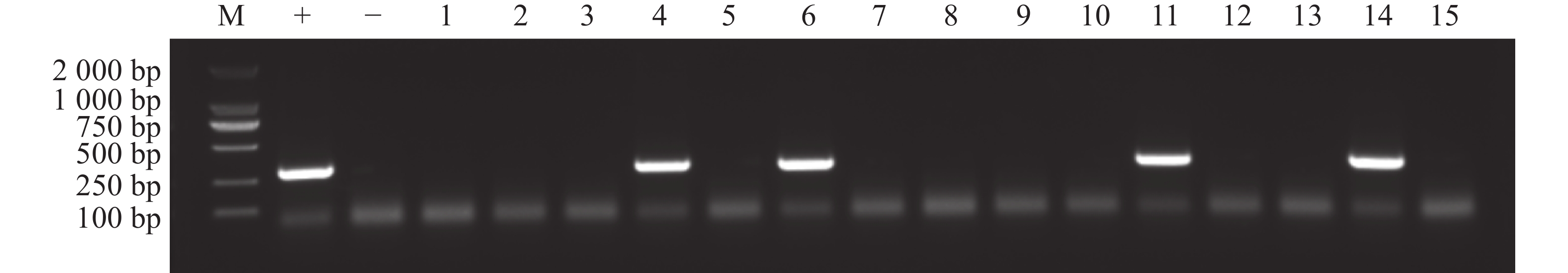
对再生的叶片进行取样,利用CTAB法提取DNA,以引物对DsRed-F(5′-GGAGGGCACCGTGAACGGCCACGAG-3′)和DsRed-R(5′-ACCTTGTAGATGAAGCAGCCGTCCT3′),进行PCR,程序是:95 ℃ 预变性 5 min;95 ℃ 变性 30 s,58 ℃ 复性 30 s,72 ℃ 延伸 1 min,34个循环后,72 ℃延伸 5 min,4 ℃保存。对PCR产物进行 1%的琼脂糖凝胶电泳验证,含有目的条带的即为阳性植株。
-
实验数据采用Microsoft Excel 进行统计,IBM SPSS Statistics 20 软件进行差异显著性分析。
-
为了找到合适的农杆菌接种浓度,选取2个不同的浓度(OD600 = 0.4或0.8)进行接种实验(供试受体为‘K122’)。结果显示(表1),当利用OD600 = 0.8的农杆菌接种时,所含DR为AtGRF5的载体(pW502)平均再生效率为26.67%,含DR为TaGRF4-OsGIF1的载体(pW503) 平均再生效率为33.33%,要明显高于OD600 = 0.4时菌液接种的效率(8%和5.30%)。说明农杆菌接种浓度为OD600 = 0.8时利于植株再生。
农杆菌浓度
(OD600)接种农杆菌
所含质粒接种
数/株再生
数/株再生效
率/%0.4 pW502 150 12 8±2.31b pW503 150 8 5.30±1.33b 0.8 pW502 150 40 26.67±3.71a pW503 150 50 33.33±1.76a 注:不同小写字母表示不同处理间差异显著,相同小写字母表示差异不显著,下同。 -
为了明确Fast-Tracc方式诱导植株再生是否受基因型的影响,本实验选取了‘K122’和‘K103’2个苦瓜基因型利用质粒pW502进行接种。以OD600 = 0.8的浓度接种,‘K122’的平均再生效率为26.67%(质粒pW502),而‘K103’的再生效率为4.7%(质粒pW502)。结果显示(表2),利用同一种DR诱导,在相同接种浓度下,K122的再生效率高于K103,表明不同基因型间的植株再生具有明显差异 。
品种名 接种农杆菌所含质粒 农杆菌浓度(OD600) 接种数/株 再生数/株 再生效率/% ‘K122’ pW502 0.8 150 40 26.67±3.71a ‘K103’ pW502 0.8 150 7 4.70±3.71b -
为了明确不同DR对诱导再生效率的影响,利用含有pW502和pW503的农杆菌在OD600 = 0.8时接种,‘K122’的平均再生效率分别为26.67% 和33.33%,两者差异不明显。而将2种农杆菌分别接种K103,平均再生效率分别为4.7%和4%,差异也不明显。结果表明,含有2种质粒的农杆菌分别接种两个不同基因型材料,再生效率都没有明显差异(表3)。
品种名 接种农杆菌所含质粒 农杆菌浓度(OD600) 接种数/株 再生数/株 再生效率/% ‘K122’ pW502 0.8 150 40 26.67±3.71a pW503 0.8 150 50 33.33±1.76a ‘K103’ pW502 0.8 150 7 4.70±3.71b pW503 0.8 150 6 4.00±2.00b -
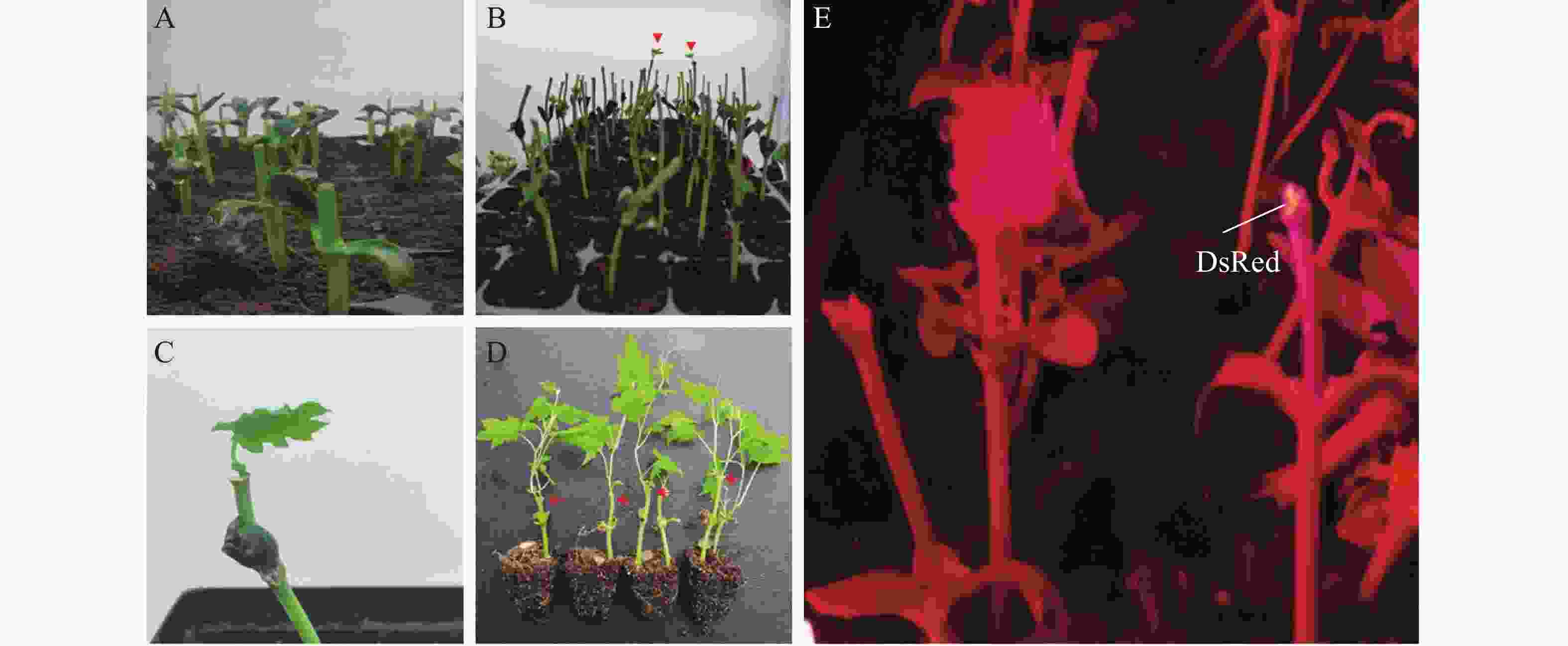
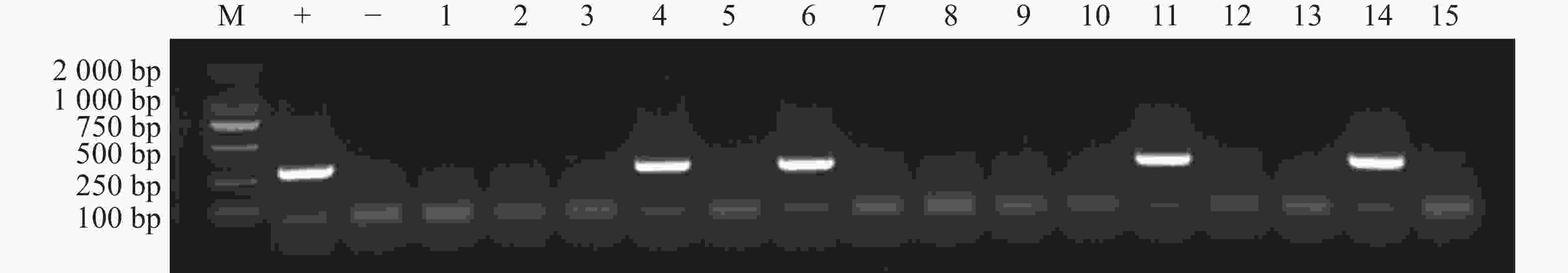
为了筛选再生植株中的阳性植株,用手持式荧光仪观察切口处再生组织是否有红色荧光信号,结果显示,部分再生组织处可以观察到红色荧光信号(图2)。将再生芽持续培养至叶片展开后进行取样,提取DNA后进行PCR检测显示部分再生植株可以扩增出分子量为312 bp的目的片段,即为DsRed转化成功的阳性植株(图3)。统计结果显示(表4),当OD600 = 0.8时,含有pW502或者pW503农杆菌对K122进行侵染,平均阳性率都达到10%,对‘K103’进行侵染时,则不能获得阳性植株。
品种名 接种农杆菌所含质粒 农杆菌浓度(OD600) 接种数/株 再生数/株 再生效率/% 阳性植株数/株 阳性转化效率/% K122 pW502 0.8 150 40 26.67±3.71 4 10.00±1.04a pW503 0.8 150 50 33.33±1.76 5 10.00±1.60a K103 pW502 0.8 150 7 4.70±3.71 0 / pW503 0.8 150 6 4.00±2.00 0 / -
与植物组培方式的遗传转化相比,Fast-Tracc方式的直接再生方法步骤相对简单,避免了传统方法中的从愈伤组织经脱分化、再分化形成再生芽的阶段[18];费时较少,本研究从播种到植株再生约耗时50 d,而传统离体培养则需要3个月以上,这些优点有利于对植物进行功能研究和遗传改良,尤其是生长期较长的植物(果树等)。
本研究虽然获得了苦瓜转基因阳性株,但是不能排除其是嵌合体的可能,与传统的组培方式获得的转基因植株一样,后续需要对转基因植株后代进行进一步鉴定,筛选可以稳定遗传的转基因植株后代。另外,转基因植株自交后代是否符合3∶1孟德尔遗传规律,也需要后续进一步调查与分析。
据报道,当农杆菌接种浓度OD600 = 0.2~0.3时诱导再生效率较高[19],但是本研究中OD600 = 0.8有较高的诱导效率,可能是由于不同转化方法导致的差异,前者是利用农杆菌侵染外植体进行离体培养,本研究是将农杆菌接种到切口处进行直接再生,当OD600 = 0.8时可以获得较高的诱导效率,表明该浓度的农杆菌侵染可以使苦瓜成功再生。众所周知,不同农杆菌接种浓度,对再生效率影响较大,并且针对不同作物的最适农杆菌接种浓度也可能存在差异,因此,在对不同作物进行Fast-Tracc方式的诱导体系的建立时,需要摸索最适接种浓度。在西瓜离体培养的遗传转化体系的建立中,pW502和pW503诱导再生效率均高于13.00%,暗示载体中含有的AtGRF5, TaGRF4-OsGIF1可能对瓜类作物有诱导再生的作用[15],本研究利用这两个载体,利用Fast-Tracc方法实现了苦瓜的直接再生,进一步证明了AtGRF5, TaGRF4-OsGIF1在瓜类作物中的高效诱导再生的作用。
研究表明,在离体培养中基因型差异是影响遗传转化的重要因素[20],本研究发现,K122的再生效率显著高于K103,可能原因是基因型的差异造成的,表明在Fast-Tracc方法进行直接再生中基因型也是影响再生的重要因素之一。本实验中最高再生率为33.33%,而阳性转化率最高为10.00%,出现了部分假阳性苗,这可能是缺少抗性筛选所导致的。当然,此方法还有进一步改进的空间,例如苗龄,侵染菌种的选择等。在接下来的研究中可以加入不同苗龄及不同菌种等因素进一步完善此体系,从而探索出更好的遗传转化体系。
本研究明确了利用含有GRF5或TaGRF4-OsGIF1的农杆菌GV3101,在OD600 = 0.8时,对‘K122’的两叶一心期进行接种,可以不通过离体培养直接再生获得转基因阳性植株。
Establishment of an efficient genetic transformation system for Momordica charantia based on the Fast-TrACC method
DOI: 10.15886/j.cnki.rdswxb.20230082
- Received Date: 2023-06-30
- Accepted Date: 2023-09-28
- Rev Recd Date: 2023-09-25
- Available Online: 2023-10-16
- Publish Date: 2023-11-24
-
Key words:
- Fast-TrACC method /
- development regulator /
- Momordica charantia /
- genetic transformation
Abstract: In order to establish a genetic transformation method (fast-treated agrobacterium co-culture (Fast-TrACC), for regeneration of Momordica charantia, the seedlings were used to be induced by Agrobacterium tumefaciens containing the development regulator DR. The regenerated plants were confirmed by fluorescence observation for target gene DsRed and PCR molecular detection, and the positive rate was also calculated. The results showed that the concentration of A. tumefaciens plays an important role on the regeneration rate of plants, with the highest regeneration efficiency of 33.33% at OD600 = 0.8. There were significant differences in regeneration efficiency between the two different genotypes, with an average efficiency being 26.67% and 4.70%, respectively. The two vectors containing AtGRF5 and TaGRF4-OsGIF1 were both effective for the regeneration of M. charantia, with the highest positive rate of 10.00% confirmed by fluorescence observation and PCR molecular detection. The establishment of this method might provide a new solution for the subsequent gene editing of genetically transformed recalcitrant vegetable crops.
| Citation: | LIU Zhiyang, LIU Jing, NING Yu, QI Renjie, XU Hai, CHEN Longzheng. Establishment of an efficient genetic transformation system for Momordica charantia based on the Fast-TrACC method[J]. Journal of Tropical Biology, 2023, 14(6): 622-627. doi: 10.15886/j.cnki.rdswxb.20230082 |






 DownLoad:
DownLoad: