-
甲烷(CH4)是一种重要的温室气体,对全球气候变暖的贡献约占16%,仅次于CO2。2020年,大气的CH4浓度已上升至1 889 μg·L−1,是工业革命前的2.62倍[1]。水稻田是CH4主要排放源之一,所释放的CH4是CH4的产生、氧化和传输的净效应[2-4],其年平均排放量为30 Tg ,约占全球人为CH4排放量的8%[5-6]。稻田CH4的排放随水稻品种而异[7-13],人们试图靠选育和推广高产量、低CH4排放的水稻品种来实现稻田CH4的长效减排[4,8,13]。种植根系泌氧能力强的水稻品种,且适度施用铁肥就能减少持续淹水条件下水稻CH4的排放[14],其基本原理是:水稻植株的根系具有泌氧能力(Radial oxygen loss,ROL)[15-17],将根际土内的Fe2+氧化为Fe3+,并在根表沉积,形成根表铁膜(Iron plaque)[16,18-19],使得稻根和根际土成为淹水稻田中铁循环最活跃的区域[16,20-22]。土壤O2浓度和Fe2+浓度是根表铁膜形成的关键因子[16,18-19],所以,种植泌氧能力强的水稻品种和施用铁肥能极大地增强稻田土壤中的铁循环。利用Fe3+还原对产CH4的抑制作用[23-27],可实现稻田CH4的持续减排。普通野生稻(Oryza rufipogon Griff.)是亚洲栽培稻(Oryza sativa L.)的野生祖先种[28-31],两者有相同的基因组型(AA基因组),且遗传关系密切。普通野生稻具有比亚洲栽培稻更丰富的遗传多样性和更复杂的遗传背景,蕴藏着极其丰富的优异基因[32],从中挖掘强泌氧材料将有助于强泌氧水稻品种的培育。笔者曾对不同普通野生稻居群的根表铁膜形成能力进行了评价[33-34]。本研究拟选取1个根表铁膜形成能力较强的普通野生稻居群进行小区试验,旨在评价施铁措施对普通野生稻CH4的减排效果。
-
普通野生稻植株采自海南省文昌市东路镇葫芦洋野生稻保护示范点(110°68′E,19°78′N)。用木村B营养液培养[33,35],选取芽龄为11 d 的生根幼苗,从母株剪下后移栽。供试土壤为砖红壤发育的水稻土,自然风干,前茬为水稻,采自海南省农业科学院永发试验基地,其土壤理化性状为[14]:pH 6.50,全氮1.50 g·kg−1,有机碳13.80 g·kg−1,全铁23.85 g·kg−1,有效铁193.38 mg·kg−1。
-
小区试验在水泥池(长5.74 m×宽1.10 m×深0.45 m)中进行,分设对照水泥池和施铁水泥池。水稻干土加入量为286.35 kg·m−2。灌水后,池内稻土一直处于淹水状态。淹水30 d后,在施铁水泥池施用人工合成的水合氧化铁(ferrihydrite)[14],施Fe量为0.7 g·kg−1干土。每年种植2季水稻,已连续种植了3年。水稻收获后,人工清除秸秆和稻根。每小区移栽160株普通野生稻幼苗(株距13 cm×行距20 cm),持续保持约10 cm的水层。施肥时按水稻常规栽培措施进行。幼苗移栽10 d后,每处理随机放置4个土壤溶液取样器(取样头5 cm长,Rhizon flex,荷兰),垂直插放到根系旁,取样头基部距土表约3 cm。
-
CH4排放测定:采用静态顶空取样技术测定CH4的排放[14]。取样罩为透明的圆柱形有机玻璃罩(内径30 cm×高100 cm×壁厚0.4 cm),其顶部装有1个小型风扇,侧面有1个用胶塞封住的取样口。每个处理放置3个取样罩,每次测定的前一晚将取样罩的底座埋入土壤,每个取样罩内的植株数为3株。每隔10 min用1次性注射器(5 mL)抽取3 mL顶空气样,用带有火焰离子化检测器(FID)的气相色谱仪(Agilent 7890A GC System,美国)测定CH4浓度。GC所在实验室的温度设定为26 ℃。根据CH4浓度与6次气样采集时间的线性关系计算CH4排放通量[mg·(m2·d)−1]。在试验期间,每处理进行9次排放通量测定,其采样时间分别为移栽后第19、26、33、40、47、54、61、68、75 天。按任杰等[14]的方法计算取样期间的CH4总排放量。
土壤孔隙水Fe2+浓度测定:从土壤溶液采样器抽取0.5 mL孔隙水,注入盛有4.5 mL 0.5 mol·L−1 HCl的离心管中,混匀后放入冰盒内保存。采用菲洛嗪分光光度法[14],用紫外可见分光光度计(INESA 752N,中国)在562 nm下测定Fe2+浓度。
根表铁膜的测定:在移栽后第48天和第86天,每处理随机采收3株植株,剪下根系,用去离子水冲洗3次,然后用吸水纸吸去根表的水。每株普通野生稻的根系分成2部分,分别用于根表铁膜的提取,以及根含水量和根生物量的测定。采用DCB(Dithionite-Citrate-Bicarbonate)法提取根表铁膜[36],再用原子吸收分光光度计(ICE 3000 series,美国)测定铁含量[14,33-34]。根据提取液铁含量、根鲜质量和根含水量计算出根表铁膜含量(mg·g−1)。用根表铁膜含量的平均值乘以根生物量即得到单株根表铁膜数量(mg·株−1)。
-
采用Excel 2007进行数据处理和作图。采用SPSS 18.0统计软件进行统计分析和t测验。
-
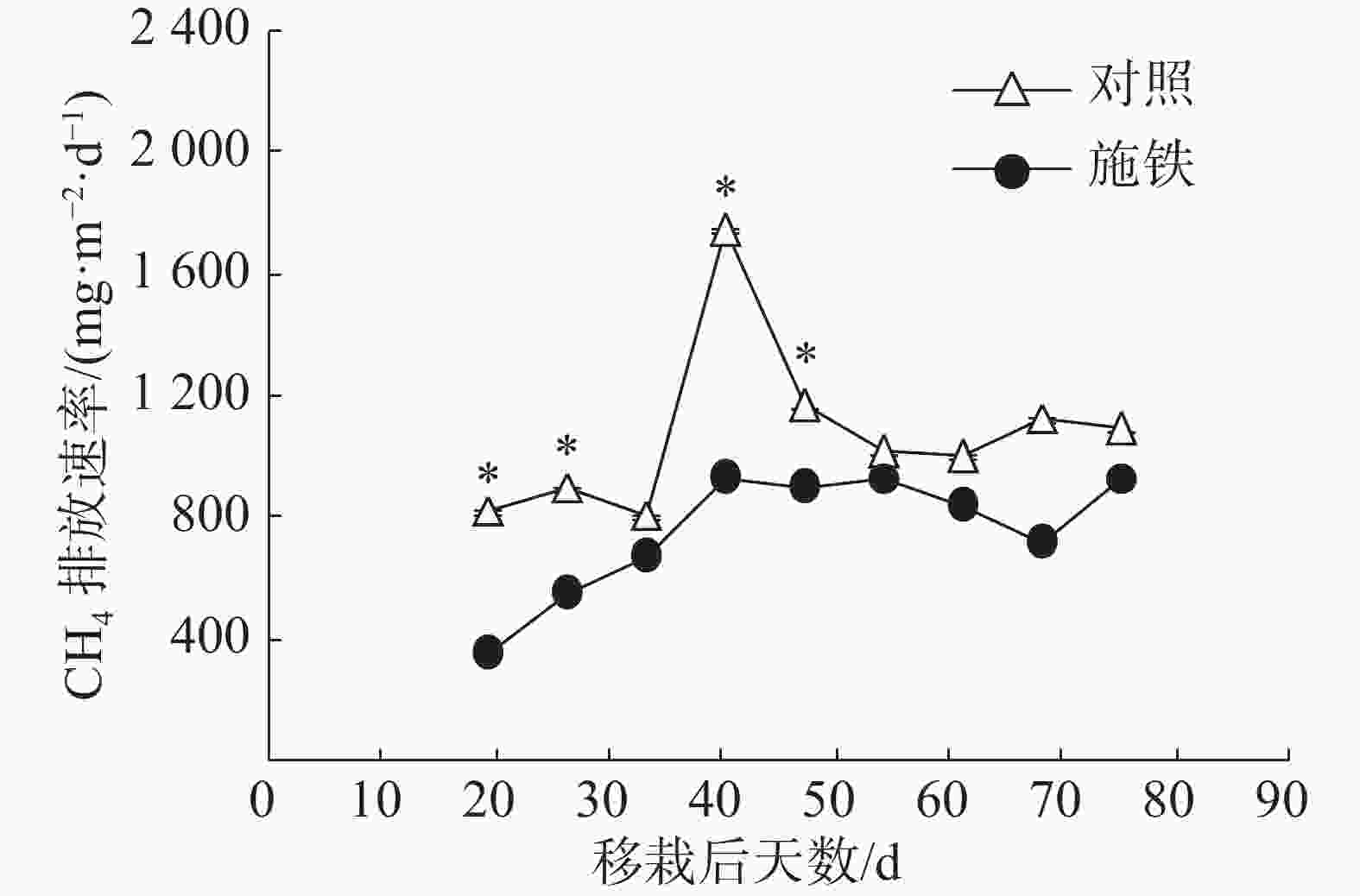
当普通野生稻生长期处于移栽后第19 天至第40 天时,施铁小区CH4排放通量从347.57 mg·(m2·d)−1持续上升到925.76 mg·(m2·d)−1,此后基本维持在这个水平上(图1)。对照小区CH4排放通量的日变化有1个高峰,出现在移栽后第40天,其值高达 1753.22 mg·(m2·d)−1;在此之前,CH4排放通量为813.87~904.98 mg·(m2·d)−1;从移栽后第47 天起,CH4排放通量维持在1 004.97~1 168.13 mg·(m2·d)−1。与对照相比,施铁措施在移栽后19、26、40和47 d的CH4减排效果均达到显著水平(P<0.05)。施铁处理和对照在57 d观测期内的CH4总排放量分别为43.22 g·m−2和61.31 g·m−2,施铁导致CH4排放减少了29.51%。
-
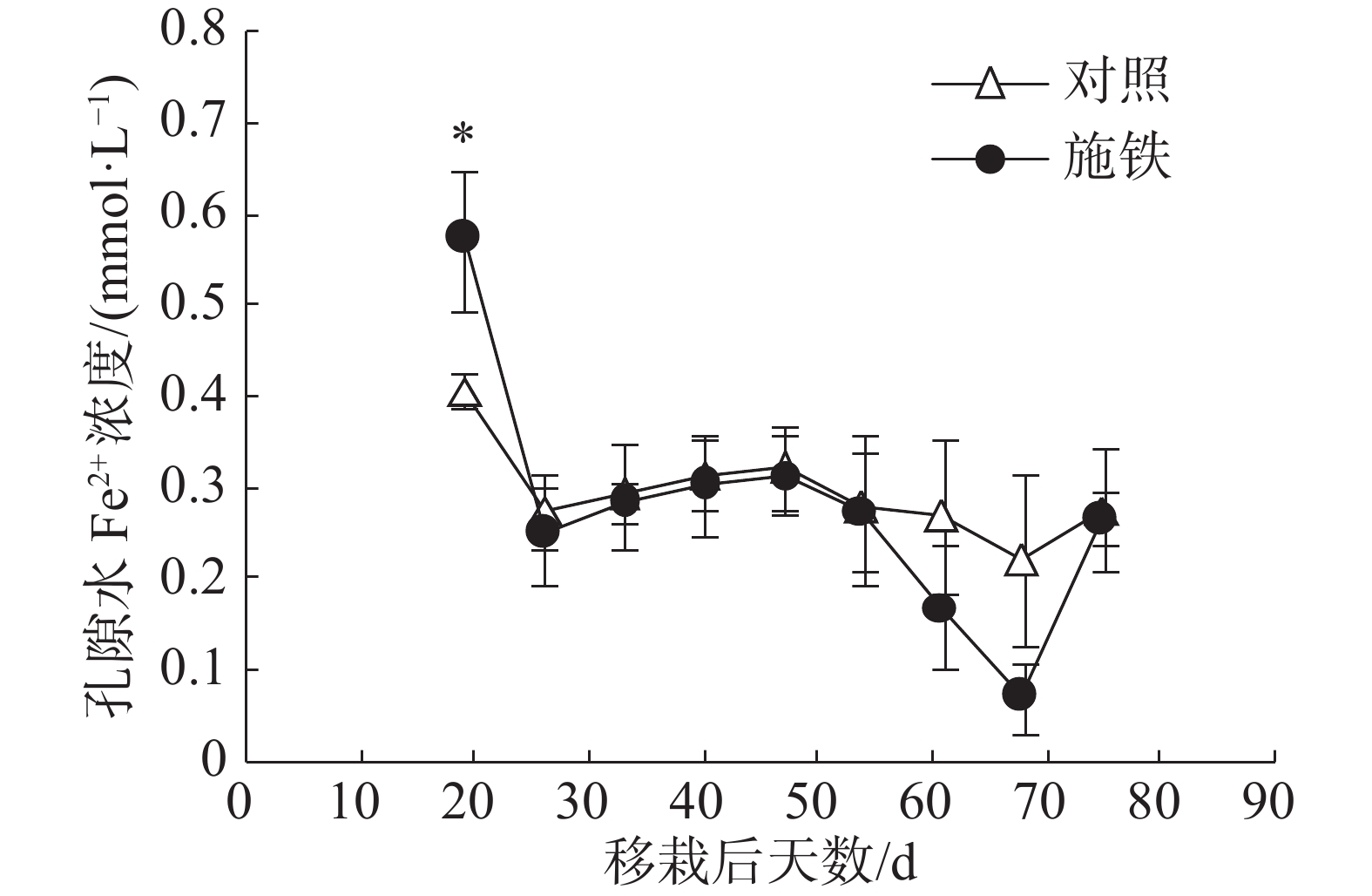
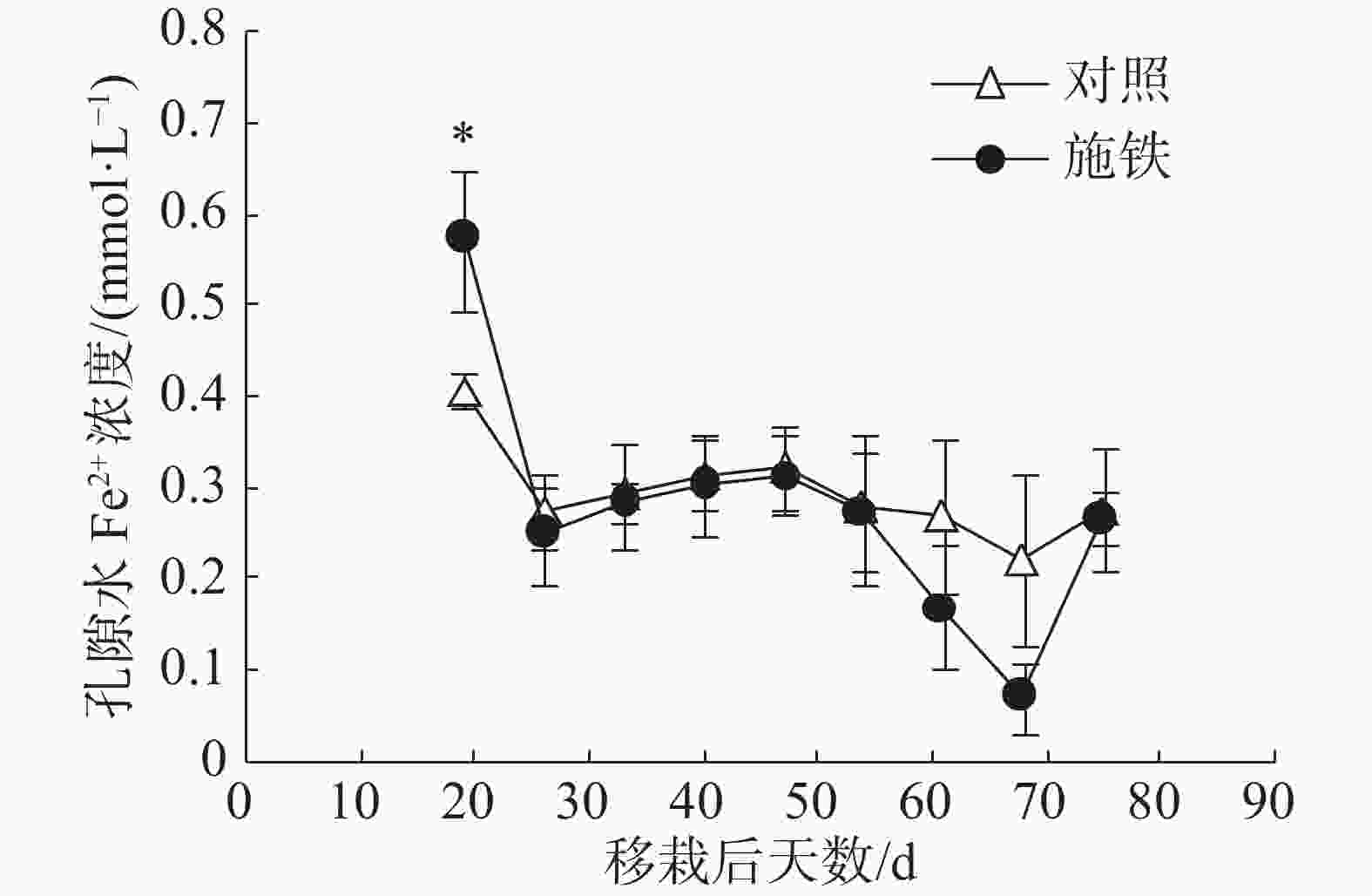
在普通野生稻移栽后第19天,施铁小区的土壤孔隙水Fe2+浓度为0.57 mmol·L−1 ,比对照小区(0.41 mmol·L−1 )高出39.02%,两者间差异显著(P<0.05)(图2)。此后,2个小区的孔隙水Fe2+浓度日变化趋势基本相似;对照小区的孔隙水Fe2+浓度处于平稳状态,介于0.22~0.32 mmol·L−1 ;施铁小区在移栽后61 和 68 d的孔隙水Fe2+浓度比对照低,分别降至0.17和0.07 mmol·L−1,但与对照相比,差异均未达到显著水平。
-
在普通野生稻移栽后48 d,施铁小区的根生物量、根表铁膜含量和单株根表铁膜数量分别为1.14 g、46.20 mg·g−1和52.44 mg·株−1,分别比对照高出10.68%、23.63%和39.65%,但两个处理之间的差异都没有达到显著水平(表1)。当普通野生稻生长至移栽后第86天,施铁小区的根生物量和单株根表铁膜数量分别提高到13.35 g和527.10 mg·株−1,而根表铁膜含量有所下降,其值为39.64 mg·g−1;虽然施铁小区的根生物量与对照相比差异不显著,但施铁小区的根表铁膜含量和单株根表铁膜数量均显著大于对照(P<0.05),分别比对照增加48.41%和81.96%。
采样时间 处理及其效应 根生物量/g 根表铁膜含量
/(mg·g−1)单株根表铁膜数量
/(mg·株−1)移栽后48 d 对照 1.03±0.19a 37.37±5.25a 37.55±1.76a 施铁 1.14±0.09a 46.20±3.49a 52.44±4.93a 施铁效应/% 10.68 23.63 39.65 移栽后86 d 对照 10.95±0.72a 26.71±3.72b 289.68±21.56b 施铁 13.35±0.66a 39.64±1.87a 527.10±14.36a 施铁效应/% 21.92 48.41 81.96 注:各处理数值为平均值±标准误;同列不同小写字母表示同天采样的施铁处理
与对照之间差异显著(P<0.05)。 -
本研究采用小区试验评价了施铁措施对普通野生稻田CH4排放的影响。在57 d的观测期内,施铁能使CH4排放减少29.51%,显著的CH4减排效应出现于普通野生稻生长前期,即移栽后19~47 d。这与任杰等[14]的水稻盆栽试验结果有所不同:施铁导致根表铁膜形成能力较强的水稻品种的CH4总排放通量比对照减少17.52%,CH4减排效应仅出现于水稻生长后期。虽然这2个试验的土壤来源相同,且施铁量一样,但是,在本研究实施前,试验小区已连续种植了6季水稻,稻土处于持续淹水状态。在普通野生稻移栽后第19天,施铁小区土壤溶液中仍含有较高浓度的Fe2+,比对照小区高出39.02%。正是施铁小区土壤中尚存的、高浓度Fe2+让普通野生稻植株的根系拥有更厚的根表铁膜。
迄今,有关根表铁膜与普通野生稻CH4排放的关系尚未见报道。但是,对水稻而言,这方面的报道比较多。水稻根系泌氧能力与水稻CH4排放之间呈负相关[37-38],泌氧(ROL)所引发的CH4减排效果应与根表铁膜有密切关系。因为水稻根表铁膜的主要组分是水合氧化铁(ferrihydrite)[16,19,22,39-42],所占比例可达50%~100%[43],该氧化物的比表面积较大,易被铁还原菌还原[19-20,27],而水合氧化铁等Fe3+氧化物的还原可有效抑制产CH4的产生[23-27]。对水稻根表铁膜的研究表明,根表铁膜的形成有2个驱动因子。1) O2浓度:淹水稻土中的O2主要来自水稻植株根系的ROL[16,18-19]。ROL随水稻的品种和生育期有所变化[44-45]。通常水稻品种泌氧能力愈强,根表铁膜愈厚[19,46-49]。同ROL一样,根表铁膜含量也随水稻生育期而变化[47]。根表铁膜形成能力可间接地表征水稻根系泌氧能力的强弱。因此,寻找根系泌氧能力强或者根表铁膜厚的水稻品种是实现CH4长效减排的一个有效途径。2) Fe2+浓度:在一定Fe2+浓度范围,水稻根表铁膜含量随土壤Fe2+浓度的增加而上升[18-19,50-53],所以,通过增施铁肥来提高水稻土壤Fe2+浓度,能进一步让泌氧多或者根表铁膜厚的水稻品种发挥其CH4减排潜能。
目前,涉及普通野生稻CH4动态的相关微生物群落结构的研究非常有限。CONRAD等[54]曾报道,普通野生稻土壤的产CH4古菌群落结构同附近亚洲栽培稻土壤没有差异。水稻和普通野生稻根际微生物组的对比研究表明,普通野生稻土壤中产CH4古菌和CH4氧化菌均比水稻土活跃[55-56]。本研究观测到的施铁措施对普通野生稻CH4的减排效果,很可能跟CH4氧化菌的功能与活性有一定的关系,因为CH4氧化菌需要O2,消耗土壤中的CH4,进而减少CH4的排放。因此,在未来的研究中,有必要探究根表铁膜形成与CH4有氧氧化对ROL的竞争关系。
在我国,普通野生稻分布于广东、广西、海南、云南、江西、湖南、福建和台湾等8省区[57]。普通野生稻作为多年生植物,栖息于长期淹水的环境中[28-30],拥有极其丰富的优异基因[32]。在普通野生稻种质资源中,有些居群具有较强的泌氧能力或较厚的根表铁膜[33-34],有些品系则拥有耐铁毒的特性[58-60],充分利用这些优良的遗传材料将有助于培育强泌氧、耐铁毒的水稻新品种。耐铁毒能力的提高意味着铁肥安全施用量上限的提升。本研究证实,施铁措施对具有厚铁膜潜力的普通野生稻居群的CH4减排展现出了明显的促进作用。因此,高效地发掘与利用普通野生稻强泌氧和耐铁毒的功能基因是培育低CH4排放水稻品种的一个重要途径,这对有效缓解全球温室气体减排和粮食安全问题之间的矛盾具有重要意义。
Mitigation of methane emission from Oryza rufipogon paddy soil with a higher Fe2+ concentration
doi: 10.15886/j.cnki.rdswxb.2022.05.010
- Received Date: 2022-05-03
- Accepted Date: 2022-05-23
- Rev Recd Date: 2022-05-20
- Available Online: 2022-07-12
- Publish Date: 2022-09-21
-
Key words:
- methane emission /
- Oryza rufipogon /
- iron plaque /
- soil pore water
Abstract: In order to explore the effect of Fe amendment on methane emission from Oryza rufipogon paddy soil, a comparison experiment was conducted in concrete ponds using a Oryza rufipogon population with higher capacity to form iron plaque on roots. The 11-day-old seedlings were transplanted to the rice paddy soil which was previously treated with or without addition of ferrihydrite at a rate of 0.7 g Fe/kg dry weight soil and already planted 6 growing seasons of rice under continuous flooding regime. Methane emission rate, Fe2+ concentration in soil pore water, root biomass and iron plaque on roots were measured. The experimental results showed that the methane emission in the Fe-amended pond during the period of 19-75 days after transplanting was reduced by up to 29.51%, comparing to control. Methane suppression observed by Fe amendment occurred only in the early growing season. There was a significant difference in Fe2+ concentration in soil pore water between two treatments only at 19 days after transplanting over the investigation period. The difference in both root biomass and amount of iron plaque per plant between Fe-amended treatment and control increased with age of plants. The present study suggests that Fe amendment can effectively mitigate methane emission from Oryza rufipogon plants with potential to have much more iron plaque on roots.
| Citation: | WANG Sheng, DAN Jianguo. Mitigation of methane emission from Oryza rufipogon paddy soil with a higher Fe2+ concentration[J]. Journal of Tropical Biology, 2022, 13(5): 496-501. doi: 10.15886/j.cnki.rdswxb.2022.05.010 |







 DownLoad:
DownLoad: