-
芋螺(Cone snail)是一种肉食性海洋软体动物[1],其分泌的芋螺毒液包含许多不同化合物的混合物,包括小分子、肽和酶。其中,含有很多种不同成分的神经活性毒素肽−芋螺毒素(Conotoxin, CTx)具有广泛的多样性,这些具有高活性和靶点选择性的神经小肽大多由6~50个氨基酸残基构成,富含二硫键,以特定的离子通道和受体为靶标,作为药理学工具和探针,具有较好的开发前景[2-4]。α−芋螺毒素 (α-conotoxin, α-CTx) 是目前研究最广泛和深入的CTxs。α-CTx分子质量较小,由12~20个氨基酸残基连接构成,是各种乙酰胆碱受体(nicotinic acetylcholine receptor,nAChR)亚型的选择性拮抗剂[5-6]。α-CTx一般含有4个半胱氨酸(Cys),根据二硫键的不同连接方式,可组成3种异构体,其中,大部分α-CTxs的活性形式为球型。另外,根据α-CTx半胱氨酸间氨基酸数量的不同将α-CTx分为:α-3/5、α-4/4、α-4/6、α-4/7等多种亚家族。α-CTx不仅作为分子探针,用以鉴别不同亚型的nAChR,在治疗与nAChR相关疾病时有着至关重要的作用[7-9],并且在成瘾、镇痛等神经生理、药理研究方面也扮演重要角色[10]。
罗素兰研究团队从疣缟芋螺中分离得到了一种 α-4/7型CTx LvIA,它由15个氨基酸残基组成,能够作用于nAChRs,对α3β2 nAChR具有较好的的活性(IC50为8.7 nmol·L−1),但是LvIA对α6/α3β2β3,α3β4和α6/α3β4 3种nAChRs亚型保持一定的高亲和力[11-13]。为了提高LvIA的选择专一性,前期实验室基于LvIA进行了设计和改造,获得了对应的共晶结构,同时也利用受体突变技术解析了它与α3β2 nAChR相互作用的分子机制[14-15]。研究发现,当第11位天冬氨酸(Asp, D)突变成丙氨酸(Ala, A)后对α3β2 nAChR的活性保持不变,共晶实验表明,第11位氨基酸影响着LvIA与α3β2 nAChR的结合作用[16-19]。第11位是否是LvIA的关键氨基酸位点,今后能否基于该位点对LvIA进行改造,有待深入研究。
本研究基于LvIA前期研究发现的关键氨基酸信息[20-22],主要是针对第11位氨基酸,对LvIA进行进一步设计改造,分别用精氨酸(Arg, R)和组氨酸(His, H)对Asp进行取代,设计了2种新型突变体,考察第11位氨基酸电性和侧链基团对LvIA活性的影响,旨在为LvIA的设计和开发提供依据。
-
α-CTx LvIA及其所有突变体线性肽粗品(吉尔生化上海有限公司),色谱级三氟乙酸(Trifluoroacetic acid, TFA)(上海阿拉丁生化科技股份有限公司),色谱级乙腈(Acetonitrile, ACN)(ThermoFisher Scientific公司),N,N−二甲基甲酰胺(DMF)、碘(I2)、铁氰化钾(K3[Fe(CN)6])、甲酸等分析纯试剂(广州化学试剂厂),质粒提取试剂盒(Omega),胶原酶(Collagenase)、乙酰胆碱(ACh)、阿托品(Atropine)(Sigma公司),DNA纯化试剂盒(TaKaRa大连有限公司),体外转录试剂盒(Fisher Scientific公司),胶原蛋白酶 A(美国 Sigma-Aldrich 公司),圆二色谱检测涉及的试剂由华中科技大学分析测试中心提供;Waters 2695制备型高效液相色谱仪,Waters e2695分析型高效液相色谱仪(美国Waters公司),岛津电喷雾电离质谱(Electrospray ionization-mass spectrometer,ESI-MS)(美国格雷斯公司),双电极电压钳系统Axon 900A 和Digidata 1550B数模转换器(美国Molecular Device公司),ELGA超纯水系统(英国ELGA公司);分光光度计(Smart SpecTM plus)(美国Bio-RAD公司),AlphaImager HP凝胶成像系统(美国Protein Simple公司);实验用非洲爪蟾(美国Nasco公司)。
-
设计2种LvIA第11位突变体序列送至上海吉尔生化有限公司,使用固相合成法(Fmoc法)进行合成(纯度为80%~85%)。然后使用制备型RP-HPLC对合成好的线性肽进行纯化,使产物的纯度达到95%以上。纯化得到的线形肽采用两步法氧化的方法进行氧化折叠,保证形成I-III, II-IV的二硫键连接结构。第1步氧化,将纯化后的线性肽冻干粉溶于B60(60%乙腈),缓慢地滴加到铁氰化钾反应液(K3[Fe(CN)6] 10 mmol·L−1, Tris 0.1 mol·L−1, pH7.5)中,室温充分反应45 min,促使其形成第1对二硫键(Cys-2和Cys-8),线性肽浓度不高于50 g·L−1。利用RP-HPLC纯化反应后得到单环产物。将第1步纯化后的多肽进行第2步氧化。在氮气保护的条件下,逐滴加入到碘(I2)反应液中(7.5 mmol·L−1 I2溶液;V水∶VTFA∶VACN= 73∶3∶24),反应时间8~12 min, 促使其连接Cys-3和Cys-16形成第 2 对二硫键,反应完毕滴加VC饱和溶液至溶液颜色从紫黑色变成无色,终止I2氧化反应,至此,得到包含2对二硫键的 LvIA突变体的最终产物。使用RP-HPLC对氧化折叠产物进行纯化(溶剂 A 为 0.075% TFA水溶液,溶剂 B为0.05% TFA、90% ACN, 梯度洗脱条件:5%~35%),使其纯度大于95%,纯化得到的氧化折叠产物利用ESI-MS鉴定其分子质量,确定LvIA及其突变体是否形成正确的二硫键连接方式。冻干保存,用于后续电生理活性实验。
-
从载入了含(rat,r)α3、β2 2种亚基基因质粒的DH5α感受态细胞中提质粒,将获取的质粒酶切成线性质粒,然后纯化此线性化DNA模板,利用0.8%的琼脂糖凝胶电泳检验纯度,并使用Nanodrop 测定浓度。使用T7 RNA polymerase体外转录试剂盒将DNA模板转录,转录后的RNA使用纯化试剂盒进行纯化,纯化的产物cRNA,再使用分光光度计测定浓度,并使用0.8%的琼脂糖凝胶进行纯度鉴定。选取1只腹部没有斑点的非洲爪蟾,冰冻1 h麻醉,使用手术刀将爪蟾的腹部下侧切开,用剪刀将肌肉剪开,取2片蛙卵置于OR-2溶液中,再用胰蛋白酶酶解成单个蛙卵,至大部分卵母细胞表面无膜,挑选其中个体均匀,无血丝,黑白分明的爪蟾卵母细胞。按照1∶1的比例混合成所需的亚型,保证每个细胞的注射量为50.6 nL,注射后的蛙卵置于含抗生素的ND96培养液中(96 mmol·L−1 NaCl,2 mmol·L−1 KCl,1.8 mmol·L−1 CaCl2,1 mmol·L−1 MgCl2,5 mmol·L−1 HEPES,pH7.1~7.7;抗生素:青霉素、10 mg·L−1链霉素和100 mg·L−1庆大霉素),17 ℃培养2~3 d,表达α3β2受体亚型。
-
将表达了特定受体的卵母细胞放置于细胞槽内,将双电极电压钳装好holder,再把灌注约1/2 3 mol·L−1 KCl溶液的电极针安装到holder上,将电极针浸入液面,调零,使其钳制电压为70 V, 基准电流低于100 nA,将电压膜片钳打开到ON的模式,钳住细胞,调节程序,每个Sweep(1 min)的第2秒的时候灌流系统给予蛙卵细胞100 μmol·L−1浓度的ACh刺激,其余时刻持续使用ND96溶液灌流。使用双电极电压钳系统检测和记录蛙卵细胞的电流,待电流大小稳定以后,逐次加入经过梯度稀释的待测定活性的LvIA突变体,孵育时间为5 min,再一次开启程序,比较蛙卵细胞加药前后2次产生电流的的大小差异,计算反应率(Response)。通过非线性拟合方程:Response=100/[1+([toxin]/IC50)nH]×100% 进行拟合分析,获取药物作用的剂量反应曲线(IC50值),其中,nH代表Hill系数。
-
纯化线性肽,使其纯度高于95%,用于后续的氧化折叠反应,连接2对二硫键,将所得产物通过HPLC和MS鉴定,具体结果见图1-A、B。[D11H]LvIA在乙腈浓度23%时洗脱,洗脱时间为14.8 min;而[D11R]LvIA在乙腈浓度为25%时洗脱,洗脱时间是14.9 min。通过质谱确定了[D11H]LvIA和[D11R]LvIA的相对分子质量,分别为1 702.95 和1 721.99 Du,与理论值一致,且均比其线性肽的相对分子量少了4 Du,说明成功连接了2对二硫键并且末端酰胺化,见图1-C、图1-D。
-
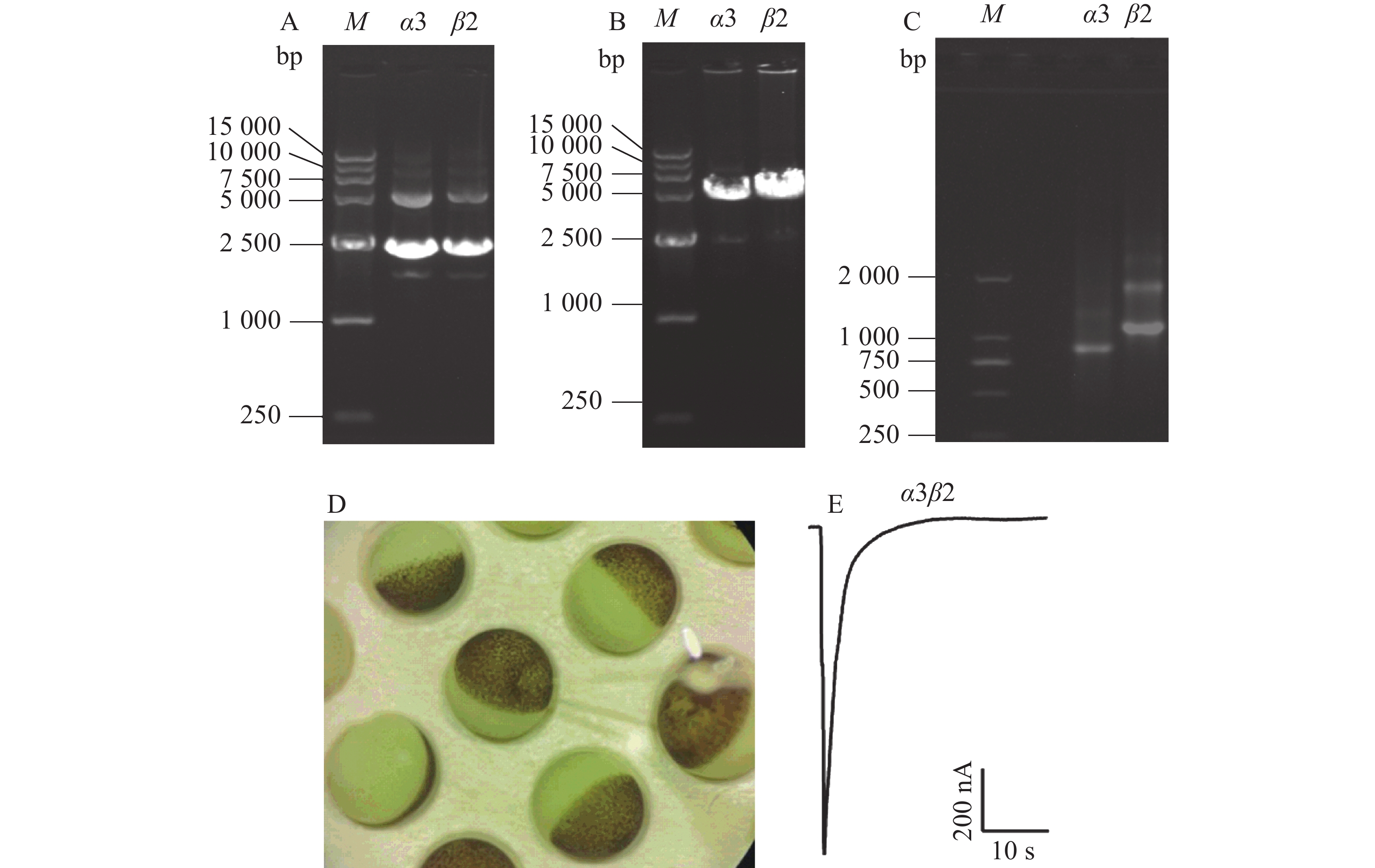
提取包含鼠源α3、β2这2种亚基的质粒,使用0.8%的琼脂糖凝胶电泳进行纯度检测。从图2-A可知,质粒条带清晰,未发生拖尾情况,超螺旋结构大小在2 500 bp左右,符合理论值大小。从图2-B可知,条带清晰可见,无弥散现象,超螺旋结构的大小在5 000~7 500 bp范围内。使用紫外分光光度计测定线性质粒的质量浓度,质量浓度皆大于0.2 g·L−1,可以保证后续试验的浓度要求。制备α3、β2这2种亚基的cRNA,所得RNA的凝胶电泳结果如图2-C,图中有1条明显的亮带,大小在750~1 000 bp之间,符合理论值,α3亚基的浓度为0.4 g·L−1,β2亚基的浓度为0.49 g·L−1。将2种亚基按照1∶1的比例混合,显微注射到酶解好的爪蟾卵母细胞中(图2-D),表达2~3 d,使用激动剂ACh刺激,可以在细胞膜上检测到内向电流,电流大小在200~2 000 nA范围内,如图2-E。
-
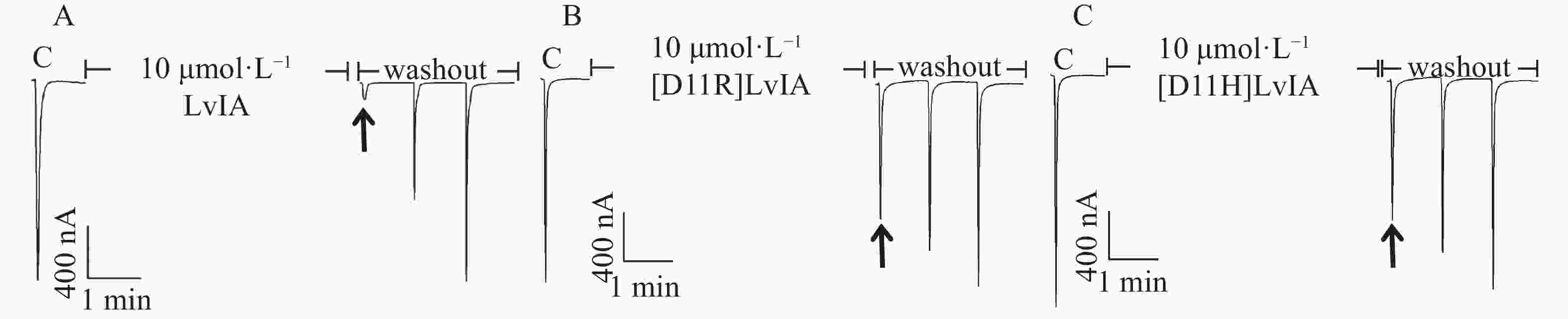
分别将LvIA和2种11位的LvIA突变体在表达α3β2 nAChR的非洲爪蟾卵母细胞上进行电生理活性检测。在终浓度为10 μmol·L−1的孵育条件下,LvIA、 [D11R]LvIA和[D11H]LvIA对α3β2nAChR的阻断率分别为(95.8±2.8)%(n=6)、(9.8±2.5)%(n=7)和(12.4±3.1)%(n=5)(图3),与野生型LvIA相比,2种11位突变体对α3β2 nAChR活性几乎完全丧失。
为了进一步确定2种LvIA 的11位突变体对于α3β2 nAChR的阻断活性,笔者做了剂量反应曲线(图4),分别测定了[D11R]LvIA和[D11H]LvIA对α3β2 nAChR的IC50值,2种突变体对于α3β2 nAChR的IC50值分别为8 976 nmol·L−1和6 390 nmol·L−1(表1),活性与比本体的活性相比,分别降低了574.38%和408.62%。证明当第11位的Asp变化后,LvIA的活性大大降低。
-
α3β2 nAChR 是一种十分重要的受体,与疼痛、成瘾等生理学作用密切相关。LvIA是一种十分重要的α-CTx,作用于α3β2 nAChR,是从疣缟芋螺中发现并且克隆得到的,对LvIA构效关系的研究将有益于获得靶向α3β2 nAChR的相关分子探针,有助于对与α3β2 nAChR相关的药理学研究。目前,发现能够作用于α3β2 nAChR的α-CTx的有很多,其中,GIC、MII、OmIA和PeIA等具有较强的靶向作用,针对这些芋螺毒素的突变改造对于本实验具有借鉴作用[24-23]。如:在对α-CTx MII的研究中发现,MII第11位的谷氨酸(Glu)与α3-β2亚基上带正电荷的残基如:(+) K153和(-)K163形成相互作用,促进MII与α3β2 nAChR的结合,11位对保证MII的活性至关重要[25]。利用共结晶技术对α-CTx GIC的研究发现其第7位的Ala、11位的Asn和12位的Asn对于GIC与受体的结合具有十分重要的作用,特别是11位的Asn可以通过形成氢键与Ac-AChBP相互作用,如果改变11位的氨基酸,会影响GIC与受体的结合,11位氨基酸对于α-4/7型CTxs与受体的结合起到至关重要的作用[26];在PeIA的相关研究中,也发现11位氨基酸分别突变为Arg和Lys之后,对α3β2 nAChRs的IC50分别变为30.9 µmol·L−1和45.4 µmol·L−1,基本丧失了活性[27],上述结果都证明了11位氨基酸对于α-4/7型CTx活性影响显著。
根据前期研究,发现β2亚基上的3个位点:T59K、V111I、F119Q对α-CTx LvIA的敏感性有显著影响,受体突变加电生理结果表明受体上59、111和119的3个位置,是与LvIA结合的关键位点。罗素兰研究团队对LvIA前期研究中,获得了LvIA与Ac-AChBP的共晶结构。借助共晶结构和分子动力学模拟发现α3亚基中的Lys-155可以与LvIA中带负电荷的Asp-11形成盐桥,增加相互作用,说明第11位的Asp对于LvIA与α3β2 nAChRs的相互作用影响较大,但是将第11位突变成Ala之后活性保持不变,所以本研究希望在前期基础上进一步深入探究第11位如何影响LvIA对于α3β2 nAChRs的活性,针对11位所设计的两个突变体[D11R]LvIA和[D11H]LvIA,当第11位氨基酸突变成碱性氨基酸Arg和His之后,2个突变体的活性都极大的降低。结合前期共晶结构和分子动力学模拟分析,当11位突变为碱性氨基酸后,与α3亚基中的Lys-155都为碱性氨基酸,同性相斥,结合力减弱,造成了LvIA活性的降低。该研究探究了11位的重要作用,为今后基于LvIA的分子设计和改造提供了基础。
Synthesis of new analogs of α-conotoxin LvIA mutated at the position 11 residue and their target activity
doi: 10.15886/j.cnki.rdswxb.2023.01.004
- Received Date: 2022-06-02
- Accepted Date: 2022-03-25
- Rev Recd Date: 2022-06-28
- Available Online: 2022-04-08
- Publish Date: 2023-01-25
-
Key words:
- α-conotoxin LvIA /
- mutant design and synthesis /
- α3β2 nicotinic acetylcholine receptor /
- target binding activity /
- electrophysiological technique.
Abstract: α-snail toxin LvIA is a small peptide targeting α3β2 acetylcholine receptor (nAChR), which was found in the South China Sea snail. The structure and function of LvIA were previously analyzed in our laboratory. After alanine scanning mutation it was found that the mutant [D11A] LvIA, which was replaced by alanine (A) with aspartic acid at the site 11 of LvIA, maintained its activity against α3β2 nAChR. In order to further explore the effect of the properties of amino acid at the position 11 on the binding activity of LvIA target, two new mutants of LvIA [D11R] LvIA and [D11H] LvIA were designed, namely, two basic amino acids - arginine (R) and histidine (H) were used to replace the original acid amino acid D, respectively. The linear peptides of the two new mutants were synthesized and then folded by a two-step oxidation method to obtain a disulfide bond between the first and third cysteines (Cys 1-3) and the second and fourth cysteines (Cys 2-4). The peptides containing Cys (1-3, 2-4) disulfide bond were successfully synthesized by high performance liquid chromatography (HPLC) and mass spectrometry. The synthesized peptides were correct in molecular weight and their purity was above 95%. The binding activity of these two mutants to α3β2 nAChR was detected by two-electrode voltage clamp electrophysiology. The results showed that when the amino acid properties of the site were changed from acidic to alkaline, LvIA activity was greatly affected, resulting in direct loss of α3β2 nAChR blocking activity. The activity of [D11R] LvIA and [D11H] LvIA was 574.38% and 408.62% lower than that of wild-type LvIA, respectively. This suggests that the acidity and basicity of the 11th amino acid is crucial to the activity of LvIA. These results provide some reference for the optimization design and modification of LvIA in the future, based on which it is expected to obtain more specific peptide tools targeting α3β2 nAChR, which can provide a better pharmacological molecular probe for the study of the structure, function and distribution of an α3β2 nAChR receptor.
| Citation: | LI Haonan, YANG Yishuai, ZHANG SUN Dongting, ZHU Xiaopeng, LUO Sulan. Synthesis of new analogs of α-conotoxin LvIA mutated at the position 11 residue and their target activity[J]. Journal of Tropical Biology, 2023, 14(1): 1-7. doi: 10.15886/j.cnki.rdswxb.2023.01.004 |







 DownLoad:
DownLoad: