-
肝簇虫是顶复门(Apicomplexa)真球目(Encoudida)肝簇虫属(Hepatozoon spp.)的原虫,属于血液寄生虫的8个属之一。目前所发现的肝簇虫属已经达到300多种,其中至少有46种肝簇虫属能感染哺乳动物[1]。肝簇虫可以通过不同种类的节肢动物进行传播,比如蜱(Ixodida),虱(Phthiraptera),蚊(Culicidae),螨(Acariformes and Parasitiformes),白蛉(Phlebotomidae),采采蝇(Glossina),锥蝽(Triatominae),水蛭(Whitmania pigra Whitman)[1-3]。
目前已知能感染犬和其他野生犬科动物的肝簇虫有2种,分别是犬肝簇虫(Hepatozoon canis)[4]和美洲肝簇虫(Hepatozoon americium)[5]。2种肝簇虫都是通过犬和其他野生犬科动物吞食含有成熟卵囊的蜱虫而进行传播的[6-7]。也有包括犬肝簇虫通过胎盘屏障感染胎儿,或新宿主猎食了作为美洲肝簇虫的原宿主等其他途径感染的报道[8]。血红扇头蜱(Rhipicephalus sanguineus),一种以犬为主要宿主,以多种哺乳动物血液为食的棕色犬蜱,在世界范围内广泛分布[9-10],已被证明是犬肝簇虫的主要传播媒介[11]。最近,也有实验研究证实,对犬肝簇虫也有传播作用的还有在意大利南部发现的图兰扇头蜱(Rhipicephalus turanicus) [12];其他蜱种如巴西的微小扇头蜱(牛蜱)[Rhipicephalus (Boophilus) microplus][13]、卵圆花蜱(Amblyomma ovale)[14]、日本的长角血蜱(Haemaphysalis longicornis)和褐黄血蜱(Haemaphysalis flava)[15]、意大利的蓖籽硬蜱(Ixodes ricinus)[16]、匈牙利的嗜群血蜱(Haemaphysalis concinna)[17]等也可能是犬肝簇虫的潜在载体。
犬肝簇虫可广泛感染世界各地的肉食性宿主,如狗、豺、狐狸、负鼠和家猫[16, 18-22]。家养犬对犬肝簇虫的感染表现出良好的适应性,大多数犬在感染后的疾病状态处于亚临床到轻度之间,而犬被美洲肝簇虫感染后往往是致命的[5, 23]。虽然美洲肝簇虫和犬肝簇虫在犬科动物感染中都有报道,但犬肝簇虫的传播更广泛,也是犬肝簇虫病的主要原因[9]。
犬肝簇虫的诊断常用镜检方法,即在染色的血涂片中检测出犬肝簇虫的配子体[24]。酶联免疫吸附试验和间接荧光抗体试验等工具也被用于犬肝簇虫抗体的血清学诊断[25]。还可以通过PCR技术检测犬体内的犬肝簇虫,这是检测犬感染犬肝簇虫最准确的诊断方法[7, 26]。
犬肝簇虫广泛分布,欧洲[16, 19]、非洲[27-28]、美洲[29]、亚洲[4, 30]、澳洲[31]等地都有犬肝簇虫的报道;在中国的邻国也有发现,如柬埔寨[32],印度[33],日本[34],韩国[35],巴基斯坦[36],泰国[37],马来西亚半岛[38]等,而美洲肝簇虫只在北美地区有报道[5, 39]。目前我国犬只数量估计在1.5亿到2亿[40]之间。国内对犬肝簇虫的研究却非常有限,仅有北京、河南、江苏、陕西、新疆[41]、广西[42]、广东[43]、台湾[44]等地区有关于犬肝簇虫的报道。
在过去的几年里,世界范围内影响家畜和野生动物的肝簇虫流行率有所增加,因此,对肝簇虫的研究在兽医领域具有重要意义。本研究旨在通过PCR和测序方法检测出海南岛犬体内携带肝簇虫的情况,可为海南岛犬蜱传疾病的流行和分布格局提供信息。
-
2017年10月至2022年5月,在海南岛18个市县共采集了1039份犬血,血液保存于含有EDTA的EP管中,放入−80℃冻存。并对每只犬的信息建立档案记录。
-
Ezup柱式血液基因组DNA提取试剂盒、琼脂糖、DL 2000 DNA Marker均购自生工生物工程(上海)股份有限公司;Goldview染料,购自苏州金唯智生物科技有限公司;2 X Taq Plus Master Mix II(Dye Plus),购自南京诺维赞生物科技股份有限公司。
-
PCR仪,TProfessional,德国Biometra公司产品;小型台式高速冷冻离心机,5415R,Eppendorf 公司产品;琼脂糖水平电泳仪,Mini-PROTEAN Tetra,北京六一仪器厂产品;凝胶成像分析系统,GelDoc XR+,美国Bio-Rad公司产品。
-
取出100 μL上述采集到的血液,分别加入到1.5 mL EP管中,参照DNA提取试剂盒说明书步骤提取犬血中DNA,并于−20 ℃保存。
-
本实验采用的引物序列参照文献[45],上游引物为HEP-F:5′-ATACATGAGCAAAATCTCAAC-3′;下游引物为HEP-R:5′-CTTATTATTCCATGCTGCAG-3′。PCR具体反应体系:2 X Taq PLus Master Mix II 12.5 μL,特异性引物F和特异性引物R(10−8 moL·L−1)各 0.5 μL,血液基因组DNA(约20 ng) 1 μL。PCR反应程序:95 ℃,预变性5 min;95 ℃,变性时间60 s;58 ℃,退火时间60 s;72 ℃,延伸时间60 s;共35个循环;最后72 ℃延伸5 min,4 ℃保存。反应结束后,取5 μL PCR产物加入1.5%琼脂糖凝胶加样孔中进行电泳,然后在凝胶成像分析系统下观察结果是否出现目的条带。将出现目的条带所对应的阳性产物送往生工生物工程(上海)股份有限公司进行测序。
-
使用NCBI中BLAST在线比对工具将肝簇虫的测序结果进行比对,选出代表性的犬肝簇虫序列与GenBank中已知序列进行比对,利用软件进行同源性分析(DNAMAN 8.0),使用Mega XI软件,选择Neighbor-joining(NJ)算法中的Kimura-2-parameter参数模型,Bootstrop值选择1000进行支序图构建,以刚地弓形虫(Toxoplasma gondii)的18S rRNA序列(登录号L37415)为外群。
-
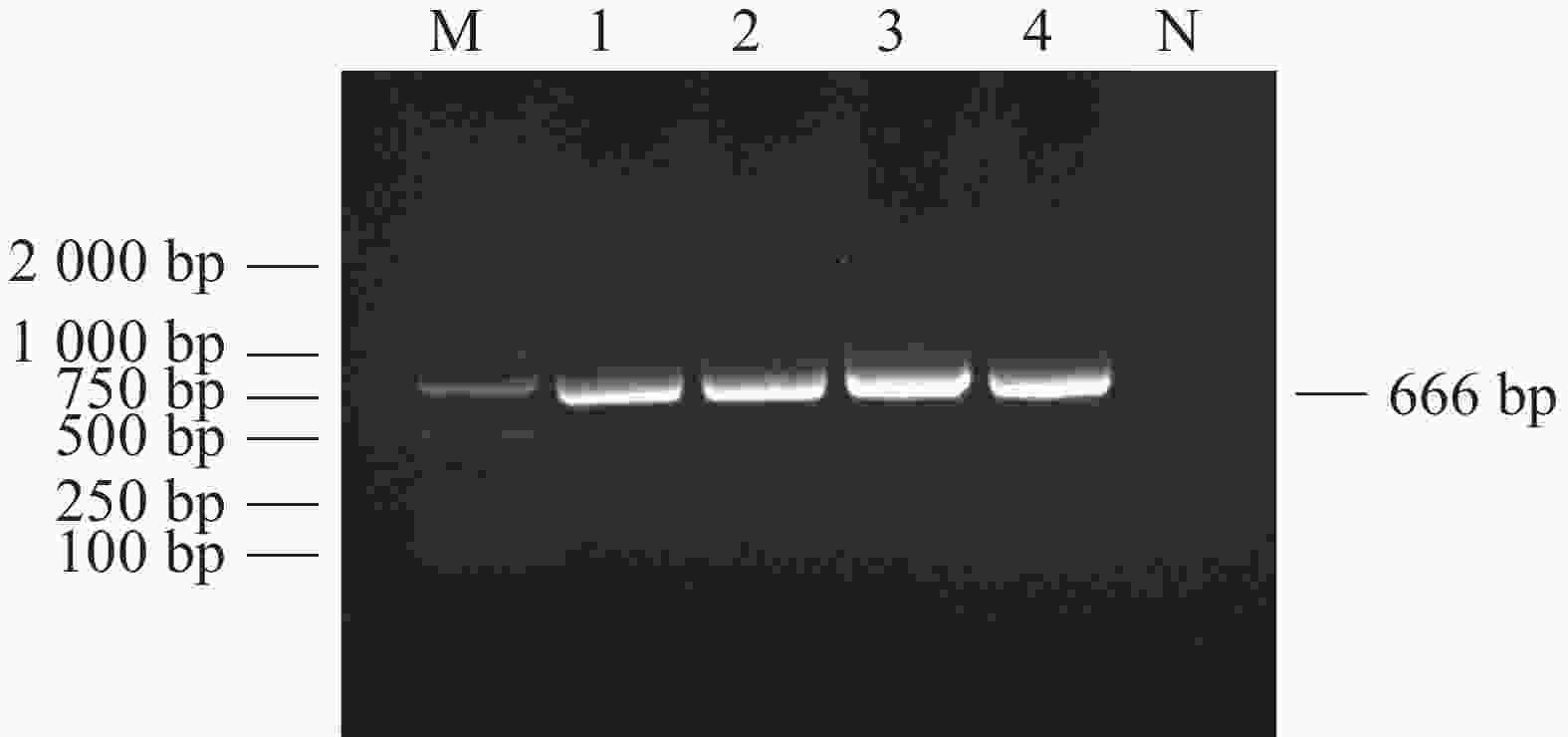
对采集到的1 039份犬全血DNA进行犬肝簇虫18S rRNA基因扩增,电泳结果显示扩增产物在666 bp处出现目的片段(图1),与犬肝簇虫18S rRNA基因预期片段大小相符。扩增序列测序拼接后经BLAST比对与GenBank上已有的犬肝簇虫序列AF176835同源性为99.85%。
-
在海南岛1 039份犬血中发现犬肝簇虫阳性血液135份,感染率为13.0%。海南岛18个市县犬肝簇虫感染率见表1。海口(20.7%)、三亚(20.6%)、屯昌(33.3%)等地犬肝簇虫感染率较高。文昌市(8.7%)、定安县(5.6%)、澄迈县(6.3%)、儋州市(5.0%)、琼中县(15.8%)、乐东县(18.8%)、昌江县(6.7%)等地均发现有犬感染犬肝簇虫。
测序方法 市/县 犬肝簇虫
样品数量/阳性数量
(感染率/%)18S rRNA 海口市 435/90(20.7) 18S rRNA 文昌市 46/4(8.7) 18S rRNA 琼海市 34/0(0) 18S rRNA 定安县 232/13(5.6) 18S rRNA 澄迈县 16/1(6.3) 18S rRNA 临高县 12/0(0) 18S rRNA 儋州市 60/3(5.0) 18S rRNA 琼中黎族苗族自治县 19/3(15.8) 18S rRNA 保亭黎族苗族自治县 24/0(0) 18S rRNA 万宁市 18/0(0) 18S rRNA 五指山市 12/0(0) 18S rRNA 三亚市 34/7(20.6) 18S rRNA 乐东黎族自治县 16/3(18.8) 18S rRNA 陵水黎族自治县 4/0(0) 18S rRNA 白沙黎族自治县 11/0(0) 18S rRNA 昌江黎族自治县 15/1(6.7) 18S rRNA 东方市 21/0(0) 18S rRNA 屯昌县 30/10(33.3) 18S rRNA 总数 1039/135(13.0) -
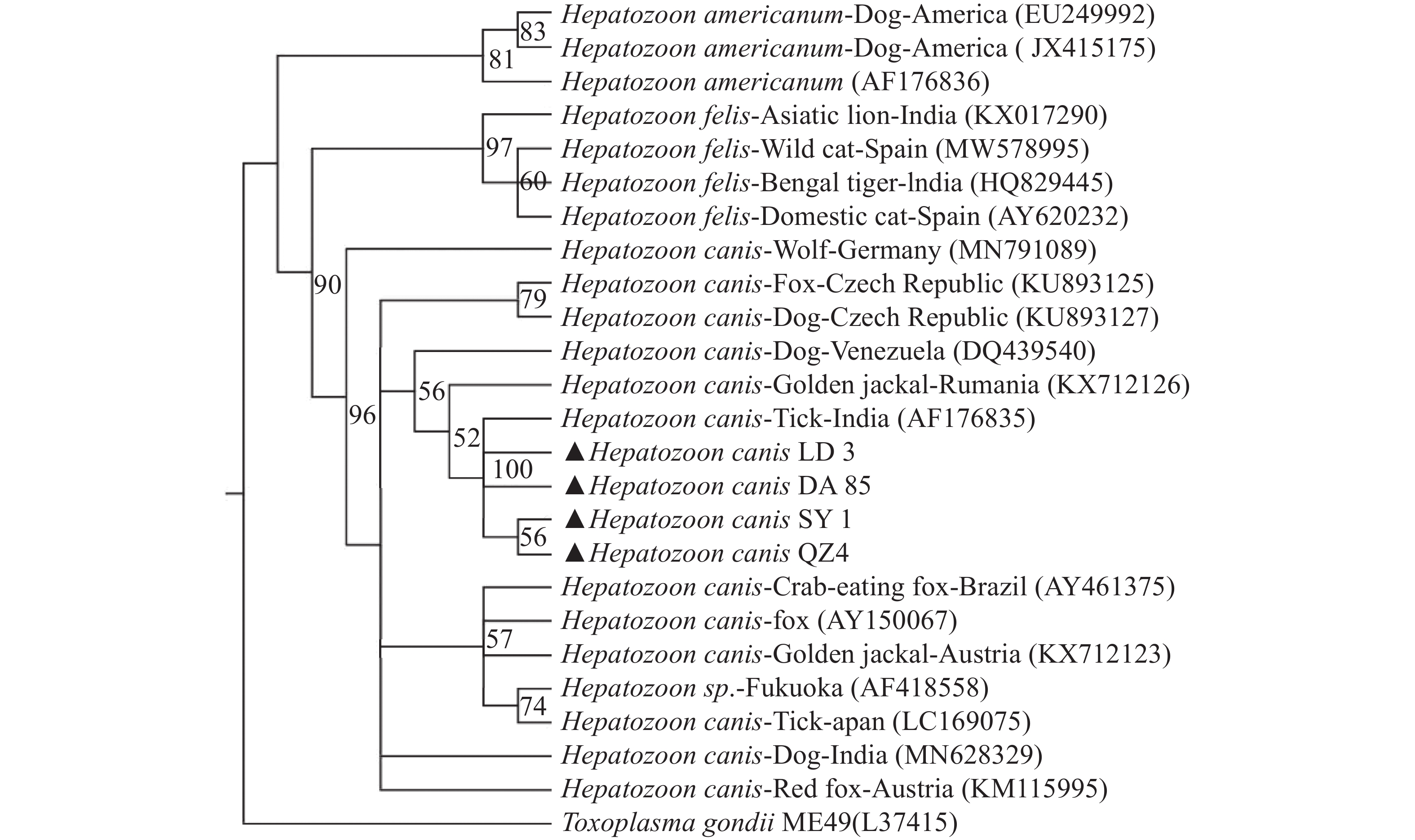
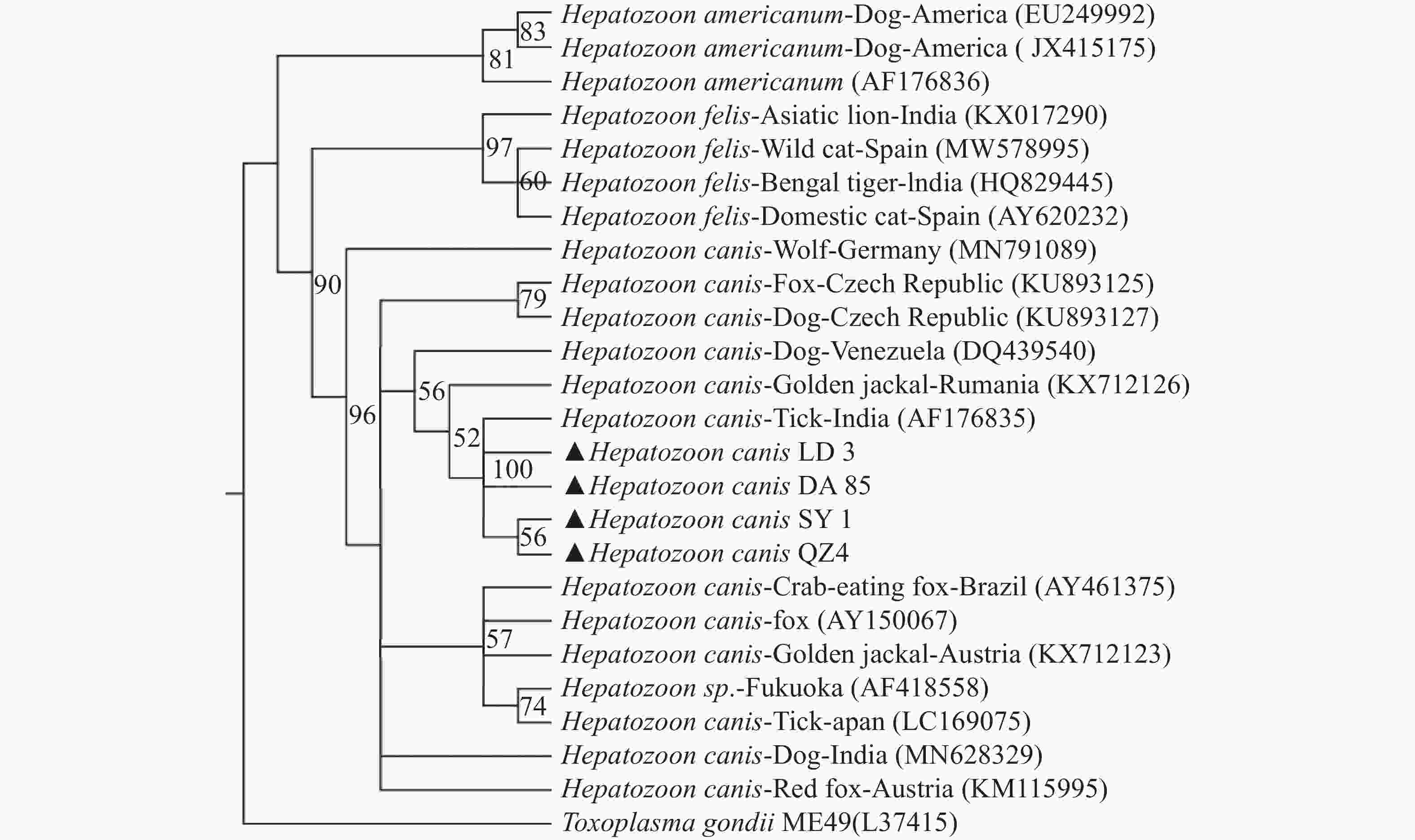
BLAST分析表明,海南的犬肝簇虫的同源性为99.4%~100%,用MEGA-X软件选择本实验中获得的代表性犬肝簇虫18S rRNA基因序列构建支序图(图2)(三亚(OP699205)、乐东(OP699206)、定安(OP699207)、琼中(OP699208)的阳性样品)。从图2中发现海南的犬肝簇虫(Hepatozoon canis LD3、Hepatozoon canis DA85、Hepatozoon canis SY1、Hepatozoon canis QZ4)与印度的犬肝簇虫(AF176835)均位于bootstrap支持度100的同一分支中,并在bootstrap支持度90的情况下与该属其他分支分离[猫肝簇虫(Hepatozoon felis ),美洲肝簇虫( Hepatozoon americium )]
-
犬肝簇虫是蜱传病原虫中分布最广的一种,主要由血红扇头蜱[46]传播。本研究采集的样本来自于海南岛18个市县,海南岛属于热带季风气候,全年温度和湿度相对较高,年平均温度在22~27 ℃。海南岛气候适合热带物种血红扇头蜱的发展和生存。目前针对犬肝簇虫对不同宿主或宿主特异性的情况尚未有较多的研究[6, 11]。因此,无法消除犬肝簇虫通过其他蜱虫物种传播的可能性[19]。
自1905年犬肝簇虫首次被提出以来[4],犬肝簇虫已在美洲、欧洲、亚洲、非洲和澳大利亚的几个国家被报道。大多数的报道通常是通过组织病理学或显微镜诊断[35]的方法来确定肝簇虫的感染,缺乏对其种群的遗传结构和基因变化的理解。本实验采用PCR技术检测犬体内的犬肝簇虫,并通过测序和建立支序图的方式对该血液寄生虫的物种进行鉴定[47],使调查结果的准确性更高。
本研究使用了一套肝簇虫属的特异性引物对海南岛1039份犬血样进行检测,琼脂糖凝胶电泳结果显示,其中135份样品(13 .0%)为阳性。通过对获得的阳性扩增子进行测序分析来确定肝簇虫的种类。
本研究结果支持并证实了海南犬体内存在犬肝簇虫,也是海南岛犬肝簇虫的首次分子生物学报道。据报道,犬肝簇虫在非洲国家流行率如苏丹42.3%[48]、尼日利亚41.4%[49]和赞比亚13%[50]。犬肝簇虫在欧洲国家流行率如法国1%[51]、西班牙3%[52] 捷克50%[53]、意大利南部50.6%[7]。犬肝簇虫在北美洲流行率如古巴47.5%[54]、美国犬肝簇虫与美洲肝簇虫同时出现,感染率为2.5%[55]。犬肝簇虫在南美洲流行率如巴西(53.3%)[56],犬肝簇虫在亚洲流行率如印度(30%)[33]、日本(42.9%)[34]。
文献报道我国犬肝簇虫感染率为河南登封市4.08%[43],河南郑州4.9%,北京4.5%,江苏南京2.3%,陕西杨凌8.9%,新疆乌鲁木齐1.2%[41],中国农业大学动物医院有确诊一例犬肝簇虫阳性病例[57],广州也有疑似犬肝簇虫的病例报道[58]。
海南岛犬肝簇虫的感染率为13.0%,与其他省市感染率相比偏高,比国外其他国家相比感染率偏低,这些犬肝簇虫感染率的差异可能是由多种因素造成的,如目标犬的种群特征、采样季节、社会因素(犬类饲养、使用药物杀蜱)、地理位置和气候条件等,这些因素最终会影响病媒蜱物种的数量和分布[59]。因犬肝簇虫的传播需要通过吞食含有成熟卵囊的蜱虫进行,使得犬肝簇虫病的感染率不高。但目前没有可用的犬肝簇虫疫苗,使得犬肝簇虫病的治疗比较困难。防治这种疾病的最佳方法是采取适当的蜱虫控制策略来预防感染。
支序图分析结果表明,来自海南岛的犬肝簇虫与其他地区的犬肝簇虫属于同一支系,与海南样品同源性最接近的序列是来自印度的犬肝簇虫(AF176835)。这一发现可能表明,该病原体可能是由于旅游业、自然灾害和社会因素等,人与宠物的流动性日益增加的原因而带入海南。
此外,本研究中犬肝簇虫基因型与猫肝簇虫、美洲肝簇虫分类不同。由于该类群的成员已在全球广泛分布的犬科动物和家养犬中被报道,因此可能对进一步研究该类群的新类群划定提供一定的帮助。
Epidemiological investigation and genetic relationship of canine hepatozoonosis in Hainan Island
DOI: 10.15886/j.cnki.rdswxb.20220114
- Received Date: 2022-12-15
- Accepted Date: 2023-03-21
- Rev Recd Date: 2023-03-09
- Available Online: 2023-09-21
- Publish Date: 2023-09-25
-
Key words:
- Hepatozoon canis /
- PCR /
- infection rate /
- epidemiology /
- Hainan
Abstract: In order to understand the prevalence of Hepatozoon canis disease in Hainan in recent years, the prevention and control measures were evaluated for better control of Canine hepatozoonosis in Hainan. There were 1039 dog blood samples collected from 18 cities and counties in Hainan Province from October 2017 to May 2022, and they were detected for H. canis by PCR. Among the dog blood samples collected, 135 samples were found to be positive for H. canis, with an infection rate of 13.0%. Of the samples higher infection rates of H. canis were found in Haikou (20.7%), Sanya (20.6%), Tunchang (33.3%), and some infections of H. canis were also found in Wenchang (8.7%), Dingan (5.6%), Chengmai (6.3%), Danzhou (5.0%), Qiongzhong (15.8%), Ledong (18.8%) and Changjiang (6.7%). The positive samples from Sanya (OP699205), Ledong (OP699206), Dingan (OP699207) and Qiongzhong (OP699208) were selected to construct a phylogenetic tree based on the 18S rRNA of the H. canis. It was found that the homology of H. canis in Hainan was the closest to that of H. canis (AF176835) in India, which was 99.85%, and was also separated from that of the branches of H. felis and H. americanum. This shows that the dogs in Hainan Island has a high infection rate of H.canis. The prevention and treatment of H.canis requires regular elimination of the transmission vector Rhipicephalus sanguineus .
| Citation: | WANG Kaidong, WANG Jinhua, ZU Haiyue, LIN Yang, WANG Shiming. Epidemiological investigation and genetic relationship of canine hepatozoonosis in Hainan Island[J]. Journal of Tropical Biology, 2023, 14(5): 467-473. doi: 10.15886/j.cnki.rdswxb.20220114 |







 DownLoad:
DownLoad: