-
火龙果又名红龙果,属仙人掌科(Cactaceae)量天尺属(Hylocereus),是极具发展前景的热带和亚热带水果[1]。火龙果因具有较高的经济价值和营养价值,集蔬菜、水果、花卉和保健于一体,被誉为“21世纪保健食品和果品珍品”, 受到人们的青睐,具有广阔的国际、国内市场[2]。火龙果20世纪80年代才引入我国台湾,虽然在我国栽培历史较短,但发展迅速,目前主要在台湾、海南、广西、广东、福建、贵州等省区种植。溃疡病是近年来火龙果种植园发生的严重真菌病害之一,发病率高达60%[3-7]。溃疡病造成火龙果枝条腐烂,果实变黑,失去食用价值,有些果园甚至颗粒无收。该病害在夏秋季高温高湿的气候条件下发病尤为严重,尤其台风过后更容易大面积暴发,这也是该病在海南地区盛行的主要原因之一。该病害由新暗色柱节孢(Neoscytalidium dimidiatum (Penz.) Crous & Slippers)侵染引起的[4-11],开展病原菌的致病机理研究可以为病害防治所需新药以及抗性种苗的研发提供相关的理论依据,从而达到对病害长期、有效的防控。病原菌基因功能缺失突变体的构建是研究病原菌致病机理的有效手段,这一方法的关键技术是建立遗传转化体系。农杆菌介导的遗传转化(Agrobaeterium tumefaciens-Meidated Transformation)可快速构建真菌T-DNA(transfer DNA)随机插入突变体,因其操作简单,可转化完整的细胞,比如分生孢子、菌丝、子实体等,免去了制备原生质体的繁琐,广泛应用于多种真菌[12]。目前已有100多种真菌使用该方法进行成功转化[13],比如玉米大斑病菌[14]、稻瘟病菌[15]、尖镰孢菌[16]、玉米灰斑病菌[17]等。本研究拟建立根癌农杆菌介导的火龙果溃疡病菌的遗传转化体系,为火龙果溃疡病菌致病机理的研究奠定技术基础。
-
火龙果溃疡病菌野生型菌株由本实验室分离、鉴定和保存。农杆菌菌株EHA105由海南大学热带农林学院陈银华教授馈赠。载体pXEH8279 (KanaR, HygBR)由吉林大学植物科学学院潘洪玉教授馈赠。实验用培养基IM、IAM[12]。
-
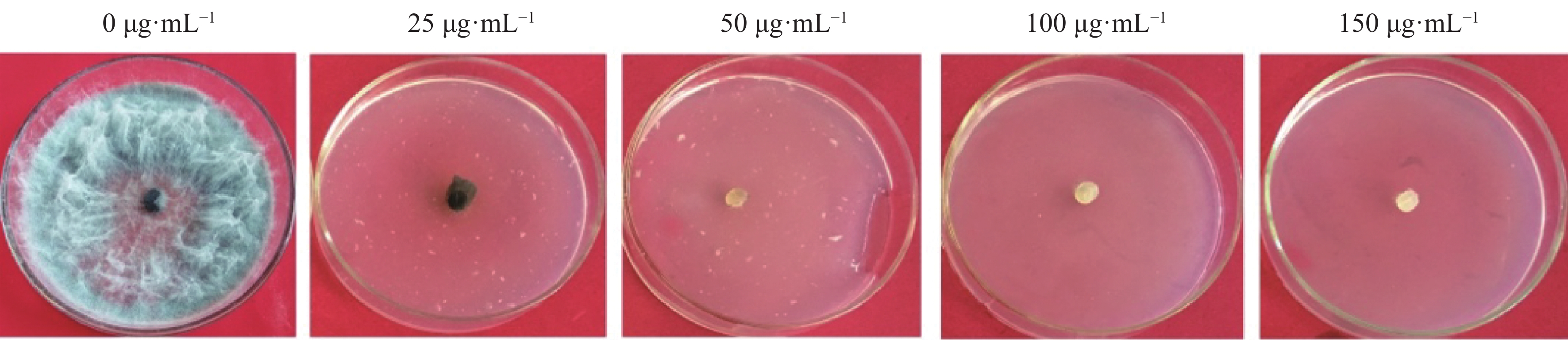
将火龙果溃疡病菌野生型菌株接种在PDA培养基上活化,待长至菌落直径约5 cm时,用5 mm打孔器在菌落边缘打孔取菌饼,并将菌饼转接在加有不同浓度 HygB(0、25、50、100、150 µg·mL−1)的PDA培养基上,置于25 ℃培养箱中避光培养。每处理3重复,培养7 d后,观察菌落生长情况,确定最佳的HygB筛选浓度。
-
将携带有质粒pXEH8279的农杆菌菌株EHA105在卢里亚−贝尔塔尼平板培养基(LB)[利福平(rif) 10 µg·mL−1,卡那霉素(Kana) 50 µg·mL−1]上划线,置于28 ℃培养至长出单菌落。挑取单菌落转移至新的LB平板培养基(rif 10 µg·mL−1, Kana 50 µg·mL−1)上划线,28 ℃培养48 h后,用灭菌的药匙刮取农杆菌,使其分散在IM培养基内,28 ℃,200 r·min−1,培养4~6 h,使其OD600达到0.5,置于50 mL无菌管中备用。
-
将活化的火龙果溃疡病菌野生型菌株接种在PDA培养基上,28 ℃培养7 d后,吸取0.01%吐温20于培养基上,用无菌棉棒将分生孢子轻轻洗下,然后用4层灭菌纱布过滤,制成分生孢子悬浮液。取适量分生孢子悬浮液于PD培养基中,调整分生孢子浓度至5×105个·mL−1,避光放置在摇床上,转速为150 r·min−1,室温下萌发 24 h。
-
诱导培养的农杆菌与萌发的分生孢子悬浮液等体积混合后,于25 ℃,80 r·min−1,摇培20~30 min。吸取100 μL混合液,涂布在共培养培养基(IAM, Induced Agar Medium)上,置于25 ℃黑暗培养。共培养 48 h后,在IAM上倒筛选培养基,置于25 ℃培养。筛选培养基即加有潮霉素(HygB,50 μg·mL−1)、头孢霉素(Cef, 200 μg·mL−1)、氨苄青霉素(Amp, 200 μg·mL−1)的马铃薯葡萄糖琼脂(PDA)培养基。培养5~6 d后即可见有单菌落长出。
-
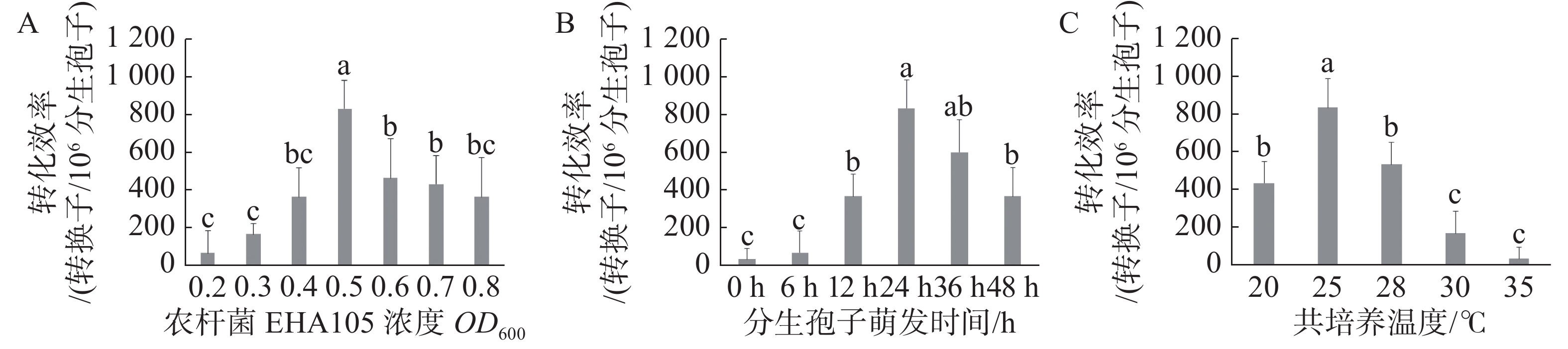
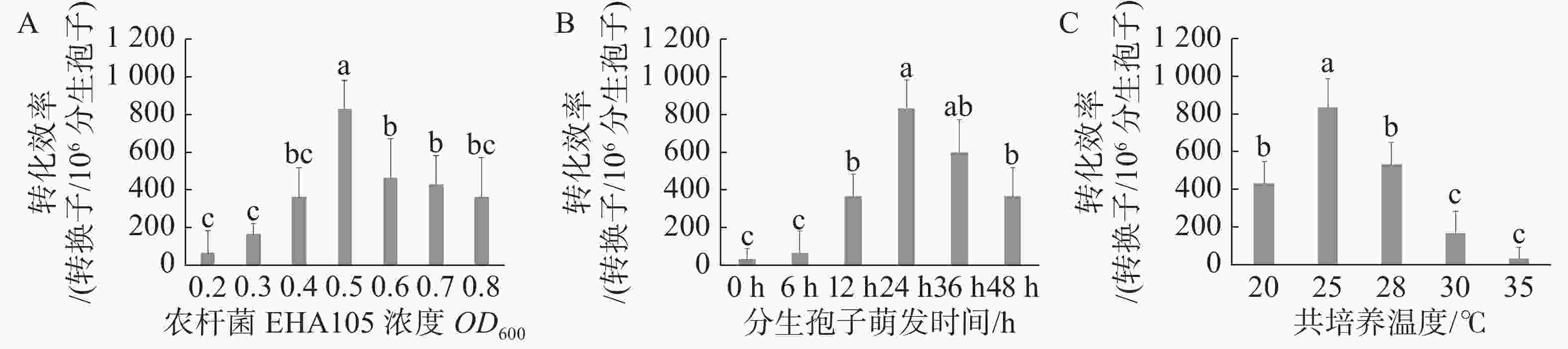
根据以上遗传转化方法,依次验证以下因子对火龙果溃疡病菌遗传转化效率的影响,包括农杆菌浓度OD600(0.2、0.3、0.4、0.5、0.6、0.7、0.8)、分生孢子萌发时间(0、6、12、24、36、48 h)以及共培养温度(20、25、28、30、35 ℃),采用单因素试验,每个处理设置3个重复。
-
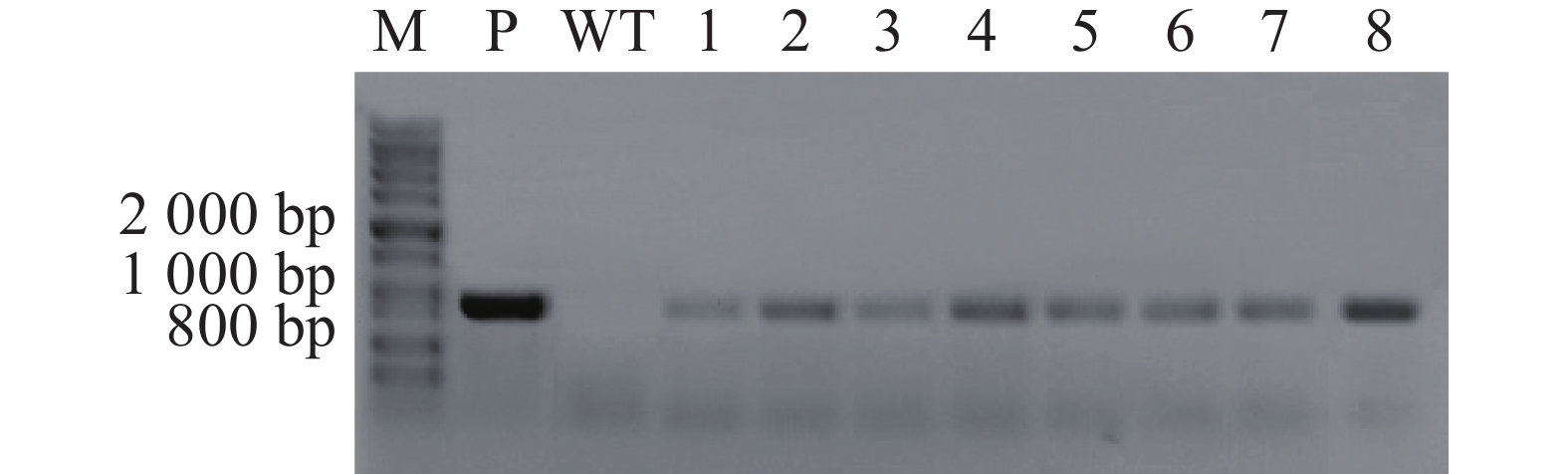
随机挑选8株转化子以及野生型菌株接种在PDA培养基上,置于28 ℃培养箱中培养。4 d后,用无菌枪头分别刮取培养基中的菌丝,转移至1.5 mL离心管中。分别向装有菌丝的离心管加入液氮,并用无菌塑料研磨棒迅速将菌丝研磨成粉状,利用CTAB(Hexadecyl trimethyl ammonium bromide, 十六烷基三甲基溴化铵)法提取转化子及野生型菌株基因组DNA。以HygB抗性基因片段为模板设计引物hygF (5′-gaagaatctcgtgctttcag-3′)和hygR(5′-gtacttctacacagccatcg-3′),通过PCR方法验证T-DNA片段是否插入,目标片段长度880 bp。PCR扩增程序:94 ℃ 预变性5 min;94 ℃变性40 s,56 ℃退火40 s,72 ℃延伸1 min,30个循环;72 ℃ 延伸7 min。
-
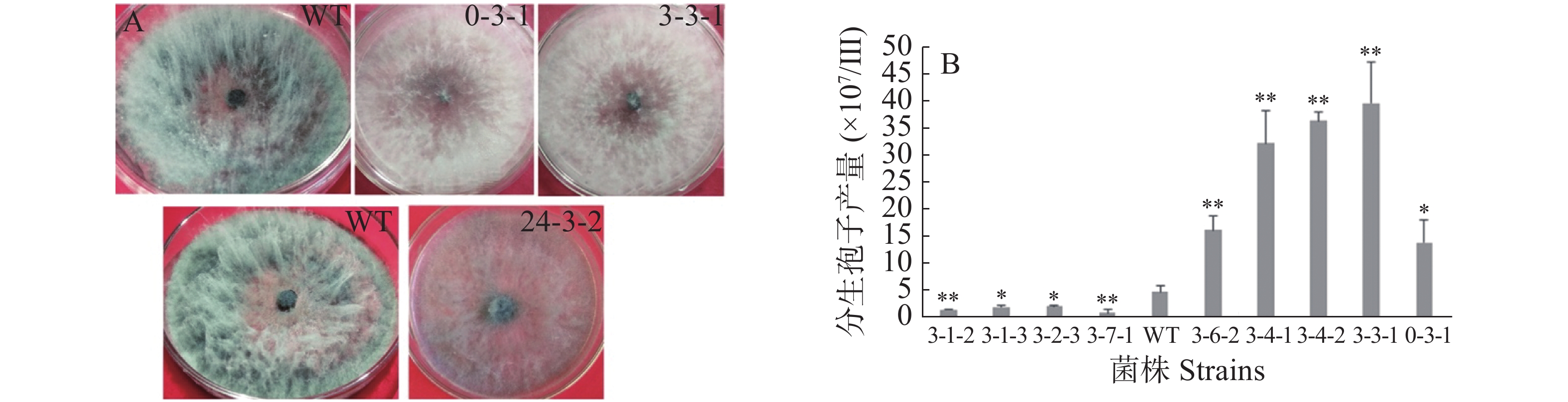
将随机选取的200株转化子以及野生型菌株接种在PDA培养基上,28 ℃培养4 d后,用5 mm打孔器分别在菌落边缘取菌饼,并将菌饼转移至新的PDA培养基上,置于28 ℃培养箱中培养7 d,观察菌落形态特征并拍照后,收集分生孢子,计算每皿产生的分生孢子数量。每个处理设置3个重复。
-
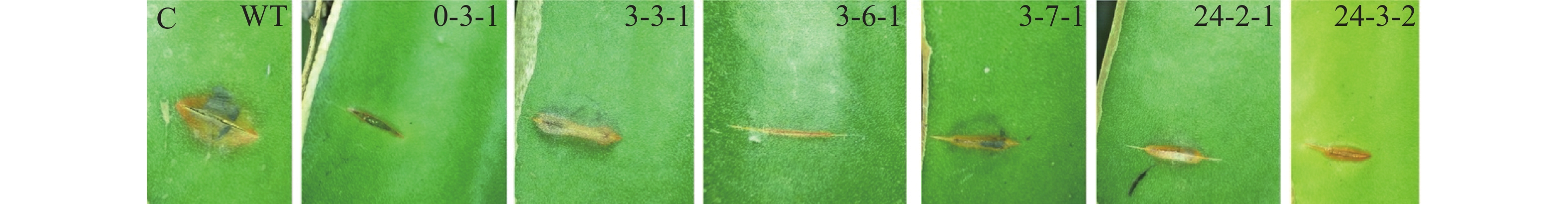
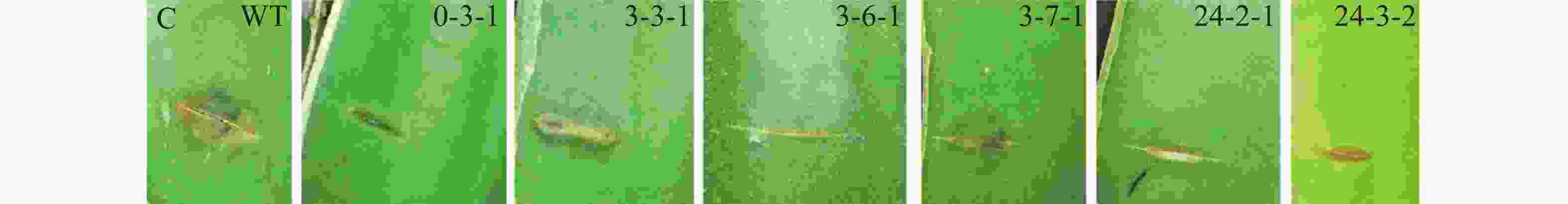
采用田间接种方法,筛选致病力降低的转化子。具体操作如下:将转化子及野生型菌株分别接种在PDA培养基上,置于28 ℃培养箱中培养4 d后,用5 mm打孔器分别取菌落边缘菌饼;在海南大学儋州校区农科基地火龙果种植园选取健康的火龙果植株,用无菌刀片在茎上划出5 mm左右伤口,将菌饼转移至伤口处,并用保鲜膜包裹以保持湿度[(28±2)℃,RH80%]。3 d后观察发病情况并拍照。
-
当HygB浓度为50 μg·mL−1时该菌完全不能生长(图1),故选取该浓度为火龙果溃疡病菌对HygB的最低耐受浓度,用于后续实验中转化子的筛选。
-
当农杆菌浓度OD600为0.5时遗传转化效率最高,与其他处理差异显著(图2-A);当分生孢子萌发时间为24 h时遗传转化效率最高,与萌发时间为36 h时差异不显著,而与其他处理差异显著(图2-B),但分生孢子萌发36 h实验周期较长,且菌丝萌发造成实验操作难度加大,故选取分生孢子萌发24 h作为最优;当共培养温度为25 ℃时遗传转化效率最高,且与其他处理差异显著(图2-C)。通过以上研究,农杆菌浓度OD600 =0.5、分生孢子萌发时间24 h、共培养温度25℃时遗传转化效率最高,达到833个转化子/106个分生孢子。
-
提取转化子基因组DNA,通过PCR的方法扩增T-DNA片段上HygB抗性基因,结果显示,8株转化子均能扩增出HygB抗性基因片段(880 bp),初步可以确定T-DNA成功插入以上转化子基因组中(图3)。
-
野生型菌株气生菌丝旺盛,直立,后期颜色为灰黑色;通过与野生型菌落形态比较,发现转化子0-3-1和3-3-1菌落形态差异较大,气生菌丝浓密,呈白色,且转化子3-3-1不产生分生孢子;转化子24-3-2气生菌丝稀疏,颜色变淡(图4-A)。转化子与野生型菌株产孢能力比较,发现4株产孢量减少的菌株分别为3-1-2、3-1-3、3-2-3和3-7-1,其中,3-1-3、3-2-3与野生型差异显著(P<0.05), 3-1-2、3-7-1与野生型差异极显著(P<0.01);发现5株产孢量增加的菌株分别为3-6-2、3-4-1、3-4-2、3-3-1和0-3-1,其中,0-3-1与野生型差异显著(P<0.05),其余4株与野生型差异极显著(P<0.01)(图4-B)。通过接种实验,发现6株致病力下降的菌株分别为0-3、3-3-1、3-6-1、3-7-1、24-2-1、24-3-2(图4-C)。以上转化子可用于后续实验。
-
据文献报道,影响根癌农杆菌介导丝状真菌遗传转化效率的因素有很多,比如根癌农杆菌菌株的选择、农杆菌浓度、真菌分生孢子浓度、共培养时间及共培养温度等[12]。有研究表明,不同根癌农杆菌菌株对遗传转化效率的影响很大,已报道的用于丝状真菌遗传转化的农杆菌菌株有AGL-1、EHA105、LBA1100、LBA4404 等,本研究选用EHA105菌株,实验证实可以成功转化。乙酰丁香酮(As)是该体系能否转化成功的决定性因素,根据文献报道,多数丝状真菌在As浓度为200 μmol·L−1时遗传转化效率最高[18],本研究中As选用该浓度。转化受体的细胞浓度对遗传转化效率亦有影响,受体孢子浓度较高,转化效率也较高,本研究中发现分生孢子浓度过高会导致真菌过量生长而无法挑出转化子,设置分生孢子浓度为5×105可以获得较高的转化效率。据文献报道,共培养时间以48 h转化效率最高[19],共培养时间过长反而会导致遗传转化效率降低,本研究选取共培养48 h。
本实验主要研究了农杆菌浓度、分生孢子萌发时间及共培养温度对遗传转化效率的影响。理论上,农杆菌浓度越高,受体细胞被成功转化的概率越高,遗传转化效率越高,然而事实上,当农杆菌浓度过高时遗传转化效率反而降低,本研究中,当农杆菌OD600为0.5时火龙果溃疡病菌的遗传转化效率最高,高于0.5则遗传转化效率降低。有研究表明,未萌发的分生孢子作为受体不能够成功转化[14, 20]。本研究发现,当分生孢子萌发24 h时遗传转化效率最高,证实分生孢子受体的形态对火龙果溃疡病菌的遗传转化效率影响很大。研究者对20 ~37 ℃内农杆菌的转化效率进行研究,发现22~25 ℃是最适的共培养温度[21]。本研究亦发现,25 ℃是火龙果溃疡病菌遗传转化的最适共培养温度。
通过农杆菌介导的遗传转化构建病原真菌的T-DNA 随机插入突变体库,从中筛选致病力降低的突变体,并获得T-DNA侧翼序列,从而获得致病相关基因,这是目前研究病原真菌致病机制的主要方法之一。本研究通过建立的根癌农杆菌介导的遗传转化体系,获得T-DNA插入转化子,并从中筛选致病力降低的转化子,这些转化子可用于后续研究中,以获得致病相关的基因,通过致病相关基因功能研究,以阐明火龙果溃疡病菌的致病机理。
Establishment of Agrobacterium tumefaciens-mediated transformation system for Neoscytalidium dimidiatum and Screening of Transformants
doi: 10.15886/j.cnki.rdswxb.2022.03.006
- Received Date: 2021-06-24
- Accepted Date: 2021-12-28
- Rev Recd Date: 2021-11-15
- Available Online: 2022-03-21
- Publish Date: 2022-05-23
-
Key words:
- pitaya stem canker /
- Agrobacterium tumefaciens-mediation /
- genetic transformation /
- screening
Abstract: Pitaya is a nutrient-rich fruit with high economic value and great development potential in tropical and subtropical region. Pitaya stem canker caused by Neoscytalidium dimidiatum is one of the most serious fungal diseases infecting pitaya, which has become a main factor restricting the development of pitaya. In order to control the disease effectively, it is necessary to study the pathogenic mechanism of the pathogen causing pitaya stem canker. Agrobacterium tumefaciens-mediated genetic transformation was hence used to establish a genetic transformation system for N. dimidiatum. The effects of three main factors, concentration of A. tumefaciens, germination time of conidia, and co-culture temperature, on the transformation efficiency were analyzed, and transformants were screened. The results showed that the genetic transformation efficiency of N. dimidiatum was the highest, as high as 833 transformants / 106 conidia, when the OD600 of A. tumefaciens was 0.5, the germination time of conidia was 24 h, and the co-culture temperature was 25 ℃. Two hundred transformants were randomly selected from the genetic transformation for screening, of which 3 transformants with different colony morphology, 4 with decreased spore production, 5 with increased spore production, and 6 with decreased pathogenicity were generated.
| Citation: | YU Weiwei, AN Xinyuan, CHEN Junjing. Establishment of Agrobacterium tumefaciens-mediated transformation system for Neoscytalidium dimidiatum and Screening of Transformants[J]. Journal of Tropical Biology, 2022, 13(3): 243-248. doi: 10.15886/j.cnki.rdswxb.2022.03.006 |








 DownLoad:
DownLoad: