-
5−羟基色氨酸(5-Hydroxytryptophan,5-HTP)是一种色氨酸衍生物,由色氨酸羟化酶催化,使色氨酸苯环上5′位氢原子被羟基取代[1]。在哺乳动物体内,5-HTP可脱羧转化为5-羟色胺(5-Hydroxytryptamine,5-HT),也称为血清素,参与调节情绪、认知、学习、记忆、睡眠和其他生理过程[2]。5-HT可进一步转化为褪黑素(Melatonin,MT),该激素由松果体释放,具有调节睡眠的作用[3- 4]。5-HTP是5-HT和MT的重要前体,在抗抑郁、减肥、治疗失眠和头痛等方面有重要作用。但是,摄入过量的5-HTP可能导致血清素综合征,产生一定的副作用,例如躁动、神志不清、心动过速、出汗和血压波动等[5]。
5-HTP主要来源是从非洲豆科植物加纳(Griffonia simplicifolia)种子中提取,但是植物的生长周期长,并受季节和地域的限制,产率低,不适合工业化应用[6]。化学合成方法的周期长、耗资大,需在高温高压环境下合成,易造成环境污染,且色氨酸区域选择性羟基化较难实现,同样不适用于大规模合成[7- 8]。微生物合成具有生产周期短、成本低、产量高和易于大规模生产等优点,具有巨大的应用前景。笔者着重阐述定向进化提高芳香族氨基酸羟化酶的羟化活性、辅酶因子合成和再生途径的引入和代谢工程优化5-HTP合成效率等方面阐述了微生物合成5-HTP的进展,旨在为5-HTP的规模化生产研究提供资料。
-
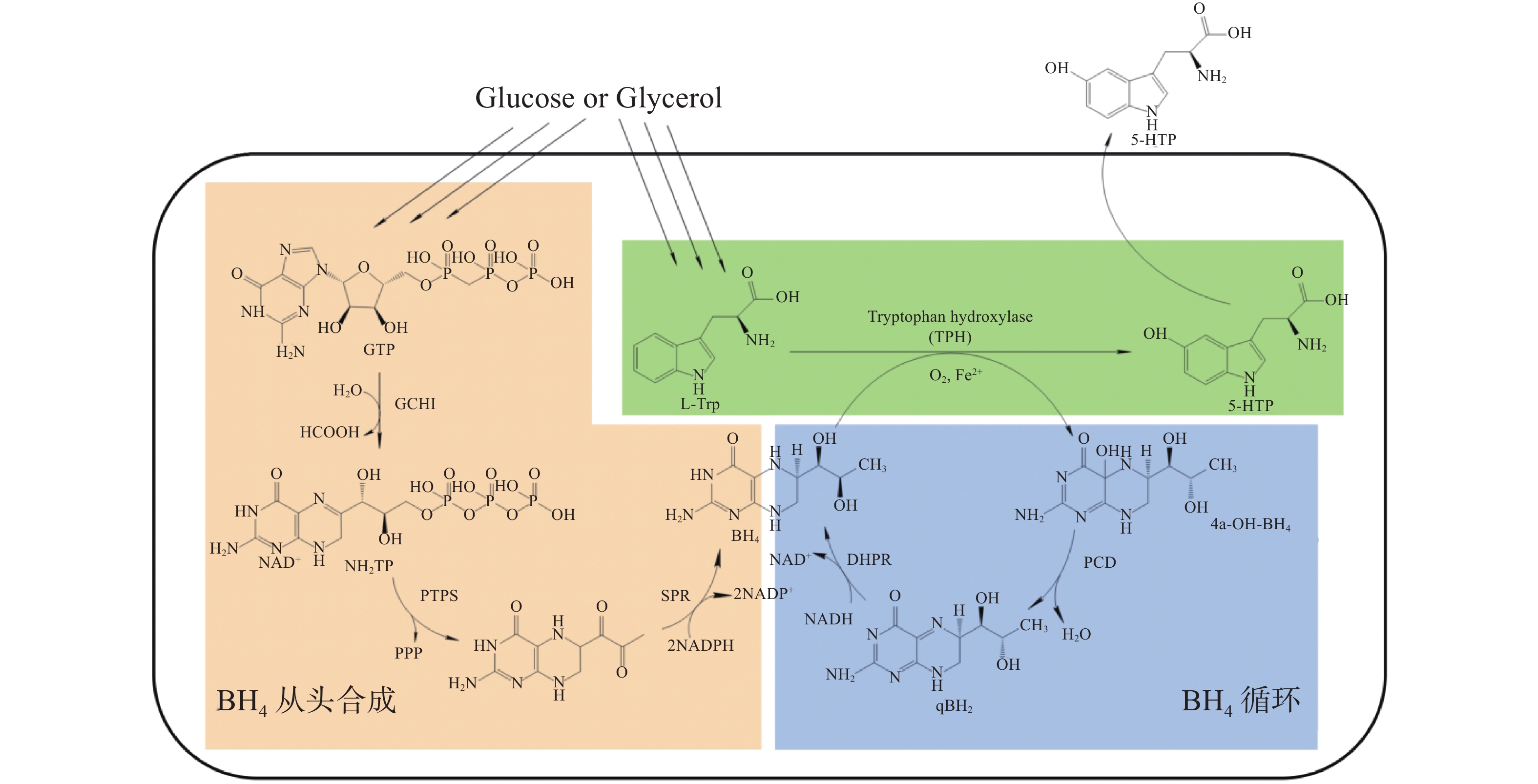
在生物体内,5-HTP由色氨酸羟化酶(Tryptophan hydroxylase,TPH)催化L−色氨酸(L-Tryptophan,L-Trp)和O2合成,此过程需要四氢生物蝶呤 ( Tetrahydrobiopterin,BH4)和Fe2+作为辅助因子[9]。TPH是芳香族氨基酸羟化酶(Aromatic amino acid hydroxylases,AAAH)家族的一员,它是单加氧酶,催化时将O2中一个氧原子与底物结合,并将另一个氧原子还原为H2O,还原为H2O所需的2个电子由BH4提供[10]。在这个过程中,辅助因子BH4至关重要。BH4合成从GTP开始,GTP被GTP环化水解酶Ⅰ(GTP CyclohydrolaseⅠ,GCHI)催化生成7,8−三磷酸二氢新蝶呤(7,8-Dihydroneopterin triphosphate,H2NTP),再在6−丙酮四氢蝶呤合成酶(6-Pyruvoyl Tetrahydropterin Synthase,PTPS)的作用下生成6−丙酮−5,6,7,8−四氢蝶呤(6-Pyruvoyl-5,6,7,8-tetrahydropterin,PTP),最后由墨蝶呤还原酶(Sepiapterin Reductase,SPR)催化,转化成终产物BH4[11]。在哺乳动物体内,BH4作为辅酶被消耗后,会转化成蝶呤−4α−甲醇胺(BH4-4α-carbinolamine,4α-OH-BH4),在蝶呤−4α−甲醇胺脱水酶(Pterin-4α-carbinolamine dehydratase,PCD)的作用下生成醌型双氧蝶呤(Quinonoid Dihydrobiopterin, qBH2), 在双氢蝶啶还原酶(Dihydropteridine Reductase,DHPR)的催化下再生成BH4,完成BH4循环(图1)[12]。
-
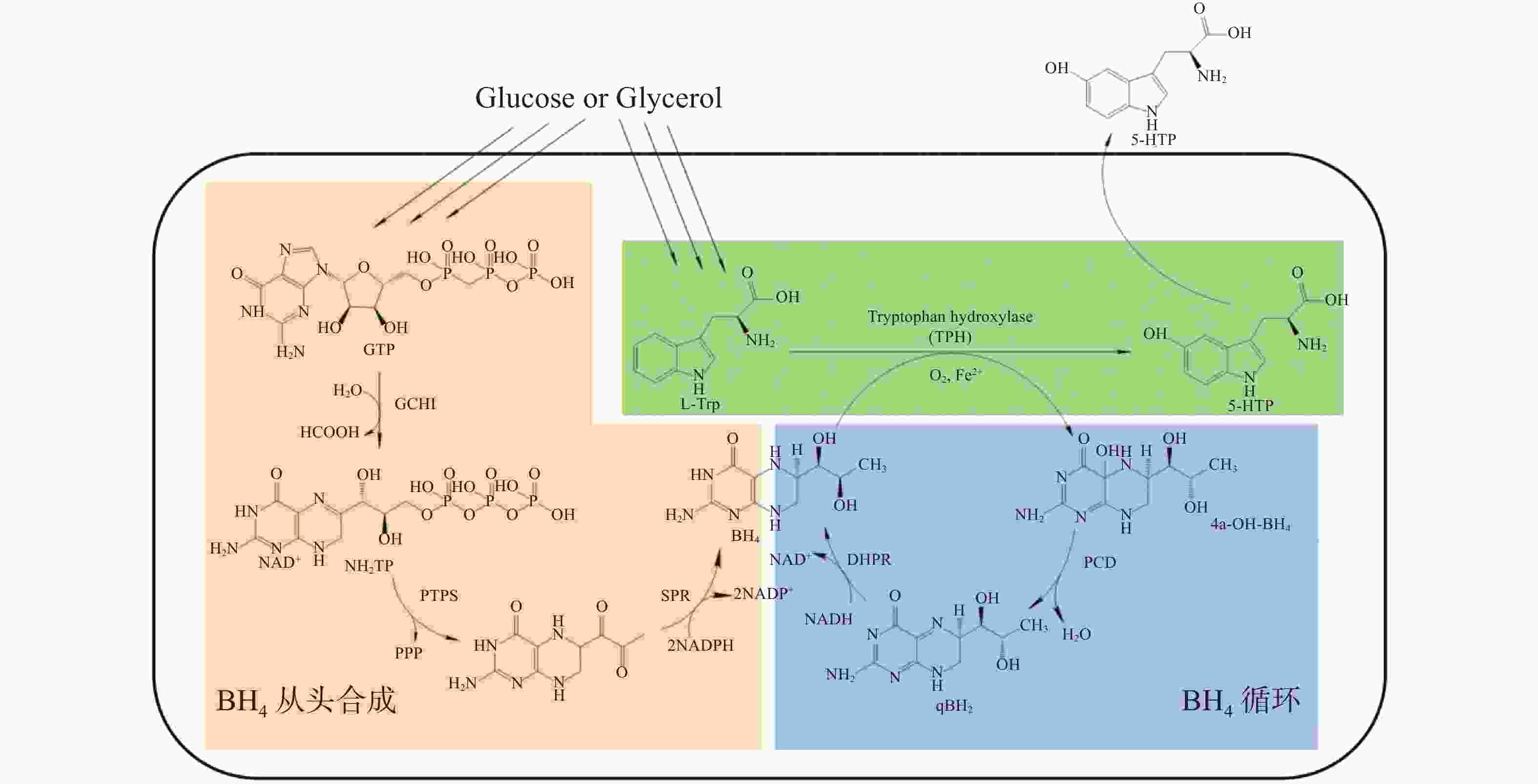
在大肠杆菌内构建5-HTP生物合成途径需要3个模块,分别为色氨酸羟化酶模块、BH4从头合成模块和BH4循环模块(图2)。
-
AAAH是非血红素亚铁和BH4依赖的单加氧酶,它们包括色氨酸羟化酶(Tryptophan hydroxylase,TPH)、苯丙氨酸羟化酶(Phenylalanine hydroxylase,PAH)和酪氨酸羟化酶(Tyrosine hydroxylase,TH)。这3种酶都使用BH4和O2为辅助因子,分别催化色氨酸、苯丙氨酸和酪氨酸转化为5-HTP、酪氨酸和左旋多巴[13]。其中,人类TPH有2种亚型,由2个不同的基因编码。最早被发现且研究最多的是TPH1,主要存在于松果体和肠道系统,而TPH2主要存在于神经细胞[14]。
Moran等[15]首先在大肠杆菌中表达了野生型兔源色氨酸羟化酶(TRH)以及该酶2种截短的突变蛋白,发现野生型兔源色氨酸羟化酶表达水平较低,且删除N端调节域101个氨基酸的突变蛋白主要以包涵体的形式存在,但同时删除N端101个氨基酸与C端28个氨基酸的蛋白质TRH102-416表达量为细胞总蛋白的30%。其结果表明,兔源TRH102-416是一种稳定、高活性的TRH形式,可在大肠杆菌中高水平表达。MCKINNEY等[16]在大肠杆菌中表达了全长的人TPH,将其与麦芽糖结合蛋白(Maltose binding protein,MBP)结合(MBP-TPH),使TPH迅速而有效地折叠成天然结构,促进TPH的可溶性表达,与6×His-TPH融合蛋白相比,MBP-TPH比酶活高出3倍。WINDAHL等[17]对鸡的色氨酸羟化酶晶体结构进行了研究,发现与色氨酸结合的疏水性口袋由残基Tyr236、Thr266、Pro269、His273、Phe314、Phe319和Ile367等组成,且其中Fe2+配位结构是非血红素依赖的配位结构,与人类TPH1的结构相比,其整体结构更紧凑,在活性位点周围有两个闭合环。
由于真核生物酶的低溶解性、低稳定性以及特定的翻译后修饰,真核生物的AAAH难以在大肠杆菌中以可溶的和稳定的形式表达[16, 18]。虽然使用截短的蛋白或融合蛋白可获得可溶性和有活性的酶,但这类AAAH在大肠杆菌中羟化活性仍然较低[18]。相反,细菌AAAH在大肠杆菌内表达更稳定,更有利于提高芳香族氨基酸的羟化活性。KINO等[19]利用紫色色杆菌(Chromobacterium violaceum)PAH(CviPAH)的晶体结构信息,对CviPAH的第101位亮氨酸(L101)和第180位色氨酸(W180)进行饱和突变后发现,第101位亮氨酸突变为酪氨酸(L101Y)和第180位色氨酸突变为苯丙氨酸(W180F)后,L-色氨酸羟化活性提高至5.2倍。
-
色氨酸羟化酶催化过程需要BH4作为辅助因子,但大肠杆菌不含内源性BH4,因而BH4合成与再生途径的构建是色氨酸羟化酶途径的重点。Yamamoto等[20]在大肠杆菌内表达GCHI、PTPS和SPR 等3个酶基因,在大肠杆菌内成功构建BH4合成途径,并进一步提高BH4产能,最大产率达到约4.0 g·L−1。Ryotaro等[19, 21]在2009年研究的基础上,在大肠杆菌内表达CviPAH的突变体(CviPAH -L101Y-W180F),敲除宿主内降解L-Trp和5-HTP的色氨酸酶基因(∆tnaA),并导入BH4辅酶因子的再生系统(PCD,DHPR)和NADH再生系统,促进5-HTP的合成。通过添加BH4和L-Trp,并优化反应条件,最终5-HTP产率为2.5 mmol·L−1,实现了BH4再生系统的应用,提高了BH4的利用率。但该研究仍需添加BH4和L-Trp作为底物,并不是微生物合成5-HTP的最佳方法。一项专利[22]在大肠杆菌中表达截短的兔源TPH1,同时导入BH4的生物合成途径和再生系统,实现了GTP从头合成BH4以及再生BH4。该专利在大肠杆菌(∆tnaA)内共表达6个酶(TPH1、GCHI、PTPS、SPR、PCD、DHPR),在不添加BH4以及L-色氨酸的培养基中生产了0.9 mmol·L−1(相当于198 mg·L−1)的5-HTP。Wang等[23]在大肠杆菌(∆tnaA)内异源表达曼氏血吸虫(Schistosoma mansoni)TPH(SmTPH)以及BH4从头合成和循环途径,在含有2 g·L−1的L-Trp的培养基中发酵60 h生成0.93 g·L−1的5-HTP。
-
真核生物AAAH在大肠杆菌中常以包涵体的形式表达,而原核生物AAAH L−色氨酸羟化活性非常低。但据报道,当紫色色杆菌(Chromobacterium violaceum)PAH突变时将提高L−色氨酸羟化活性[19]。同时有实验表明[24-25],部分细菌PAH可利用四氢苏式新蝶呤(Tetrahydromonapterin,MH4)代替BH4作为辅酶进行羟化反应,MH4参与反应后可转变为4α-羟基四氢喋呤(4α-Hydroxytetrahydropterin,4α-MH4),再经PCD催化为二氢单氢蝶呤(Dihydromonapterin,MH2),MH2可以进一步还原为MH4,完成MH4循环。大肠杆菌含有内源性MH4,但缺少MH4循环系统,MH4是大肠杆菌中蝶呤的主要存在形式(图2)。
Lin等[26]比对5个微生物PAH后发现,野油菜黄单胞菌(Xanthomonas campestris)PAH(XcPAH)对苯丙氨酸和色氨酸的活性较高,在其预测的晶体结构中,W179位于活性口袋内,将其突变可改变XcPAH的底物偏好,结果表明,XcPAH-W179F突变体色氨酸羟化活性是野生型的17.4倍,同时突变体苯丙氨酸羟化活性降低了约20%。XcPAH-W179F可以利用大肠杆菌内源MH4作为辅酶,因此,研究人员共表达XcPAH-W179F、PCD和DHMR成功构建色氨酸羟化途径和MH4循环系统。将该系统转入大肠杆菌内,同时添加2 g·L−1的 L-Trp作为底物,在30℃培养16 h可合成1.1 g·L−1 5-HTP,但不添加L-Trp时,仅可生成152.9 mg·L−1 5-HTP。由于在大肠杆菌中已实现了L-Trp的高水平生产(高达48.7 g·L−1)[27],将该系统导入色氨酸高产菌株并对其进行优化有望获得5-HTP高效生产菌株。
Mora-Villalobos等[28]比较了10种细菌AAAH对色氨酸的亲和性,发现台湾铜绿假单胞菌(Cupriavidus taiwanensis)AAAH(CtAAAH)对色氨酸亲和性较高,通过与色氨酸偏好的酶进行比较发现CtAAAH-W192与L-Trp的吲哚环的结合有关,对该位点突变可改变其底物偏好。与野生型相比,突变体CtAAAH-W192F的色氨酸羟化活性提高了5.5倍,苯丙氨酸羟化活性降低了约10%。将该突变体转入大肠杆菌(∆tnaA)内,同时表达MH4循环系统,在添加5 mmol·L−1 L-Trp的培养基中培养24 h后,可合成2.5 mmol·L−1的 5-HTP。
-
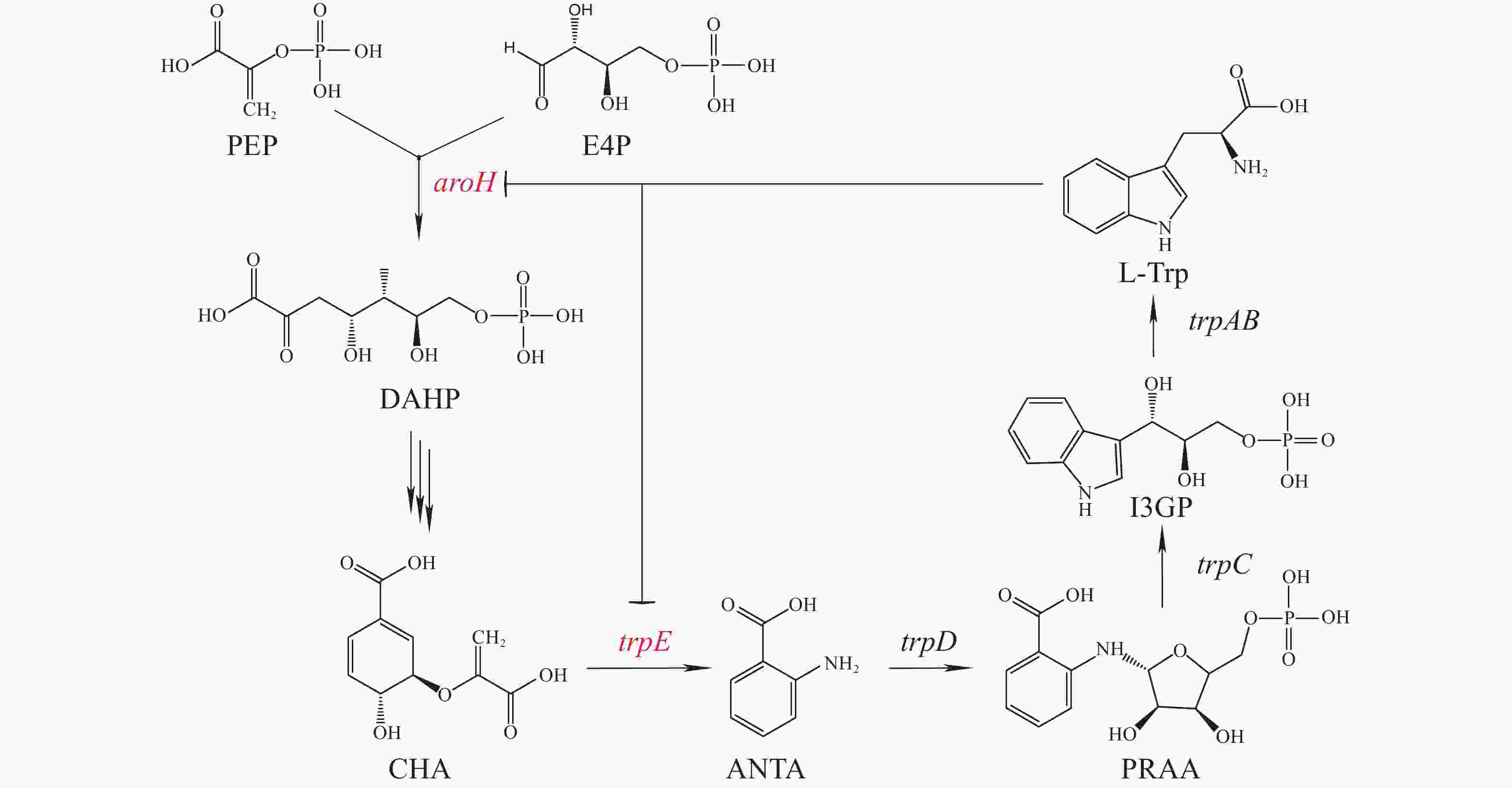
利用代谢工程平衡细胞代谢网络,可进一步提高5-HTP产量。细胞内L−色氨酸的合成起始于3−脱氧−D−阿拉伯庚糖酮酸−7−磷酸( 3-Deoxy-D-arabino-heptulosonic acid 7-phosphate,DAHP)。磷酸烯醇式丙酮酸(Phosphoenolpyruvate,PEP)与赤藓糖−4−磷酸(Erythrose-4-phosphate,E4P)经aroH基因编码的DAHP合成酶(DAHP synthase)催化合成 DAHP,再经分支酸途径合成分支酸(Chorismate,CHA),CHA在色氨酸操纵子的催化下合成L−色氨酸(图3)[29]。这一过程中,aroH 与trpE基因的表达与蛋白活性都受到色氨酸的反馈抑制。点突变与启动子替换可以解除色氨酸对AroH 与TrpE的反馈抑制,将代谢流向有利于色氨酸合成的方向,增加色氨酸在宿主细胞内的累积量,以提高5-HTP的合成[30]。
大肠杆菌trpR基因编码一种阻遏蛋白TrpR,它能够控制色氨酸操纵子的表达,当有高浓度的L-Trp存在时,TrpR可与L-Trp形成二聚体,与色氨酸操纵子结合,阻止转录,L-Trp的生物合成途径停止。当L-Trp含量低时,TrpR处于失活状态,色氨酸操纵子可正常转录[31]。在大肠杆菌内敲除trpR基因可实现L-Trp的累积,有利于5-HTP的合成。
Mora-Villalobos等[32]在2017年研究的基础上对CtAAAH再次进行优化,构建了双突变体CtAAAH-F197L/E219C(CtAAAH-LC),与单突变体CtAAAH-W192F相比,CtAAAH-LC的Km值更低(0.95 mmol·L−1),反应速度更快(Vmax=1.9 mmol·L−1·s−1)。同时,构建了菌株TrpD-Pl,该菌株以LIN等[30]人的色氨酸高产菌株S028为出发菌株,敲除了基因trpR,并含有人MH4再生系统(PCD,DHMR)。将CtAAAH-LC转入菌株TrpD-Pl 中进行分批补料发酵,60 h后,色氨酸含量达(23.4±1.4)g·L−1,5-HTP产量为(962±58)mg·L−1。
Wang等[33]进一步对L-色氨酸生产模块、色氨酸羟基酶模块和BH4模块进行优化。作者在大肠杆菌内表达人源TPH,将BH4的合成与再生系统同时导入大肠杆菌内,并解除色氨酸对TrpE和AroH的反馈抑制(aroH fbr 和 trpE fbr),在未添加L-Trp和BH4的摇瓶中发酵116 h,可合成3.97 g·L−1的 L-Trp和314.8 mg·L−1 的5-HTP。再经代谢工程调控,对L-色氨酸模块、色氨酸羟基酶模块和BH4模块优化。研究表明,截短的TPH在大肠杆菌中可溶性和稳定性将提高[34, 35],将截短的TPH145(TPH2 NΔ145/CΔ24)代替TPH进行摇瓶发酵,最终合成424.7 mg·L−1的5-HTP,产率提高34.9%。前期的实验表明,L-Trp在宿主内大量累积却未转化成5-HTP,推测合成L-Trp将消耗大量的营养物质,不利于5-HTP的合成。用低拷贝质粒表达aroH fbr 和 trpE fbr,L-Trp产量显著降低(207.9 mg·L−1),同时,5-HTP的产量增加至532.6 mg·L−1。再经启动子优化,降低BH4合成和循环途径过度表达的负担,最终5-HTP和L-色氨酸产量显著增加,摇瓶发酵分别为1.3 g·L−1和1.7 g·L−1。在以甘油为碳源的补料分批发酵罐中发酵,5-HTP最终产量为5.1 g·L−1。由于多质粒表达在发酵后期稳定性较差,为了增加羟化途径的稳定性,将aroH fbr和trpE fbr整合到大肠杆菌基因组中,同时降低了色氨酸羟化质粒拷贝数和aroH fbr启动子的强度,以重新平衡和优化代谢途径,最终5-HTP摇瓶发酵产量为1.61 g·L−1,提高了24.8%,L-Trp产量为0.20 g·L−1,降低了88%[36]。
除了大肠杆菌异源表达生产5-HTP外,Zhang等[37]首次在酿酒酵母BY4741内成功合成了5-HTP,通过异源表达原核PAH或真核色氨酸3/5-羟化酶,以MH4或BH4两种辅助因子增强合成5-HTP。此外,酿酒酵母中天然基因DFR1与大肠杆菌中的folM基因具有相似的功能,经过基因DFR1敲除,证实在酿酒酵母中DFR1基因通过再生MH4对5-HTP的合成起着关键作用,为利用代谢工程促进酵母生产5-HTP奠定了基础(表1)。
菌株 羟化酶 辅酶 代谢工程优化 5-HTP产量 参考文献 E. coli CviPAH -L101Y-W180F BH4 ∆tnaA 550 mg·L−1 [21] E. coli 兔源TPH1 BH4 ∆tnaA 198 mg·L−1 [22] E. coli SmTPH BH4 ∆tnaA 0.93 g·L−1 [23] E. coli XcPAH-W179F MH4 ∆tnaA 1.1 g·L−1 [26] E. coli CtAAAH-W192F MH4 ∆tnaA 550 mg·L−1 [28] E. coli CtAAAH-F197L/E219C MH4 色氨酸高产菌株TrpD-Pl 962±58 mg·L−1 [32] E. coli TPH145(TPH2 N∆145/C∆24) BH4 低拷贝质粒表达aroH fbr 和
trpE fbrBH4合成和
循环途径启动子优化5.1 g·L−1 [33] E. coli TPH145(TPH2 NΔ145/CΔ24) BH4 aroH fbr 和 trpE fbr整合至基因组
低拷贝质粒表达TPH145降低
aroH fbr启动子强度1.61 g·L−1 [36] S. cerevisiae 原核:XcP4H
真核:T5H或T3HBH4或MH4 — 0.337±0.187 μg·L−1 [37] -
在微生物内合成5-HTP需要芳香族氨基酸羟化酶、辅酶因子合成系统和辅酶因子再生系统3个模块。前期研究表明对TPH进行突变、截短或融合标签可提高该酶在宿主内羟化活性,将BH4合成系统和再生系统导入宿主细胞与TPH共表达,可成功构建色氨酸羟化途径[34- 35]。部分细菌PAH可利用MH4代替BH4作为辅酶进行羟化反应,提高PAH色氨酸羟化活性、表达MH4循环系统,亦可构建色氨酸羟化途径[24-25]。经代谢工程调控增加L-Trp在宿主内的积累量可进一步提高5-HTP的产率。目前,微生物发酵生产5-HTP最高产量为5.1 g·L−1,但仍无法大规模商业化生产,今后的研究重点可集中在以下几个方面:(1)5-HTP合成与宿主细胞生长相关,高强度表达将影响细胞生长,无法高效合成5-HTP[36]。因此,代谢途径的优化与平衡更为重要,可集中研究3个模块和L-Trp代谢工程调控中不同表达强度对5-HTP合成的影响,提高5-HTP合成率,降低L-Trp在宿主细胞内的残留量;(2)需进一步优化培养基组成以及发酵过程中温度、pH、溶氧等参数对宿主细胞内L-Trp转化率和5-HTP积累量的影响,确定最优发酵工艺条件;(3)仍需研究5-HTP分离纯化技术,探索发酵液固液分离和脱色等工艺流程,结合纳滤膜和微滤膜等新兴技术提高回收率、获得高纯度产品,实现微生物合成5-HTP商业化生产。在生物活性方面,已有研究表明过量摄入5-HTP将产生一定副作用,仍需深入探索药理毒理机制,为临床使用5-HTP提供数据支持。此外,酿酒酵母被认为是安全无毒的模式生物[38],在酿酒酵母内合成5-HTP研究较少,可通过基因组挖掘进一步研究不同芳香族氨基酸羟化酶在酿酒酵母内的高活性表达以及两种辅酶因子合成与再生途径的构建,同时需改造酿酒酵母内L-Trp的代谢通量和协调代谢平衡,为研究酿酒酵母合成5-HTP提供新的技术支持。
Advances in microbial synthesis of 5-hydroxytryptophan
doi: 10.15886/j.cnki.rdswxb.2023.01.016
- Received Date: 2022-09-07
- Accepted Date: 2022-10-22
- Rev Recd Date: 2022-10-17
- Available Online: 2023-01-01
- Publish Date: 2023-01-25
-
Key words:
- 5-Hydroxytryptophan /
- biosynthesis /
- tetrahydrobiopterin /
- tryptophan hydroxylase
Abstract: 5-Hydroxytryptophan (5-HTP) is an intermediate metabolite in the biosynthetic pathway of serotonin and melatonin, which can effectively treat a variety of diseases, such as depression, headache, obesity and insomnia. Traditional methods to produce 5-HTP are natural extraction or chemical synthesis. However, these methods do not meet the growing needs of market due to inefficiency and inability to mass production. With the development of metabolic engineering and synthetic biology, it is an inevitable trend to use microorganisms to synthesize 5-HTP. A comprehensive review is made of the advances in microbial synthesis of 5-HTP. Microbial synthesis of 5-HTP is achieved through directed evolution of hydroxylase, the construction of coenzyme factor biosynthesis and regeneration pathways, and further metabolic engineering efforts are made to balance metabolic flow in the host cell to increase the production of 5-HTP. This review might provide a technical support for industrial biosynthesis of 5-HTP which is of guiding significance for large-scale industrial production of 5-HTP.
| Citation: | WENG Kexin, ZHANG Mingliang, LI Li. Advances in microbial synthesis of 5-hydroxytryptophan[J]. Journal of Tropical Biology, 2023, 14(1): 42-49. doi: 10.15886/j.cnki.rdswxb.2023.01.016 |







 DownLoad:
DownLoad:

