-
四氯虫酰胺(Tetrachlorantraniliprole)是我国自主研发的双酰胺类杀虫剂,2013年在农业农村部获得登记,具有高效、低毒、广谱的特点[1]。已有研究报道,10%四氯虫酰胺悬浮剂对玉米田棉铃虫(Helicoverpa armigera)[2]和玉米螟(Pyrausta nubilalis)[3]有较好的防治效果。四氯虫酰胺对水稻二化螟(Chilo suppressalis)[4]和水稻纵卷叶螟(Cnaphalocrocis medinalis)[5]田间防治效果较好,但药剂在水稻田的喷洒使用行为会造成水体环境污染,从而对水生生物产生毒性危害。四氯虫酰胺与甲维盐复配对草地贪夜蛾(Spodoptera frugiperda)有较好的防治效果[6],但由于农药在生态环境中的迁移转化,会对非靶标生物产生一定的影响,如双酰胺类农药氯虫苯甲酰胺和溴氰虫酰胺对螟黄赤眼蜂(Trichogramma chilonis Ishii)成蜂表现为低风险性[7];在水生生态系统中,溴氰虫酰胺会 影响羊角月牙藻(Selenastrum bibraianum)的光合作用并抑制生长[8];10%四氯虫酰胺悬浮剂对家蚕(Bombyx mori Linnaeus)的毒性为中毒,96 h的LC50为27.28 mg·L−1;10%四氯虫酰胺悬浮剂对水生生物鲤鱼(Cyprinus carpio)为低毒,96 h的LC50为89.11 mg·L−1[9]。黑色素是通过一个复杂的过程被合成为紫外线防御机制,该过程涉及一系列酶和多种信号转导途径。黑色素生成是由酪氨酸酶基因(Tyr)催化的酪氨酸经中间体3,4-二羟基苯丙氨酸氧化为多巴醌而引发的;在没有巯基(例如半胱氨酸或谷胱甘肽)的情况下,黑色素合成途径中的第二种酶酪氨酸酶相关蛋白2(Trp-2)将多巴醌迅速转化为多巴色素,随后将多巴色素转化为5,6-二羟基吲哚(DHI)或吲哚5,6-醌2-羧酸(DHICA);参与黑色素合成的最后一种酶,即酪氨酸酶相关蛋白1 (Trp-1),催化DHICA的氧化形成直链黑色素[10]。Tyr是一种糖蛋白,它的糖基化和降解作用在抗黑素形成剂的开发中起着至关重要的作用。小眼症相关转录因子(MITF),通过转录调节Tyr,Trp-1和Trp-2表达在黑色素生成中起重要作用,为此MITF为黑色素生成的主转录因子[11]。SRY-box转录因子10 (Sox10)可以调节小眼症相关转录因子Mitf/mitfa的表达,Sox10在分化前的神经嵴细胞中广泛表达。Sox10功能的丧失会导致斑马鱼(Danio rerio)和小鼠(Mus musculus)中黑色素细胞和大多数其他神经嵴衍生细胞完全或部分缺失,因此Sox10对于黑色素细胞的发育非常重要[12]。
目前未见有四氯虫酰胺原药对模式生物斑马鱼的毒性影响力的研究报道。本研究以斑马鱼为研究对象,通过静态染毒观察农药暴露对斑马鱼胚胎生长发育的影响;同时通过RT-qPCR方法研究四氯虫酰胺原药对斑马鱼胚胎黑色素合成的相关基因的影响,以期为四氯虫酰胺农药的生态风险评估提供一定的理论依据。
-
四氯虫酰胺(纯度96%,CAS:1104384-14-6),购自海南青峰生物科技有限公司;Tween-80、二甲基甲酰胺(DMF)购自Solarbio科技有限公司;磷酸盐缓冲液(PBS)购自上海立菲生物技术有限公司;Trizol试剂购自Invitrogen科技有限公司;Transcriptor First Strand cDNA Synthesis Kit 试剂盒购自Roche公司;SYBR Green I Master(Roche diagnostics A/S)试剂盒购自Roche公司;Triple Quad 6500+超高效液相色谱-三重四极杆串联质谱联用仪UPLC-MS-MS(SCIEX美国Waters公司);LightCycler96 R实时荧光定量PCR仪(Roche公司)。
-
试验分别在24,48,72,96 h对各试验浓度四氯虫酰胺药液取样,并对样品进行前处理。样品前处理:将试验药液稀释成设定的浓度的10倍,取1mL稀释后的药液并加入9 mL的甲醇,使得水药液与甲醇的体积比为1∶9,再涡旋,摇匀,取1 mL经0.22 μm过滤膜过滤后转移到样品瓶。
-
根据试验设定标准曲线浓度为0.005,0.01,0.05,0.1,0.2 μg·mL−1。
-
色谱柱:Waters ACQUITY UPLC BEH C18柱(2.1 mm×50 mm,1.7 μm),流动相A:超纯水,流动相B:乙腈,梯度洗脱程序:0~0.5 min,保持初始10%的乙腈;0.5~2 min,乙腈的比例线性增加从10%~95%;2~5 min,保持95%的乙腈3 min;5.0~5.2 min,乙腈的比例线性下降从95%~10%;5.2~8.0 min,保持10%的乙腈2.8 min。每个样品进样结束,仪器平衡5 min。流速:0.25 mL·min−1。
-
离子化方式:电喷雾离子源(ESI);检测模式:多反应检测模式(MRM);扫描方式为负离子扫描(ESI−),一级扫描范围(m/z):201.9~535.8,二级扫描范围(m/z ):499.7~535.8;气帘气:0.28 Mpa;碰撞气:0.06 Mpa;去簇电压(DP):70 V;碰撞能量(CE):5 eV;离子喷雾电压:5 500 V;离子源温度:550℃。
-
设定加标浓度分别为0.01、0.02、0.05 μg·mL−1。然后按照上述的分析方法测定,并根据建立的基质标准方程,采用外标法计算回收浓度,根据 SANCO/12571/2013[13] 的要求计算回收率和相对标准偏差(RSD)。
-
试验用AB野生型斑马鱼(Danio rerio)引自国家斑马鱼中心,并参照OECD标准[14],采用北京爱生科技公司的斑马鱼养殖系统饲养,饲养中严格控制饲养条件。饲养条件:室温28 ℃,水的pH为7.5左右,水中电导率550 μS·cm−1左右,通过加氧泵使水中含氧量在80%以上,调节养殖时的光暗比为14 h∶10 h。
斑马鱼胚胎的获取:将5 cm左右的成年斑马鱼按照雌雄比例为1∶2的标准放到孵化盒中用挡板隔开,并控制光暗比(14 h∶10 h),次日拔掉挡板,使得雌雄鱼之间自由交配,收集交配后30 min之内的胚胎,并小心地用孵化液清洗。
-
按照OECD标准[14]开展斑马鱼胚胎急性毒性试验,采用静态染毒法将暴露于四氯虫酰胺的斑马鱼胚胎放到24孔板中观察,根据预实验的结果,设计6个实验浓度为:16.00,19.20,23.04,27.65,33.18,39.80 mg·L−1(公比为1.2)。在试验过程中设置溶剂对照SC和空白对照CK,并且每个浓度选取20个观察胚胎。
-
四氯虫酰胺原药试验质量浓度:16.00、19.20、23.04、27.65、33.18、39.80 mg·L−1。溶剂和助剂采用DMF和Tween-80,其最高用量低于0.1%。
-
根据OECD标准[14]分别于暴露24、48、72、96 h,使用体视显微镜观察统计各项指标(死亡率、自主运动、孵化率及体长等)。
-
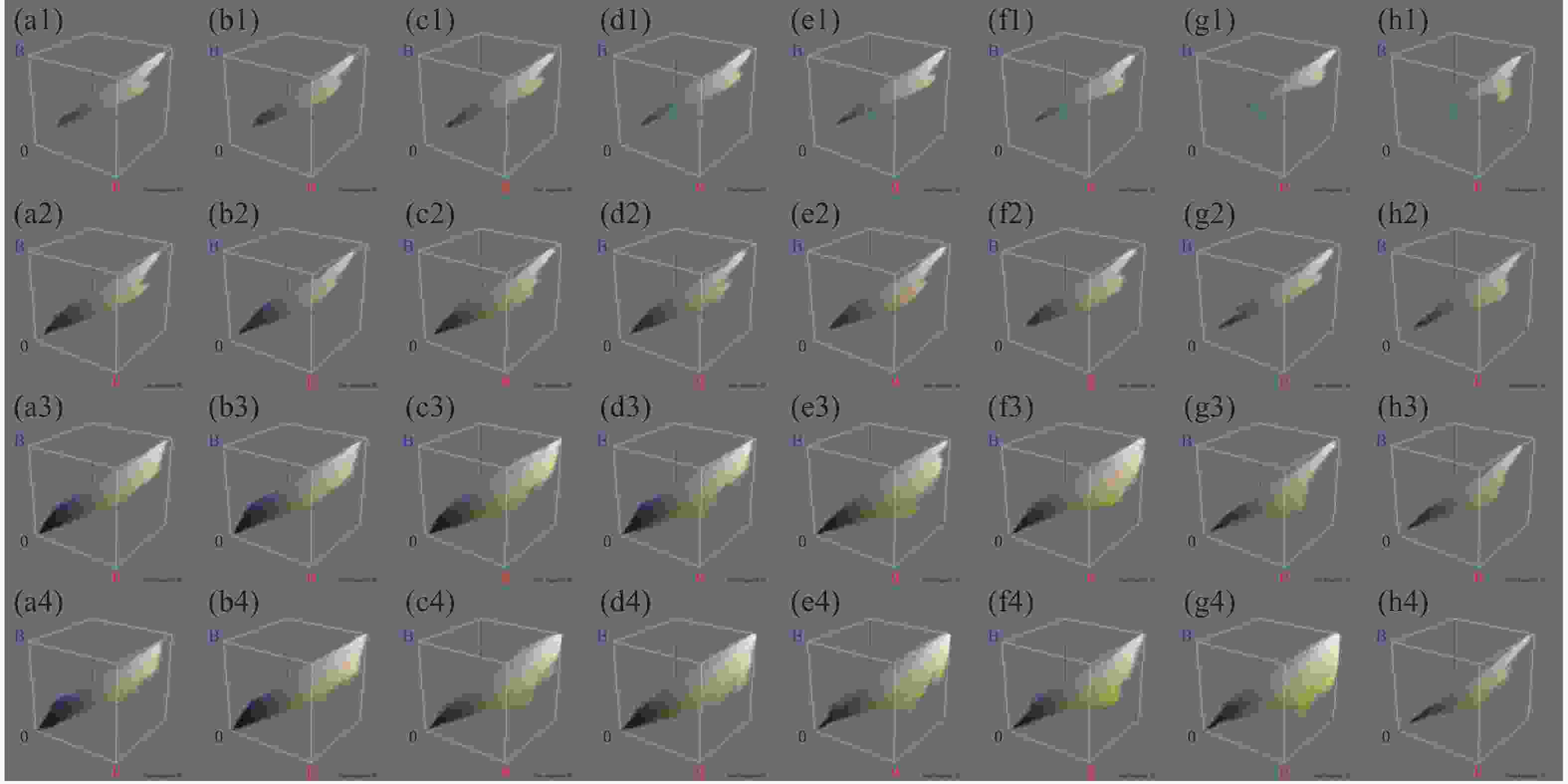
通过image-J软件对斑马鱼胚胎黑色素颜色区域进行提取,得到黑色素整体面积。将图片进行Color Inspector 3D软件分析得到RGB三维颜色分析图片,通过直观图片中黑色区域来表现黑色素的变化。
-
使用Trizol 试剂提取斑马鱼胚胎染毒24 h后各浓度的总RNA,包括溶剂对照SC和空白对照CK。采用普通琼脂糖凝胶电泳检测RNA完整性,并使用Roche公司的Transcriptor First Strand cDNA Synthesis Kit 试剂盒将RNA逆转录成cDNA,检测的基因和实时荧光定量PCR引物序列见 表1。将各浓度反转录成的cDNA分别稀释到同一浓度后,使用SYBR Green I Master(Roche diagnostics A/S)试剂盒方法在LightCycler96 R(Roche diagnostics A/S)仪器上进行实时定量PCR扩增。扩增条件:95℃预变性10 min,循环1次,然后95℃进行15 min,在60℃下退火20 min,以及72℃下延伸20 min,40个循环。RT-qPCR结果数据分析采用2−ΔΔCt方法,每个基因进行3个技术重复。
基因 上游引物序列5′→3′ 下游引物序列5′→3′ β-actin GTCCCTGTACGCCTCTGGTCG GCCGGACTCATCGTACTCCTG GAPDH CTGGTGACCCGTGCTGCTT TTTGCCGCCTTCTGCCTTA Tyr GACAGACGAGGAGACGGAAG CCTTGGGTTTACGGATGAGA Dicer ATCGCTAGCTGGTGCAATTT GTCTCTGGCTCCATCTCCAG Sox10 AAAACACTGGGGAAGCTGTG ACGTGGCTGGTACTTGTACT Mitfb CAAACCGCAACCTCTTGCTC GTCTTTGTGCGTGCTTTCGT Trp-1a GGGAACTACAGCGGGTTTGA GGAGAAATCCCAGAACGGCA Dct TCTTCCCACCTGTGACCAAT CTGATGTGTCCAGCTCTCCA Mitfa CTGGACCATGTGGCAAGTTT GAGGTTGTGGTTGTCCTTCT Trp-1b CGACAACCTGGGATACACCT AACCAGCACCACTGCAACTA Trp-2 GGATGACCGTGAGCAATGGC CGGTTGTGACCAATGGGTGC -
使用体视显微镜观察统计各项指标(死亡率、自主运动、孵化率及体长等)。试验结果用SPSS Statisitics 22.0软件分析计算LC50。并采用Dunnett’s方法对每个浓度处理的结果和空白对照的结果进行比较,差异不显著用P>0.05表示,差异显著性用P<0.05和P<0.01表示。
-
标准曲线的拟合方程为y=5.07e+0.05x(R2=0.999 1);四氯虫酰胺的加标浓度0.01,0.02和0.05 μg·mL−1对应的平均回收率分别为97.98%,101.60%和103.92%,相对标准偏差(RSD)在4.3%~6.0%,说明该方法有较好的回收率。
按照上述仪器条件和样品前处理方法,以甲醇为阳性对照,对水中四氯虫酰胺的浓度进行检测,通过检测24、48、72 、96 h中各样品分别在甲醇和水中的浓度(表2),四氯虫酰胺在水中96 h的最高降解率为7.6%,浓度大于80%。因此,四氯虫酰胺在水中96 h稳定性良好,满足OECD标准中静态染毒法的要求。
样品 不同暴露时长四氯虫酰胺的浓度/(μg·mL−1) 0 h 24 h 48 h 72 h 96 h 平均浓度 1 0.090 6 0.094 0 0.089 5 0.083 9 0.083 8 0.088 4 2 0.096 4 0.096 1 0.096 7 0.099 7 0.089 7 0.095 7 3 0.100 2 0.095 6 0.097 8 0.094 5 0.089 4 0.0955 4 0.089 9 0.097 9 0.088 3 0.096 3 0.093 8 0.093 2 5 0.091 5 0.096 7 0.087 9 0.090 1 0.087 9 0.090 8 6 0.093 1 0.095 2 0.089 1 0.096 8 0.097 4 0.094 3
7 0.084 0 0.097 8 0.082 2 0.081 4 0.096 4 0.088 4 8 0.099 1 0.099 2 0.095 4 0.098 9 0.094 9 0.097 5 9 0.099 9 0.094 9 0.096 6 0.094 7 0.092 7 0.096 4 10 0.090 4 0.098 6 0.097 6 0.095 9 0.096 4 0.095 8 11 0.094 1 0.097 4 0.099 6 0.089 9 0.092 4 0.094 7 12 0.092 3 0.093 2 0.099 6 0.095 1 0.095 1 0.095 1 注:四氯虫酰胺样品1~6溶解甲醇中为阳性对照,四氯虫酰胺样品7~12溶解水中。 -
斑马鱼胚胎暴露在四氯虫酰胺24 h和48 h的LC50均为112.88 mg·L−1,72 h的LC50 为30.421 mg·L−1,95%置信区间(CI)为27.652~34.480 mg·L−1;96 h的LC50 为23.775 mg·L−1,95%置信区间(CI)为22.066~25.582 mg·L−1(表3)。
暴露
时长/h回归
方程LC50/
(mg·L−1)95%置信区间/
(mg·L−1)24 y=2.63x-4.9 112.88 -- 48 y=2.63x-4.9 112.88 -- 72 y=6.03x-8.93 30.421 27.652~34.480 96 y=8.63x-11.89 23.775 22.066~25.582 -
试验结果表明,溶剂对照组和空白对照组没有明显差异。处理后24 h,随着四氯虫酰胺浓度的增加斑马鱼胚胎每分钟自主运动频率加快(图1),当四氯虫酰胺暴露浓度高于27.65 mg·L−1时,斑马鱼胚胎自主运动频率与对照相比差异即达显著水平(P<0.01),其中自主运动对照组为0.25次·min−1,暴露浓度为39.8 mg·L−1时,斑马鱼胚胎自主运动频率达到5.5 次·min−1,为对照自主运动频率的22倍。
-
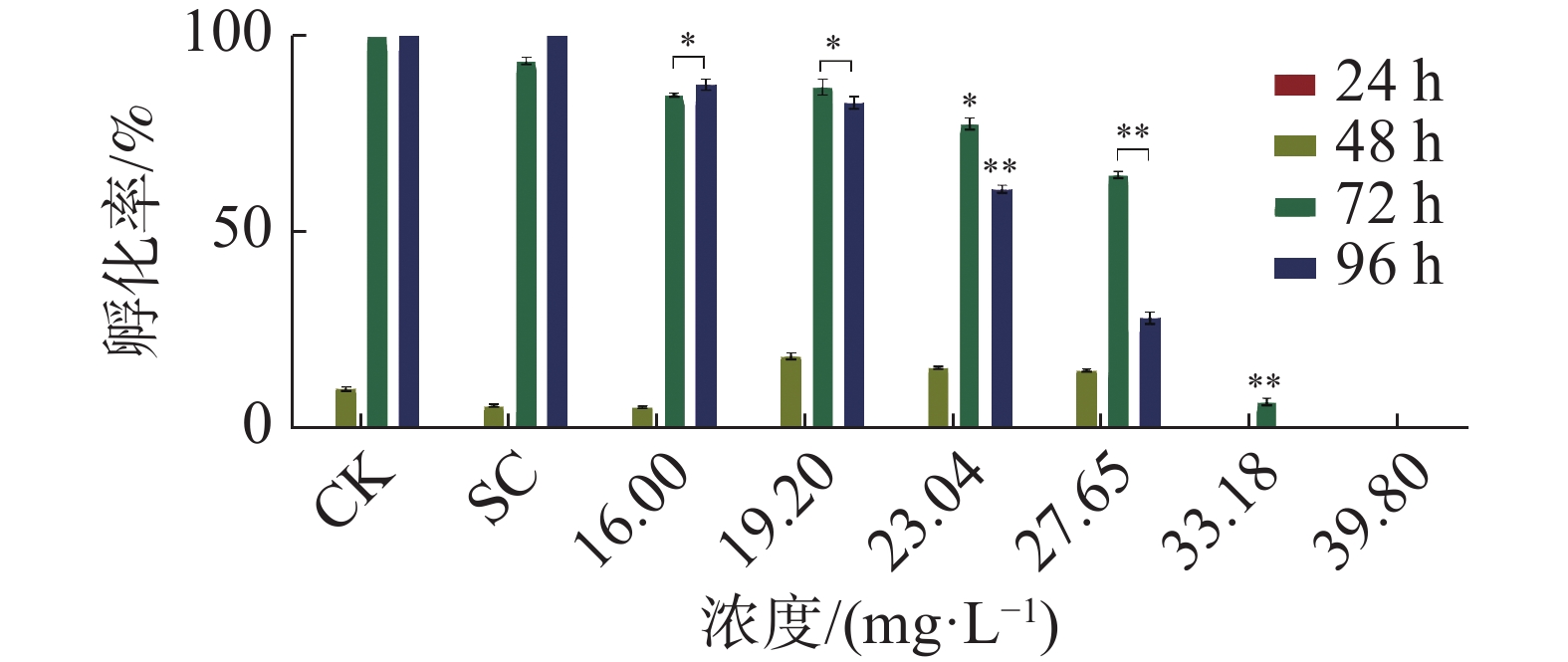
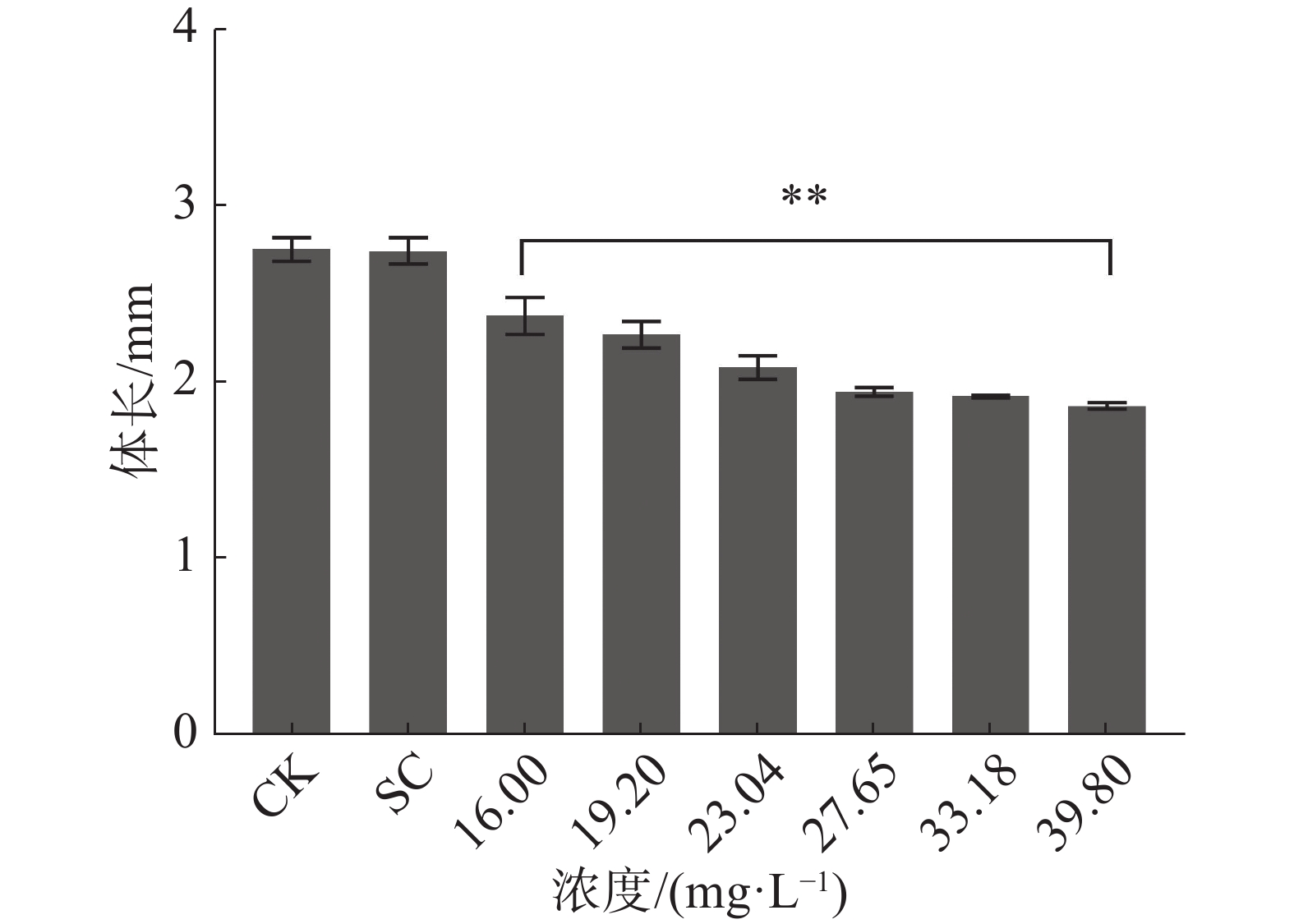
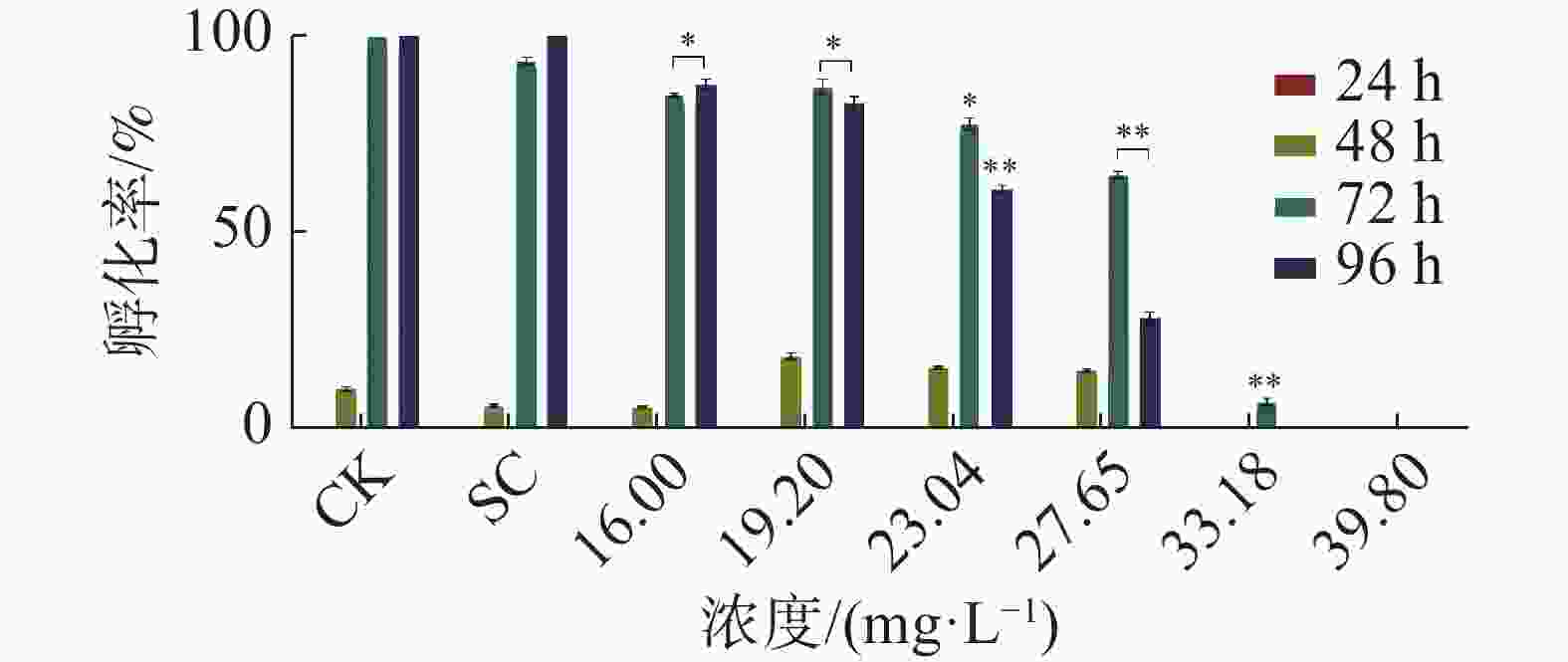
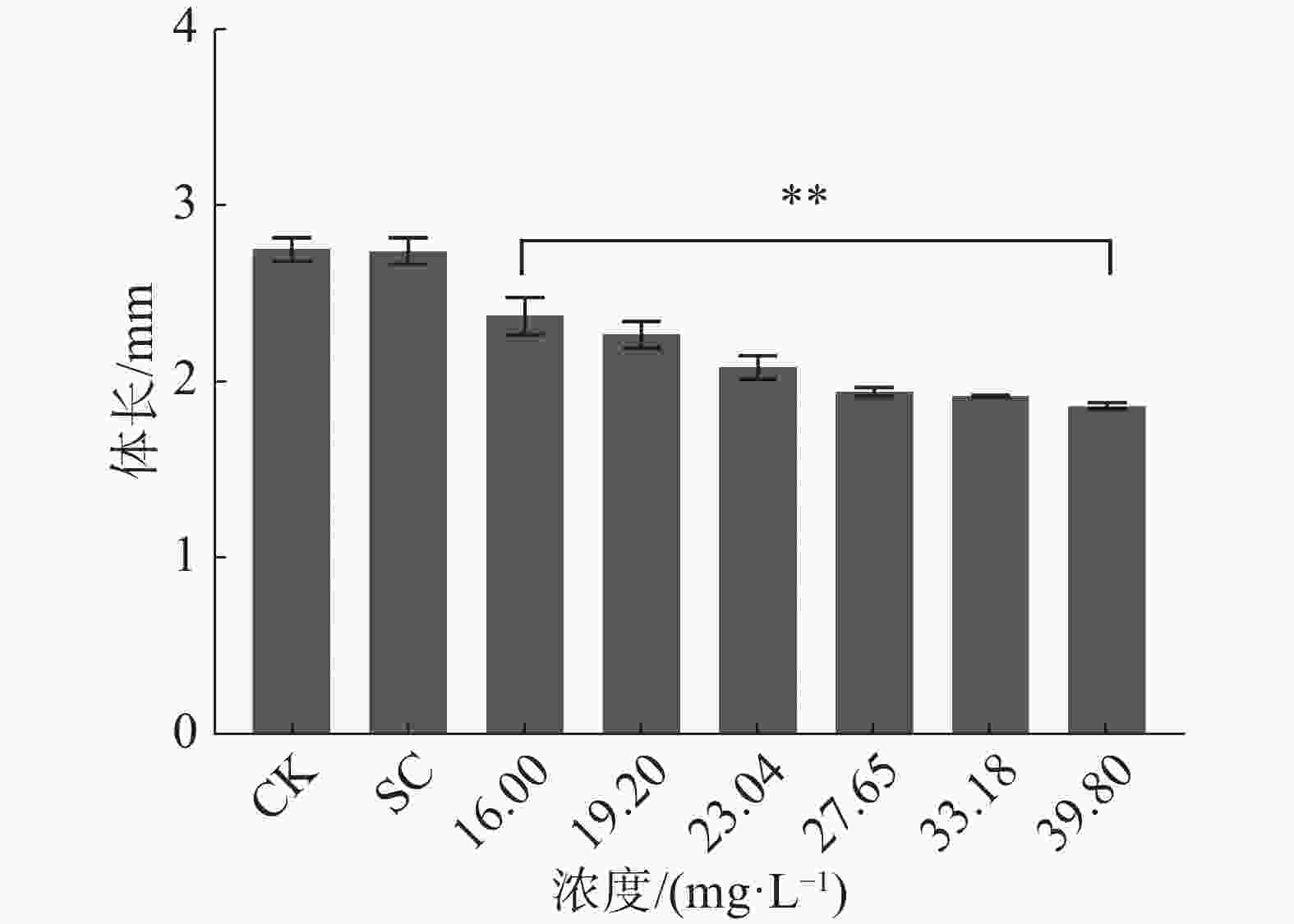
暴露时间72 h和96 h四氯虫酰胺对斑马鱼胚胎孵化率有明显的抑制作用。暴露96 h斑马鱼胚胎的孵化率也受到明显抑制,其中,暴露于四氯虫酰胺浓度在19.02 mg·L−1以上,与对照相比,暴露浓度在19.02 mg·L−1时的孵化率下降了15%,暴露浓度在27.65 mg·L−1时斑马鱼胚胎孵化率下降75%以上(图2) 。用16.00 mg·L−1四氯虫酰胺溶液暴露斑马鱼胚胎,四氯虫酰胺暴露96 h显著抑制斑马鱼胚胎的正常生长发育,斑马鱼胚胎的体长随着四氯虫酰胺浓度的增加而降低(图3)。当四氯虫酰胺暴露浓度高于16.00 mg·L−1时,斑马鱼胚胎的体长与对照相比差异即达显著水平(P<0.01),其中体长对照组为2.755 mm,暴露浓度为39.8 mg·L−1时,斑马鱼体长为0.677 mm,较对照减少了32.27%。
-
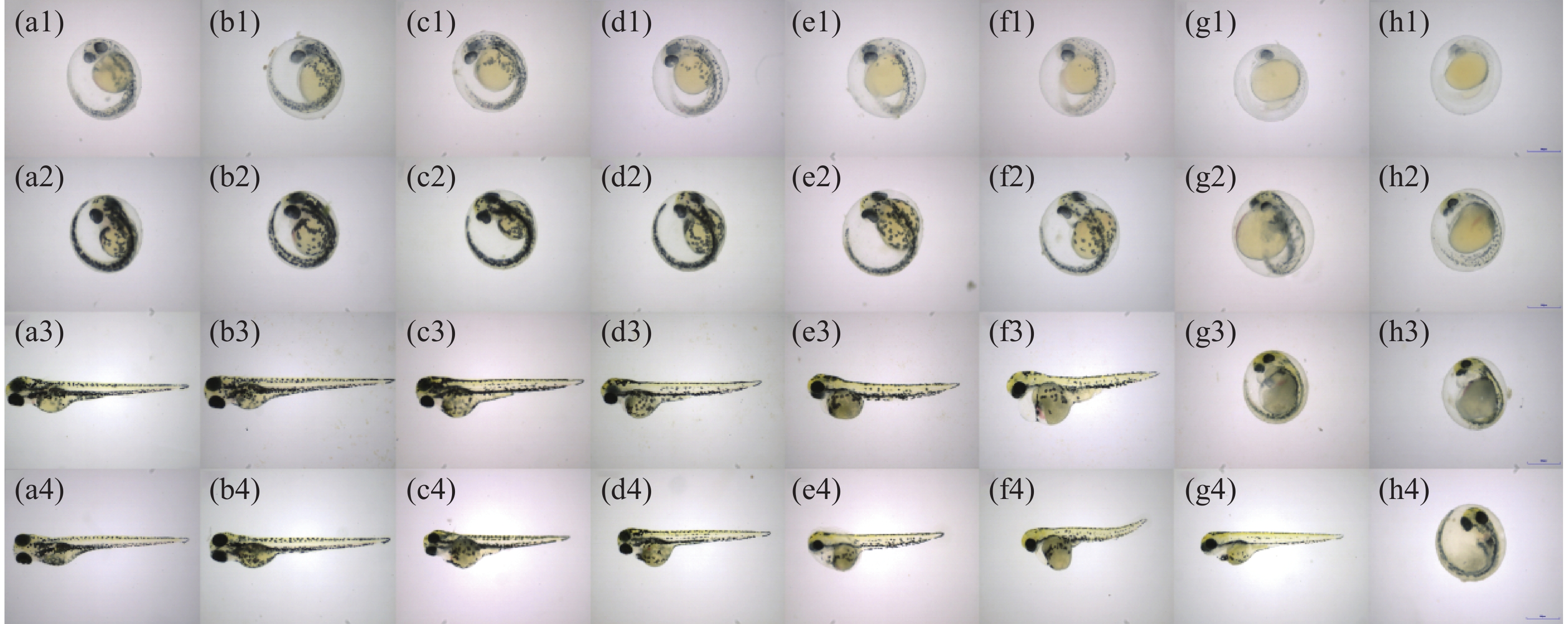
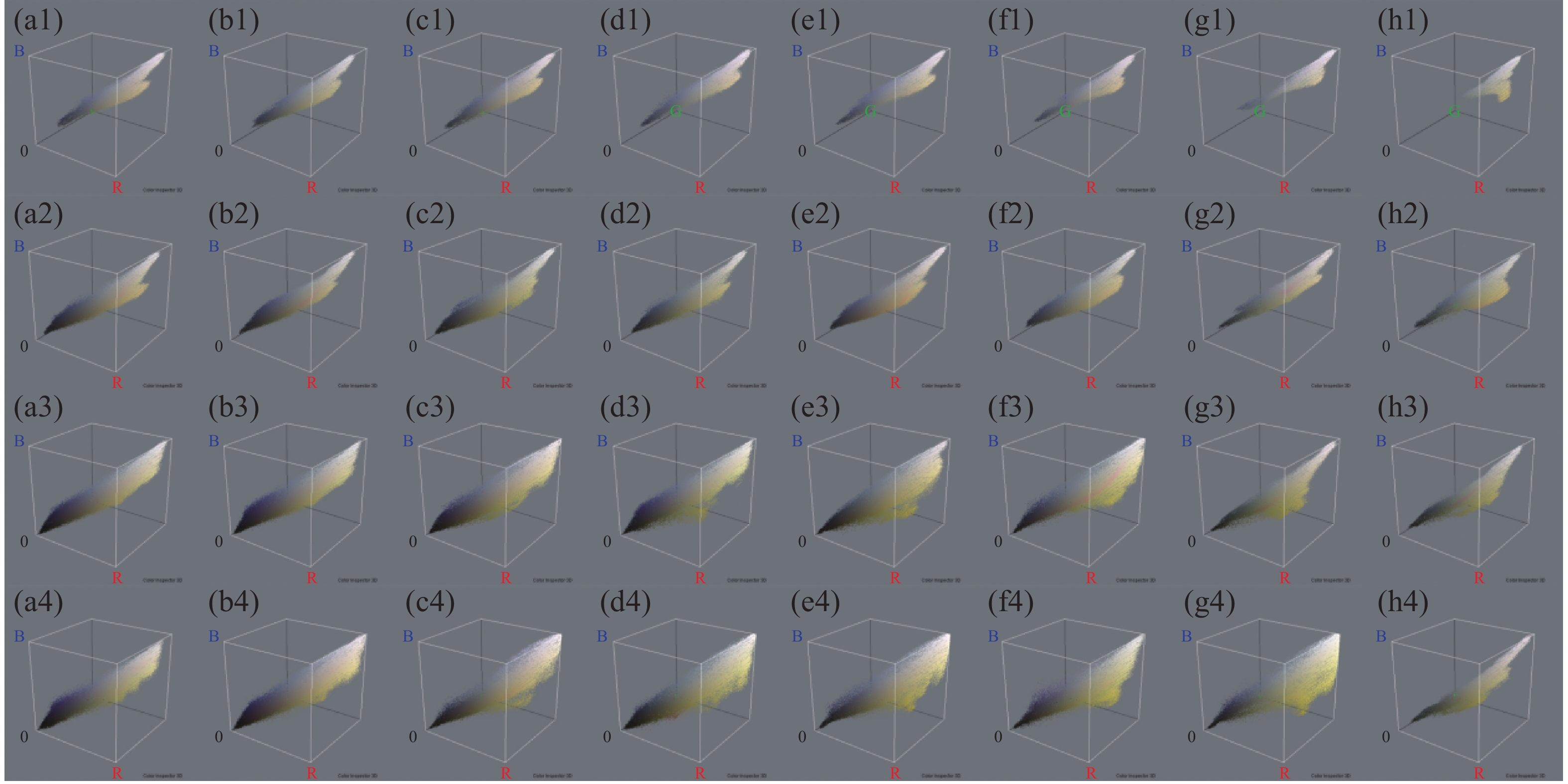
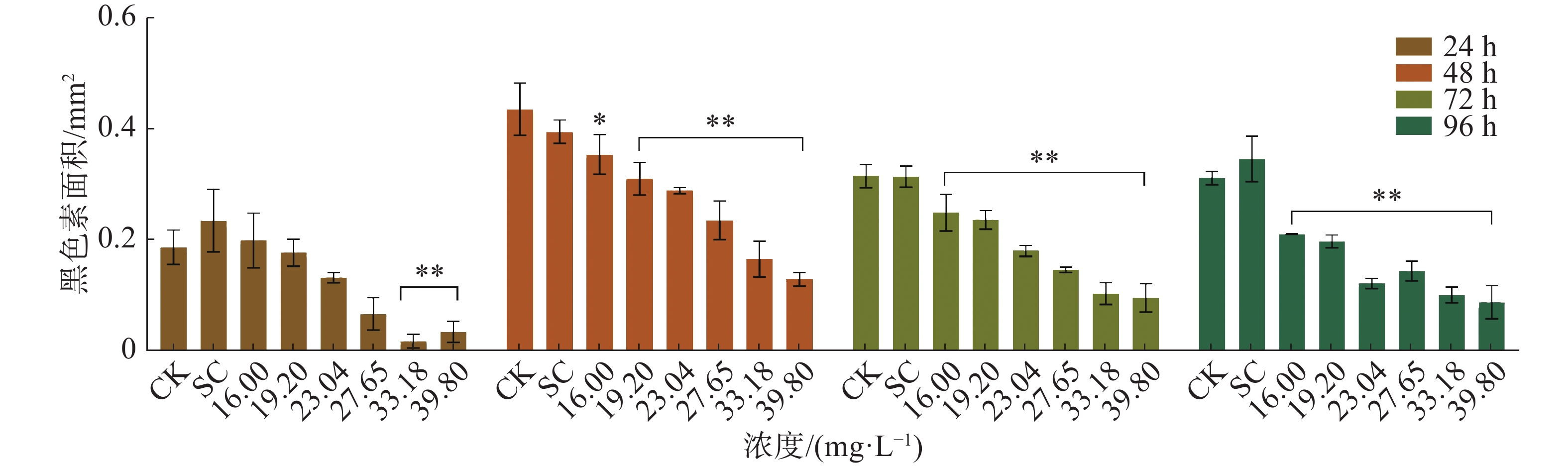
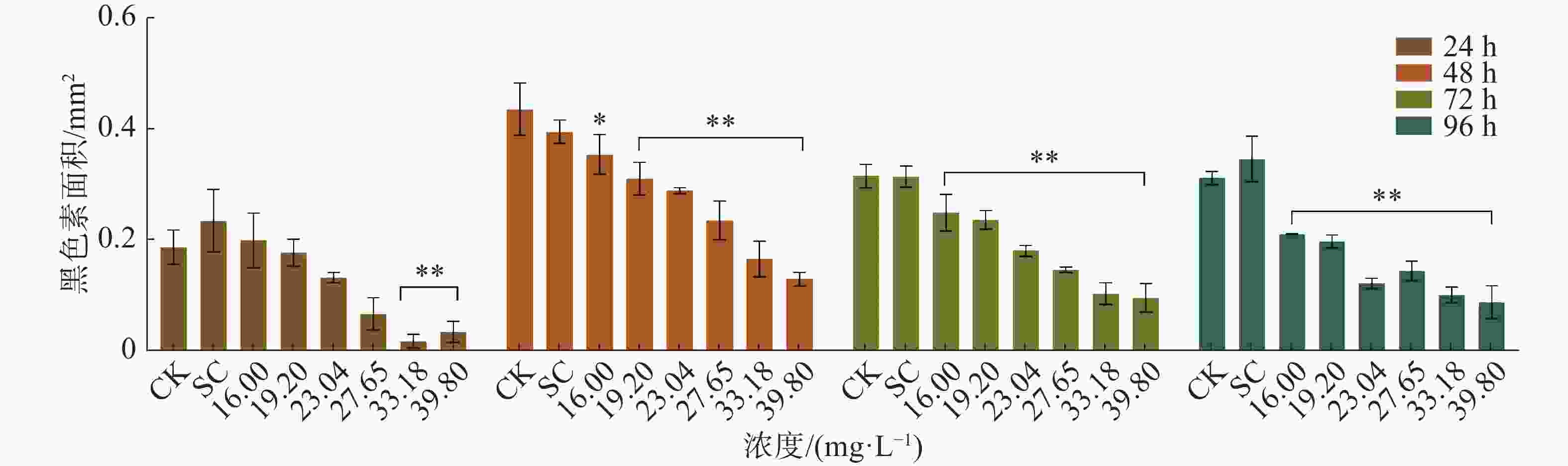
四氯虫酰胺暴露24、48 、72 、96 h的斑马鱼胚胎与空白对照相比,发现四氯虫酰胺对斑马鱼胚胎的色素沉着产生影响(图4),随着四氯虫酰胺处理浓度的增加斑马鱼黑色素区域显著减少。通过Image-J使用RGB三维色素模型分析结果表明,与空白对照相比,黑色素区域的密度随着暴露浓度的增加而显著降低(图5)。四氯虫酰胺暴露24 h通过计算黑色素面积发现随着四氯虫酰胺浓度的增加,斑马鱼黑色素面积明显减少,最高四氯虫酰胺暴露浓度39.8 mg·L−1与空白相比黑色素面积减少了80%(图6)。四氯虫酰胺暴露48 h和72 h随着四氯虫酰胺浓度的增加,斑马鱼胚胎黑色素面积显著减少,并且和暴露浓度之间有着良好的剂量效应关系。暴露浓度33.18 mg·L−1与空白相比黑色素面积减少了60%。四氯虫酰胺暴露96 h斑马鱼胚胎黑色素面积随着四氯虫酰胺暴露浓度的增加明显减少,最高暴露浓度39.8 mg·L−1与空白相比黑色素面积减少了65%。
-
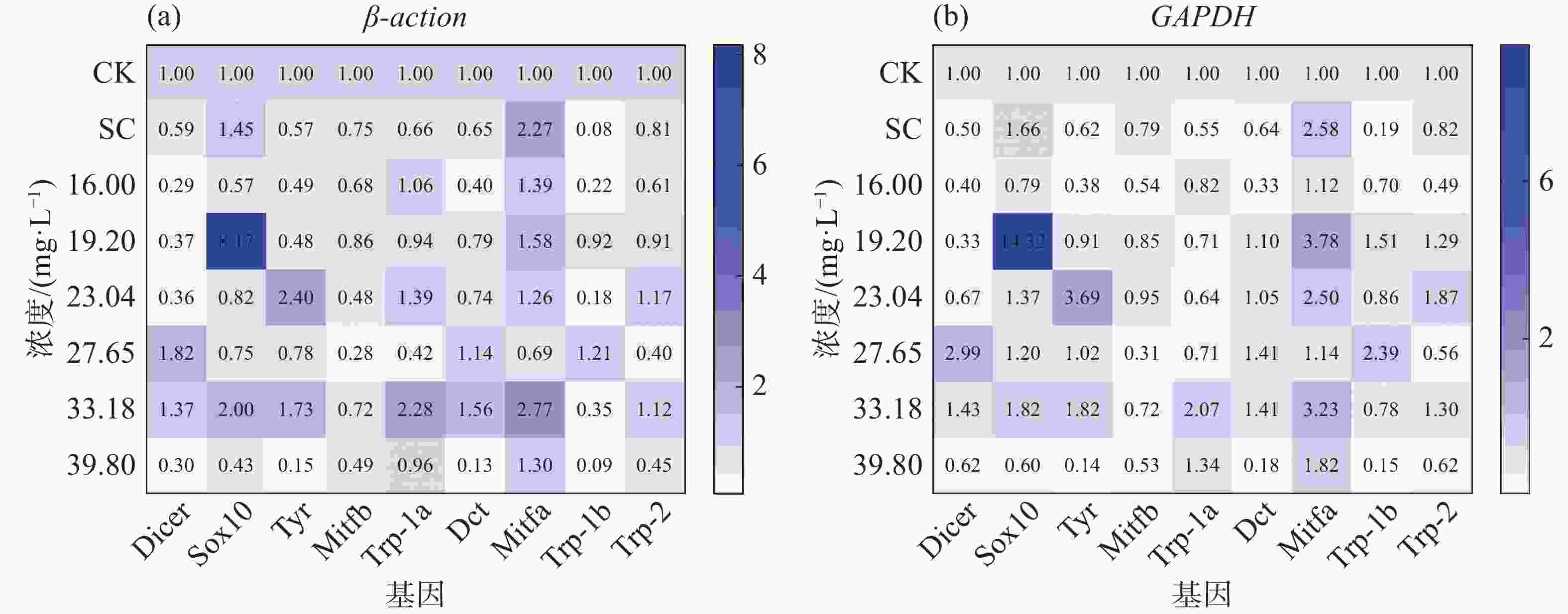
对RT-qPCR结果分析表明,在急性浓度暴露下四氯虫酰胺会影响斑马鱼黑色素合成相关基因的变化,分别使用β-actin和GAPDH两个内参基因对斑马鱼黑色素合成相关基因进行分析(图7、图8),发现2个内参基因趋势一致。
-
以β-actin内参基因进行结果分析,图7-a中Tyr,Sox10,Trp-1a,Mitfa基因在处理浓度23.04 mg·L−1以上的表达量随着浓度的增加先上调后下调,相比对照CK最高处理浓度的基因表达量分别是对照的0.15倍、0.43倍、0.96倍和1.30倍;图8-a表明四氯虫酰胺暴露24 h诱导斑马鱼胚胎黑色素合成通路中Tyr,Sox10,Trp-1a,Mitfa,Dicer,Trp-1b等基因表达量变化趋势,其中Sox10基因与Mitfa基因变化幅度较大,且趋势相对较一致,均呈现上调趋势,Trp-1a次之,说明这3个基因在四氯虫酰胺诱导斑马鱼胚胎黑色素合成过程中起关键作用(图7-a、图8-a)。
-
以GAPDH内参进行结果分析,图7-b中,Sox10,Tyr,Trp-1a,Mitfa基因在处理浓度27.65 mg·L−1以上的表达量随着浓度的增加呈现出先上调后下调的现象,相比对照CK最高处理浓度的基因表达量分别是对照的0.60倍、0.14倍、1.34倍和1.82倍。图8-b表明 四氯虫酰胺暴露24 h诱导斑马鱼胚胎黑色素合成通路中Tyr,Sox10,Trp-1a,Mitfa,Dicer,Trp-1b等基因表达量变化趋势,其中Sox10、Mitfa基因变化幅度较大,Trp-1a次之,说明这3个基因在四氯虫酰胺诱导斑马鱼胚胎黑色素合成过程中起关键作用(图7-b、图8-b)。
-
斑马鱼为评价除草剂、杀虫剂和杀菌剂等农药对环境的影响提供了有前景的生物平台。有机磷杀虫剂杀螟松暴露72 h对斑马鱼的急性毒性平均 LC50为0.341 mg·L−1,并诱导卵黄囊肿等躯体畸形[15]。杀菌剂三氯生暴露96 h对斑马鱼胚胎急性毒性平均LC50为0.42 mg·L−1,显著延迟孵化[16];有机磷农药久效磷 (MCP) 对斑马鱼胚胎具有中等毒性,暴露96 h的LC50为(37.44 ± 3.32)mg·L−1[17];除草剂2,4-D对斑马鱼胚胎的急性毒性暴露96 h的LC50为15.010 mg·L−1,表明毒性为低毒[18]。乙基多杀菌素对斑马鱼胚胎的毒性为低毒并具有致畸作用,暴露96 h的LC50为9.713 mg·L−1[19],黄伟康 [20]研究了溴氰虫酰胺对斑马鱼胚胎的急性毒性,暴露96 h的LC50为1.57 mg·L−1,属于中毒范围。四氯虫酰胺是我国自主研发的具有低毒、高效等特点的杀虫剂,本研究按照OECD的准则开展了四氯虫酰胺原药对斑马鱼胚胎的急性毒性试验,暴露96 h的LC50为23.775 mg·L−1,结果表明为低毒,这与张琦[9]报道的10%四氯虫酰胺悬浮剂对水生生物鲤鱼的结果相似。本试验结果表明与对照相比,随着四氯虫酰胺浓度的升高斑马鱼胚胎在暴露96 h下的孵化率降低、自主运动频率增加以及体长降低,并且较高浓度四氯虫酰胺显著地减少斑马鱼黑色素的面积。新型吡啶基恶唑甲酰胺可以引起斑马鱼胚胎的黑色素细胞缺失现象[21],说明化学物质在水体中会对斑马鱼黑色素细胞的生成产生影响,本研究结果表明四氯虫酰胺对斑马鱼胚胎黑色素的合成有显著的抑制作用,这与前人的结果具有相似性。
与黑色素合成相关基因的报道中,Mitfa是在黑色素母细胞前体中表达的早期基因之一,Mitfa置于产生黑色素和最终分化黑色素细胞所必需的多个基因的上游,包括多巴色素互变异构酶,酪氨酸酶,酪氨酸酶相关蛋白1,C-kit和Bcl2转录因子[22]。也有研究报道表明,Mitf作为主导作用的天然木质素通过下调Mitf和Tyr的表达,从而有效地抑制斑马鱼黑色素细胞的生成[23];在新型污染物三氯卡班影响斑马鱼幼虫黑色素生成的研究报道中,也指出Mitfa(小眼相关转录因子)和Tyr(酪氨酸酶)的表达水平的变化是影响黑色素合成的关键因子[24],本研究中Mitf基因是四氯虫酰胺诱导斑马鱼黑色素减少变化最大的基因。另文献[25]报道,在损害神经嵴细胞分化的过程中Sox10和Mitfa转录所涉及的成熟miRNA的生成来实现存在一致性。黑色素细胞由Sox10转录因子核心参与的神经嵴细胞生成,Sox10通过Mitfa启动子直接驱动黑色素细胞的命运,存在调控关系[26],且Mitf/mitfa作为黑色素分化多个关键基因的上游基因,决定黑色素细胞的命运,导致黑色素细胞的生成和发育受到影响。本研究结果显示,Sox10基因与Mitfa基因变化幅度较大,且趋势相对较一致,均呈现上调超势。
黑色素生物取决于酪氨酸酶家族的3种酶的功能,有研究表明酪氨酸酶 (Tyr)、酪氨酸酶相关蛋白1 (Tyrp1) 和多巴色素互变异构酶 (Dct或Tyrp2)等3种酶的功能直接影响黑色素的生物合成,其中酪氨酸酶相关蛋白的异常表达会导致黑色素细胞死亡[27],有文献报道杀菌剂恶霉灵通过抑制酪氨酸酶活性降低了斑马鱼胚胎黑色素密度[28],本研究中酪氨酸酶相关基因Trp-1a也是变化较大的3个基因之一。
Acute toxicity of Tetrachlorantraniliprole to Zebrafish and an impact on melanin biosynthesis
doi: 10.15886/j.cnki.rdswxb.2023.03.011
- Received Date: 2022-03-20
- Accepted Date: 2022-10-15
- Rev Recd Date: 2022-10-01
- Publish Date: 2023-05-25
-
Key words:
- tetrachlorantraniliprole /
- zebrafish /
- acute toxicity /
- melanin
Abstract: In order to provide a certain theoretical basis for the ecological risk assessment of pesticide tetrachlorantraniliprole, the concentration change of tetrachlorantraniliprole in water for 96 hours was determined by using ultra-high performance liquid chromatography-tandem mass spectrometry. According to the OECD standard, embryos of Danio rerio were used as assessment of the model organisms. After static exposure to different doses of tetrachlorantraniliprole, zebrafish embryos were observed in terms of growth and development, and their voluntary movement, melanin and body length were calculated. The 96 h LC50 were evaluated by mortality rate. Genes related with the melanin biosynthesis in the embryos were determined by using the RT-qPCR. The results showed that the tetrachlorantraniliprole in water for 96 h had the highest degradation rate (7.6%), with its concentration being greater than 80%, and had a good stability in water for 96 h. The LC50 of tetrachlorantraniliprole at 96 h was 23.775 mg·L−1, indicating low toxicity. The exposure to tetrachlorantraniliprole affected the development of zebrafish embryos in voluntary movement frequency, hatching rate and body length. The frequency of voluntary movement of zebrafish embryos increased with the tetrachlorantraniliprole concentration. When the exposure concentration was 39.8 mg·L−1, the zebrafish embryos had a voluntary movement frequency of 5.5 times·min−1, which was 22 times that of the control. When the exposure concentration was 27.65 mg·L−1, the zebrafish embryos decreased their hatching rate by 75%. At an exposure concentration of 39.8 mg·L−1, zebrafish decreased its body length by 32.27% compared with the control. Moreover, during the experiment it was also found that melanin pigmentation decreased after exposure to tetrachlorantraniliprole, and that the melanin area of 96 h zebrafish embryos decreased significantly with the increase of tetrachlortraniliprole concentration, and was reduced by 65% at the highest exposure concentration of 39.8 mg·L−1 as compared with the blank control. The RT-qPCR results showed that the expression of Tyr, Sox10, Trp-1a, and Mitfa genes had certain changes. The results showed that tetrachlorantraniliprole inhibited the expression ofSox10 and Mitfa, resulting in abnormal expression of tyrosinase-related transcription factors such as Tyr and Tyr-1a, which ultimately led to the impairment of melanin synthesis and pigmentation in zebrafish embryos.
| Citation: | ZHENG Shixiang, ZHANG Taiyu, GUO Yuzhao, ZHANG Jie, FAN Yongmei. Acute toxicity of Tetrachlorantraniliprole to Zebrafish and an impact on melanin biosynthesis[J]. Journal of Tropical Biology, 2023, 14(3): 338-346. doi: 10.15886/j.cnki.rdswxb.2023.03.011 |










 DownLoad:
DownLoad: