-
胆木[Nauclea officinalis (Pierre ex Pitard) Merr. & Chun],别名乌檀,是茜草科乌檀属植物,根据文献记载以枝、干、皮入药,胆木化学成分主要包括生物碱[1]、萜类及酚酸类等[2]。胆木药用价值受到了关注,目前已被开发成为浸膏糖浆[3]、饮片[4]、胶囊[5]和注射剂[6]等,在临床医学上用于抗炎镇痛[7]、抑菌[8]和抗病毒[9]清热解毒、消肿止痛 [10]等。胆木为多年生乔木,野生胆木喜生长于高山近顶或半腰荫蔽潮湿地带的杂木林中[11],在自然条件下生长缓慢,成药年限长,药用品质不佳,而人工种植往往因缺少科学管理,受到病害的干扰而导致植株早衰,药材产量不高。因此,有必要对胆木根系最直接的根际环境进行研究,特别是在根际发育过程中有可能对胆木根系造成病害或促进根系发育的细菌进行鉴定。
植物在应对胁迫环境时根际微生物起到积极作用[12],有益微生物能提升植物抗旱能力 [13-14]进而提高植物在环境胁迫下的生存能力,但在根际有害微生物使植株发生病害(比如线虫影响植物根系生理活性,进而影响根系水分和无机盐摄取)时可导致植株死亡[15]。不同微生物种类、数量以及其优势生理类群对植物生长过程中土壤有效养分的转化和吸收、植物病原菌的活性及其造成的病害均有一定影响[16]。微生物可帮助其寄生的植物抵御病害、增强抗逆性、同化和运输养分[17]、促进生长等[18],反过来植物凋落物和植物根际分泌物为根际土壤微生物提供营养。植物根系对根际微生物群落构建也具有选择性[19],其对根际微生物群落的营养选择与富集作用使得植物和微生物协同进化[20],导致根际微生物群落组成结构、群落丰度和多样性产生差异。不仅如此,植物根际分泌物及植物凋落物在很大程度上影响着林下土壤的理化性质。已有学者认为土壤理化性质与土壤微生物群落结构、群落丰度和多样性构建十分密切[21]。植物通过向土壤输入凋落物和根际分泌物,以及其吸收土壤养分的形式等方式影响土壤的理化性质[22]。植被类型、土壤有机质、土壤元素、温度、水分、pH值影响着根际微生物的群落结构和微生物的多样性[23]。土壤微生物是植物-土壤系统中比较活跃的组成成分;土壤微生物多样性代表着微生物群落的稳定性。根际微生物群落与土壤环境构建的相互关系可为植物营养环境的稳定性调控机制提供重要的理论依据[24]。高通量测序技术可快速对微生物进行有效鉴定,从而被广泛应用于微生物研究领域。本研究以五指山和琼中不同种植区的胆木根际土为样本,通过对其理化性质进行分析比较,并基于Illumina HiSeq 高通量测序技术对样本细菌多样性进行比较分析,为后续开展不同种植区胆木促生根际细菌的分离筛选工作和为不同立地条件下胆木的质量评价奠定基础。
-
样品DM4、DM7、DM10、DM12和DM14分别采自海南省五指山市畅好乡什哈村(东经 109.29°,北纬 18.40°)、海南省琼中黎族自治县上安乡长征八队(东经109.50°,北纬18.52°)、五指山市水满乡 (东经109.29°,北纬 18.40°)、海南省琼中自治县猫尾村领头十五队(东经109.56°,北纬19.05°)、海南省五指山市锦绣花园绿洲岛大酒店旁(东经109.30°,北纬 18.45°)。样地根际土的理化性质含量见表1。
样品 湿度 温度/℃ pH 有机质/(g·kg−1) 速效钾/(g·kg−1) 有效磷/(mg·kg−1) 碱解氮/(g·kg−1) DM4 0.5a 26.2±0.2ab 4.3±0.06b 28.34±0.58ab 0.29±0.02ab 35±0.2ab 0.68±0.06ab DM7 0.5a 26.7±0.3a 4.72±0.08a 24.95±1.37b 0.22±0.02b 26±0.1bc 0.65±0.02ab DM10 0. 5a 26.3±0.6b 4.38±0.08b 30.71±1.10a 0.32±0.03a 37±0.2a 0.74±0.03a DM12 0.4a 26.3±0.2a 4.78±0.02a 24.22±1.05b 0.23±0.03b 26±0.2bc 0.61±0.08b DM14 0.5a 26.2±0.7ab 4.37±0.08b 26.94±0.84ab 0.29±0.02ab 28±0.1ab 0.71±0.03ab 注:不同小写字母表示差异显著(P<0.05)。下同。 -
采用“S”形混合采样法,于各胆木种植地距离胆木树干10~20 cm 处的根系比较发达的地方挖开,将粘附在根系的土壤抖落,剔除石块和植物根系,取土,从每个种植地取9个点,每个样地取土样2 g,并混合均匀,分为3个平行装入已灭菌的离心管,并迅速置于冰盒或液氮罐中保存运回,一部分用于细菌检测,同时将其中一部分土壤带回实验室自然阴干,碾碎后过0.074 mm筛,用于pH值和有机质等土壤指标的测定。
-
采用NaHCO3-浸提-钼锑抗比色法测定有效磷[25];采用碱解扩散法测定碱解氮[26];采用原子吸收分光光度计测定速效钾[27];采用重铬酸钾容量法测有机质[28];采用pH计(DELTA 320)测定pH [29];土样湿度和温度使用土壤温度湿度测试仪(特安斯TASI)测量。将土壤温度湿度测试仪器开关打开,设置温度和湿度的参数,将探针头插入距离树干30~50 cm处根系比较发达的地方,深度20 cm,待仪器数据稳定后读数,重复3次。经纬度通过奥维定位仪获得。
-
委托百迈客生物技术有限公司对不同种植区胆木根际土壤样品的总DNA进行提取,并进行PCR、产物回收、样品建库及上机测序。操作步骤:根据保守区设计得到引物338F:5′-ACTCCTACGGGAGGCAGCA-3′,806R:5′-GGACTACHVGGGTWTCTAAT-3′,在引物末端加上测序接头,进行PCR扩增,反应条件为95 ℃预变性5 min,95 ℃变性30 s,50 ℃退火30 s,72 ℃延伸1 min,共30个循环,再72 ℃延伸5 min,最后4 ℃保存。对扩增产物进行纯化、定量和均一化,构建样品的cDNA 文库,并进行文库质检,合格的文库采用双端测序的方法对土壤细菌的16S rRNA V3+V4 区用Illumina HiSeq 2500进行测序。
-
使用R软件(VennDiagram) 进行细菌分类操作单元划分和分类鉴定,使用Mothur软件计算细菌物种丰度和多样性。采用Excel、DPS数据分析和SPSS20.0软件进行多重比较和相关性分析。
-
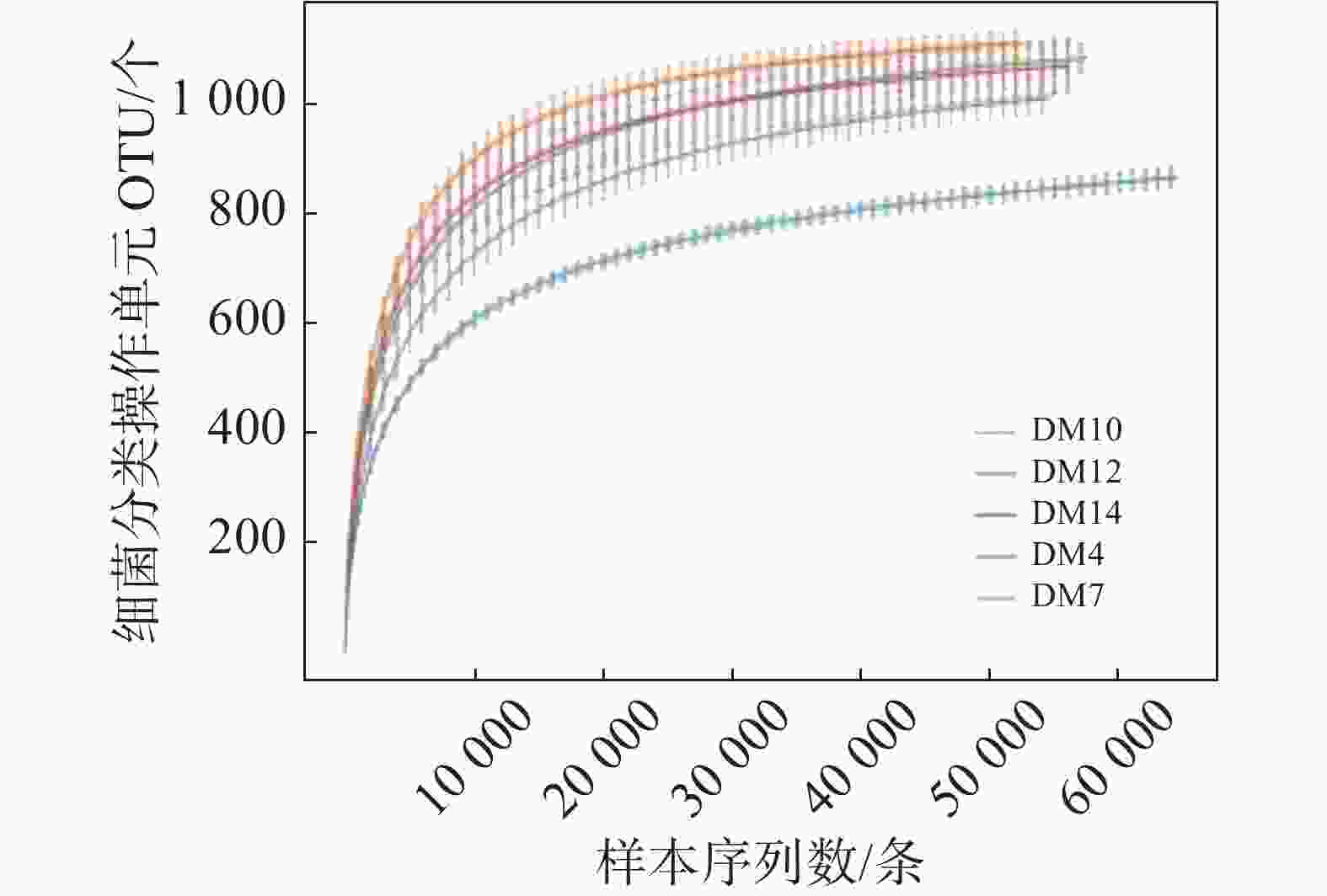
15份样品平均序列长度在414~418 bp之间。DM4、DM7、DM10、DM12和DM14组平均细菌分类操作单元个数分别为1084、870、1109、997和1012。5组样品共获得1223个细菌分类操作单元,用在97% 相似性水平下的聚类细菌分类操作单元 制作各样品的稀释曲线 (Rarefaction Curve),可作为对各样本测序量是否充分的判断,曲线急剧上升表明测序量不足。由图1可知,细菌曲线逐渐趋向平坦,说明测序数量合理,可进行数据分析。
-
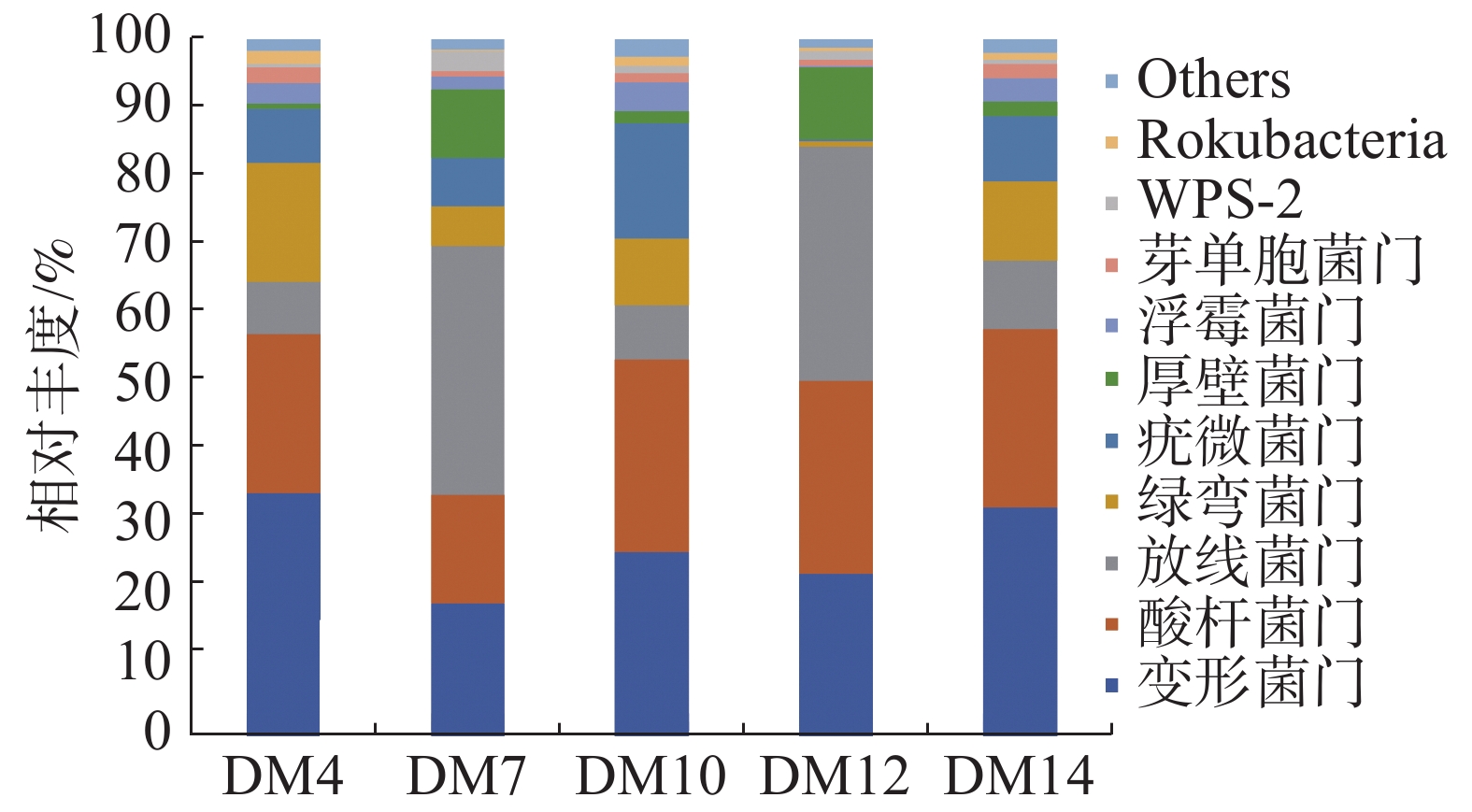
由图2可知,胆木根际土壤的优势细菌门为Rokubacteria 、WPS-2、芽单胞菌门(Gemmatimonadetes)、浮霉菌门(Planctomycetes)、厚壁菌门(Firmicutes)、疣微菌门(Verrucomicrobia)、绿弯菌门(Chloroflexi)、放线菌门(Actinobacteria)、变形菌门(Proteobacteria)、酸杆菌门(Acidobacteria)等10个细菌门。DM4、DM14第一优势菌门为变形菌门,其相对丰度分别为34.81%、32.86%;MD7和DM12的第一优势菌门为放线菌门,其相对丰度分别为35.64%和33.65%;DM10第一优势菌门是酸杆菌门,相对丰度为27.6%;MD7和 DM10的第二优势菌门为变形菌门,是DM4和DM14的第一优势菌门,其相对丰度分别为19.14%和26.43%;DM4、DM12和DM14的第二优势菌门是酸杆菌门,也是DM10的第一优势菌门,其相对丰度分别22.62%、27.65%和25.39%。
-
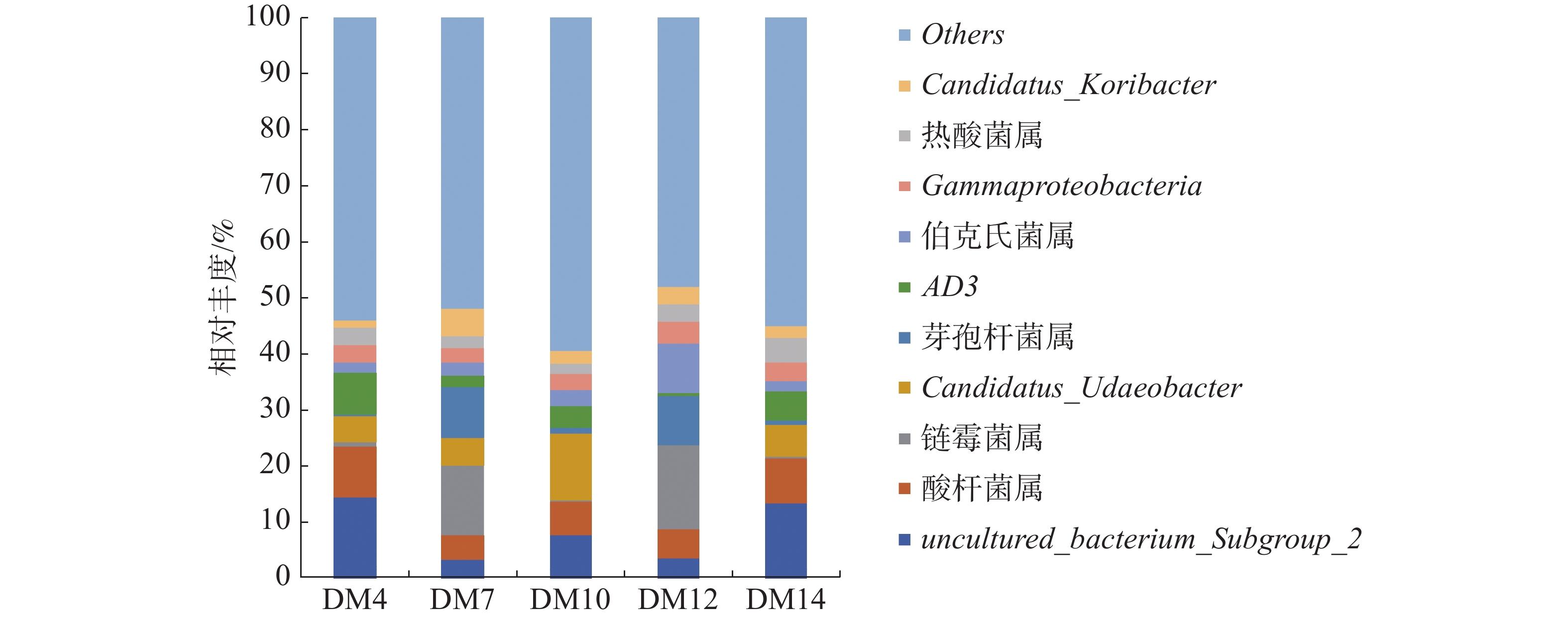
如图3可知,5组样品优势菌属有芽孢杆菌属(Bacillus)、酸杆菌属(Acidobacter)、链霉菌属(Streptomyces)、伯克氏菌属(Burkholderia)、Gammaproteobacteria、热酸菌属(Acidothermus)、以及未确定的菌属uncultured_bacterium_Subgroup_2、Candidatus_Udaeobacter、Candidatus_Koribacter和AD3。种植区为琼中的DM7和DM12第一优势菌属均为链霉菌属,相对丰度分别为12.42%和14.83%。种植区为五指山的DM4和DM14第一优势菌属是未确定的菌属uncultured_bacterium_Subgroup_2,相对丰度分别14.46%和13.46%;种植区为五指山的DM10的第一优势菌属为未确定菌属Candidatus_Udaeobacter,相对丰度为11.82%,次优势菌属是五指山的DM4和DM14的第一优势菌属即uncultured_bacterium_Subgroup_2,相对丰度为7.79%;DM4和DM14的第二优势菌属是酸杆菌属,相对丰度分别为9.09%和7.92%;DM12的第二优势菌属是伯克氏菌属,其相对丰度为9.02%,DM7第二优势菌属是芽孢杆菌属,其相对丰度为9.01%。
-
用Chao1和Ace指数衡量物种丰度,用Shannon和Simpson指数衡量物种多样性,指数值越大,说明样品的物种丰度和多样性越高。此外还统计了覆盖率(Coverage),其数值越高,则样本中物种被测出的概率越高。5个不同种植区的胆木根际土壤的细菌多样性分析结果(表2)显示,种植区为自五指山的DM4、DM10、DM14的物种丰度和多样性均高于种植区为琼中县的DM7和DM12,其中5个种植区中的物种丰度和多样性最高为五指山水满乡根际土壤DM10,最低为琼中县DM12样品。
97%相似水平指标 DM4 DM7 DM10 DM12 DM14 ACE指数 1130±21a 1077±16a 1137±18a 953±25b 1117±30a Chao1指数 1145±22a 1091±17ab 1153±16a 987±29b 1134±28a Simpson 指数 0.99±0.00ab 0.96±0.01bc 0.99±0.01a 0.96±0.00c 0.99±0.00ab Shannon 指数 7.91±0.18ab 7.0±0.33bc 8.3±0.16a 6.7±0.19bc 7.93±0.2ab Coverage覆盖率 0.998±0.000 2a 0.998±0.000 2a 0.999±0.000 2a 0.998±0.000 2a 0.999±0.000 1a -
5个种植区胆木根际土壤理化性质与根际细菌的主要菌门的相关性分析结果(表3)表明,湿度、温度、pH值、有机质含量、速效钾含量、有效磷含量和碱解氮含量对根际土壤中大多数主要细菌门有影响。土壤湿度与放线菌门(Actinobacteria)、厚壁菌门(Firmicutes)的细菌丰度呈极显著负相关,与Rokubacteria细菌丰度呈极显著正相关,与酸杆菌门(Acidobacteria)、绿弯菌门(Chloroflexi)、浮霉菌门(Planctomycetes)、芽单胞菌门(Gemmatimonadetes)的细菌丰度呈显著正相关。温度与疣微菌门(Verrucomicrobia)的细菌丰度呈极负相关,与厚壁菌门(Firmicutes)的细菌丰度呈显著负相关,与放线菌门(Actinobacteria)的细菌丰度呈显著正相关关系。pH值与放线菌门(Actinobacteria)的细菌丰度呈极显著正相关关系,与Rokubacteria 的细菌丰度呈极负相关,与酸杆菌门(Acidobacteria)、绿弯菌门(Chloroflexi)、浮霉菌门(Planctomycetes)、芽单胞菌门(Gemmatimonadetes)的细菌丰度呈显著负相关关系。有机质、速效钾、有效磷和碱解氮等的含量与厚壁菌门(Firmicutes)和变形菌门(Proteobacteria)的细菌丰度均呈显著负相关关系,其中,速效钾与厚壁菌门(Firmicutes)和变形菌门(Proteobacteria)的细菌丰度呈极显著负相关,与浮霉菌门(Planctomycetes)的细菌丰度呈显著正相关;速效钾与厚壁菌门(Firmicutes)和变形菌门(Proteobacteria)的细菌丰度呈极显著负相关,碱解氮与浮霉菌门(Planctomycetes)和疣微菌门(Verrucomicrobia)的细菌丰度呈积极显著正相关。所有土壤理化性质测量指标与变形菌门(Proteobacteria)和WS-2的细菌丰度无显著相关。
细菌 湿度 温度 pH 有机质 速效钾 有效磷 碱解氮 酸杆菌门(Acidobacteria) 0.85* −0.390 −0.85* 0.500 0.700 0.470 0.510 变形菌门(Proteobacteria) 0.400 −0.410 −0.300 0.360 0.500 0.360 0.240 放线菌门(Actinobacteria) −1.00** 0.81* 0.99** −0.87* −0.96** −0.85* −0.87* 绿弯菌门(Chloroflexi) 0.85* −0.520 −0.90* 0.660 0.7200 0.630 0.670 疣微菌门(Verrucomicrobia) 0.710 −0.94** −0.710 0.90* 0.810 0.90* 0.95** 厚壁菌门(Firmicutes) −0.99** 0.790 1.00** −0.86* −0.94** −0.84* −0.87* 浮霉菌门(Planctomycetes) 0.88* −0.90* −0.89* 0.90* 0.89* 0.89* 0.99** 芽单胞菌门(Gemmatimonadetes) 0.85* −0.390 −0.88* 0.500 0.670 0.460 0.550 WS-2 −0.780 0.410 0.740 −0.470 −0.690 −0.450 −0.440 Rokubacteria 0.92** −0.660 −0.92** 0.780 0.88* 0.750 0.680 注:*表示在5%水平上显著相关,**表示在1%水平上显著相关。下同。 -
由表4可知,湿度、温度、pH、有机质含量和速效钾含量、有效磷含量和碱解氮含量对根际土壤中主要细菌的物种丰度和多样性均有影响。湿度、有机质含量和速效钾含量对细菌物种丰度以及细菌多样性均呈显著正相关,其中,湿度和有机质对细菌物种的丰度和多样性均呈极显著正相关。温度、pH对细菌物种的丰度和多样性呈显著负相关,其中pH对细菌物种的丰度和多样性呈极显著负相关。此外,速效钾含量和细菌物种丰度呈显著正相关;碱解氮含量、有效磷含量和细菌多样性呈显著正相关。
指标 湿度 温度 pH 有机质含量 速效钾含量 有效磷含量 碱解氮含量 ACE指数 0.74** −0.59* −0.71** 0.72** 0.54* 0.3600 0.49 Chao1指数 0.74** −0.60* −0.70** 0.71** 0.55* 0.3500 0.44 Cimpson指数 0.85** −0.66** −0.72** 0.88** 0.68** 0.60* 0.59* Shannon指数 0.85** −0.71** −0.74** 0.86** 0.66** 0.4900 0.59* -
关于土壤理化性质和微生物差异的研究,杨承栋等[30]认为在不同树龄的土壤中,有机质、速效钾、有效磷、速效氮等的含量具有差异,从幼龄林至中龄林呈下降趋势;从中龄林至成熟林土壤中有机质和速效钾的含量呈上升趋势,有效磷和碱解氮的含量变化无明显规律性,根际和非根际微生物的种类和数量均存在差异;并认为这与种植区有关,在不同的土壤母质、土壤微生物和气候类型的共同作用下,土壤有机质的分解,土壤养分元素的有效性以及养分元素在土壤生成过程中的释放和迁移导致土壤理化性质的差异,适宜的土壤理化性质提供给微生物良好的生活环境,相对不好的土壤环境微生物的数量和多样性随之减少。此外,土壤理化性质和微生物的差异还有海拔高度的影响[31],说明造成土壤理化性质与微生物的差异的因素是复杂的,而不是单一的某个因素[32]。笔者在对5个胆木种植区的土壤理化性质分析中发现,样品间湿度无显著差异,含水量都较高,保持在40%~55%;种植区琼中的2组样品的温度和pH都高于种植区五指山的样品;5组样品的温度均保持在26 ℃左右,pH均为酸性。笔者分析了5个胆木种植区的土壤理化性质形成原因,认为各组样品地处海南降水丰富的种植区,pH值偏酸性可能是由于降水多导致盐基饱和度降低造成的。笔者在对5个胆木种植区的细菌分析中发现,5个种植区的细菌数量和多样性有差异,五指山样品的细菌数量和细菌多样性相对于琼中样品更加丰富一些,而同个市县的样品间也存在差异。笔者认为这是因为5个种植区所处地理环境、气候条件、海拔高度、土壤环境的差异造成的。
-
在研究土壤理化性质与人工林细菌多样性时,丁新景等[33]认为含水量、碱解氮含量、有效磷含量、速效钾含量和有机质含量与根际土壤细菌多样性相关关系显著,pH与细菌多样性指数无显著相关关系。侯建伟等[34]在对花椒根区土壤细菌群落结构研究中认为土壤pH、碳氮比、碱解氮、有效磷和速效钾总共解释了83.3%的群落变化。本研究中,5个不同的胆木种植区样品的湿度、温度、pH值、有机质含量、速效钾含量、有效磷含量和碱解氮含量对根际土壤中主要细菌物种的结构丰度以及细菌多样性均有影响;在10种优势门类细菌中,占绝对优势的菌门是变形菌门,但变形菌门类细菌未与土壤理化性质指标呈显著相关关系。宋春丽等[35]认为变形菌门为优势菌门可能与其生态位宽度较大、受环境影响较小有关。在本研究中,Rokubacteria 、芽单胞菌门、浮霉菌门、厚壁菌门、疣微菌门、绿弯菌门、放线菌门、变形菌门、酸杆菌门等细菌门与1~3种土壤理化性质指标呈极显著相关。本研究中变形菌门和酸杆菌门、放线菌门细菌的物种丰度高于其他细菌门的,这与PENG等[36]和ZHOU等[37]研究结果一致,而Rokubacteria 、芽单胞菌门、浮霉菌门、厚壁菌门、疣微菌门、绿弯菌门、变形菌门受到多种土壤环境因素限制,生长受到抑制,丰度较小[38]。在一定范围内pH值与酸杆菌门总丰度呈反比,酸杆菌门细菌在酸性土壤环境中丰度较高[39-40],而随着pH值的升高其生长受到抑制,丰度降低[41],究其原因,可能是由于酸杆菌门细菌是嗜酸性细菌,酸性土壤环境有利于细菌的生理活动[42]。在特定的土壤环境里,变形菌门细菌和放线菌门细菌的丰度会随着酸杆菌门细菌的丰度增多而下降[43],但在本研究中是细菌的结构丰度与其共同生活细菌种群类别相关;对细菌的物种丰度与多样性的研究表明,土壤细菌多样性与土壤理化性质密切相关[44-46]。本研究结果发现,5个不同胆木种植区土壤的湿度、温度、有机质含量和速效钾含量对细菌的物种丰度及多样性呈显著正相关,其中,湿度和有机质含量对细菌的物种丰度和多样性呈极显著正相关关系;温度和pH值对细菌的物种丰度和多样性呈显著负相关关系,其中pH值呈极显著负相关。此外,速效钾含量对ACE和Chao1指数,碱解氮含量对Cimpson和Shannon 指数,有效磷含量对Cimpson 指数均呈显著正相关。
-
(1)五指山样品DM4、DM10和DM14的平均细菌分类操作单元个数分别为1084、1109和1012,琼中样品DM7和DM12的平均细菌分类操作单元个数分别为870、997,五指山的平均细菌分类操作单元个数高于琼中。
(2)五指山和琼中5组样品的优势菌门组成主要是Rokubacteria 、WPS-2、芽单胞菌门、浮霉菌门、厚壁菌门、疣微菌门、绿弯菌门、放线菌门、变形菌门、酸杆菌门等10个细菌门。
(3)五指山和琼中5组样品主要优势菌属有芽孢杆菌属、酸杆菌属、链霉菌属、伯克氏菌属、Gammaproteobacteria、热酸菌属、及未确定的菌属uncultured_bacterium_Subgroup_2、Candidatus_Udaeobacter、Candidatus_Koribacter 和AD3等10个细菌属。
(4)土壤的理化性质影响细菌的优势菌门、相对丰度和多样性,如土壤湿度与放线菌门和厚壁菌门的细菌丰度呈极显著负相关,与Rokubacteria的细菌丰度呈极显著正相关;湿度、有机质含量和速效钾含量对细菌物种丰度以及细菌多样性呈显著正相关。
Bacterial community structure and diversity in the rhizosphere of Nauclea officinalis in different plantations
doi: 10.15886/j.cnki.rdswxb.2022.05.009
- Received Date: 2021-12-13
- Accepted Date: 2022-04-07
- Rev Recd Date: 2022-03-09
- Available Online: 2022-07-12
- Publish Date: 2022-09-21
-
Key words:
- Nauclea officinalis /
- rhizosphere soil /
- bacterial diversity /
- high-throughput sequencing /
- soil physical and chemical properties
Abstract: An attempt was made to understand the main bacterial community structure and bacterial diversity in the rhizosphere of Nauclea officinalis to clarify the relationship between its community structure and bacterial diversity and soil physicochemical properties for further isolation and screening of rhizosphere growth-promoting bacteria in different planting areas. Fifteen rhizosphere soil samples from 5 planting areas under N. officinalis were analyzed by high-throughput sequencing technology. Multiple comparisons and correlation analysis were performed on the data using Excel, DPS and SPSS20.0 software. The results showed that the average sequence length of the 15 soil samples was 414−418 bp. The average operational taxonomic unit (OTU) numbers of samples DM4, DM7, DM10, DM12 and DM14 were 1084, 1012, 1109, 870 and 997, respectively, and 1223 bacterial taxa were obtained. The sample community structure analysis showed that there were 10 dominant bacterial phyla, such as Rokuacteria, WPS-2, Gemmatimonadetes. The dominant genera were Bacillus, Acidobacter, Streptomyces, Burkholderia, Gammaproteobacteria, Acidophilus, and the unidentified genera Candidatus, Udaeobacter, and AD3. The diversity analysis of the bacteria from the samples showed that the species abundance and diversity of the bacteria were higher in the samples form Wuzhishan city than in the samples from Qiongzhong county. The sample correlation analysis showed that soil physicochemical properties were related to dominant bacteria. For instance, organic matter, available potassium, available phosphorus and alkali-hydrolyzable nitrogen were negatively correlated significantly with Firmicutes and Proteobacteria, and positively significantly with Planctomycetes. Humidity, organic matter, available potassium were positively significantly while the temperature and pH were negatively significantly with the species abundance and diversity of the bacteria. The composition of the main community structure in the N. officinalis plantations in Wuzhishan and Qiongzhong is generally the same, but different in abundance ratios. The bacterial community abundance and bacterial diversity were different in these two areas, indicating that the bacterial community abundance and bacterial diversity are different in different planting areas. Moreover, the bacterial community abundance and bacterial diversity in the samples from Wuzhishan were higher than those from Qiongzhong. The soil physicochemical properties had an impact on the main phyla, bacterial community abundance and bacterial diversity of rhizosphere bacteria.
| Citation: | CHEN Yanyan, XU Shitao, WANG Deli, WANG Jun, ZHOU Yuanyuan, HOU Xiangwen. Bacterial community structure and diversity in the rhizosphere of Nauclea officinalis in different plantations[J]. Journal of Tropical Biology, 2022, 13(5): 488-495. doi: 10.15886/j.cnki.rdswxb.2022.05.009 |








 DownLoad:
DownLoad: