-
蚊子是登革热、登革出血热、疟疾、基孔肯雅热、寨卡热、西尼罗河热、流行性乙型脑炎和黄热病等可怕疾病的媒介。2015年,仅疟疾就在全球造成了43.8万人死亡;登革热也是一种可怕的由蚊子传播的疾病,每年在全世界造成2万多人死亡;由于气候变化、全球化和病毒进化,登革热和其他蚊媒疾病的发病率每年都在上升。中国疾病预防控制中心统计结果表明,2000—2011年,我国登革热发病较多的省份是广东、海南和福建,就全国范围而言,登革热发病率不高;2011年后登革热发病率迅速上升,2014年出现大爆发,发病人数达到46 864例;2015—2018年的发病率呈平稳波动[1],2019年又出现明显上升,发病人数达到22 599例,是近20年来的第二个发病高峰[2]。由于迄今尚无有效的药物能治疗登革热,也没有安全有效的疫苗,因此,多数国家釆用控制登革热传播媒介的方法来进行登革热的防治。在我国,登革热主要以白纹伊蚊(Aedes albopictus)和埃及伊蚊(Aedes aegypti)为传播媒介。目前对于这2种蚊虫的防控,主要采用杀虫剂。杀虫剂的使用,一方面会对环境造成不可逆的污染,另一方面,蚊虫对化学药剂的耐受性也在不断增加。因此,迫切需要一种对环境友好的技术来控制埃及伊蚊和白纹伊蚊。RNAi是由小分子RNA引起目标基因表达沉默的现象,在农业上,被广泛用以沉默害虫发育关键基因,以达到防治农业害虫的目的。饲喂产生RNAi效应的整个过程包括取食、dsRNA从中肠内壁细胞吸收进入中肠细胞(环境RNAi效应)穿越过中肠向其他组织扩散传递(系统RNAi效应)。
蚊幼虫一般以水体中绿藻门中个体较小的微藻为食物,如衣藻、小球藻、栅藻、团藻等。在动物体内,色氨酸代谢的主要途径是:色氨酸分解产生犬尿氨酸(Kynurenine,KYN),KYN继续分解代谢产生3-羟基犬尿氨酸(3-HK)[3]。3-HK在动物体内参与氧化应激反应,会立刻氧化生成大量的活性氧,具有神经毒性。体外实验结果表明,向红尾粪麻蝇注射1 μmol·L−1的3-HK可以致使其神经麻痹而死亡。羟基犬尿氨酸转氨酶(3-hydroxykynurenine transaminase,3HKT))可以催化3-HK产生无毒中间体黄尿烯酸,具有解毒的作用[4],所以如果将3HKT基因沉默,可能导致伊蚊的死亡。在利用微藻控制媒介昆虫方面,KUMAR等[5]利用按蚊3HKT RNAi转基因衣藻控制冈比亚按蚊(Anopheles gambiae),取得一定效果。本研究设计针对伊蚊3HKT基因的RNAi序列片段,克隆、连接到中间载体T282上,得到反向重复序列,最终连接到衣藻特异的RNAi表达载体pMaa7IR/XIR上。将含有3HKT基因反向重复序列的RNAi表达载体通过玻璃珠法分别转化衣藻,利用巴龙霉素的选择和酶切鉴定最终得到含有目的基因反向重复序列的工程藻株。将上述得到的转基因工程藻株,喂养埃及伊蚊幼虫,观察统计幼虫的生长、发育、死亡数据。本研究旨在为生物杀蚊,阻断登革热、寨卡病毒病等恶性传染病的流行提供新思路。
-
莱茵衣藻(Chlamydomonas reinharditii CC425)为细胞壁缺失的藻株,购自中国科学院淡水藻种库。藻株接种在TAP固体培养基上和TAP液体培养基中培养。培养条件:温度25 ℃,摇床转速220 r·min−1,光照强度为110 μmol·m−2·s−1,24 h全天光照。RNAi干涉载体ppMaa7IR/XIR、中间载体T282由本实验室保存。其他试剂盒转化菌株购自上海生工生物工程有限公司、大连宝生物有限公司等。
-
莱茵衣藻CC425在110 μmol·(m−2·s−1)、25 ℃、24 h全天光照条件下培养,待藻株生长到对数生长中期,收集100 mL藻液,12 000 r·min−1离心3 min,去上清液,取底部藻体,液氮速冻。利用大连宝生物Trizol植物RNA提取试剂盒抽提衣藻总RNA,并利用大连宝生物公司cDNA合成试剂盒将RNA反转录合成cDNA待用[6]。
-
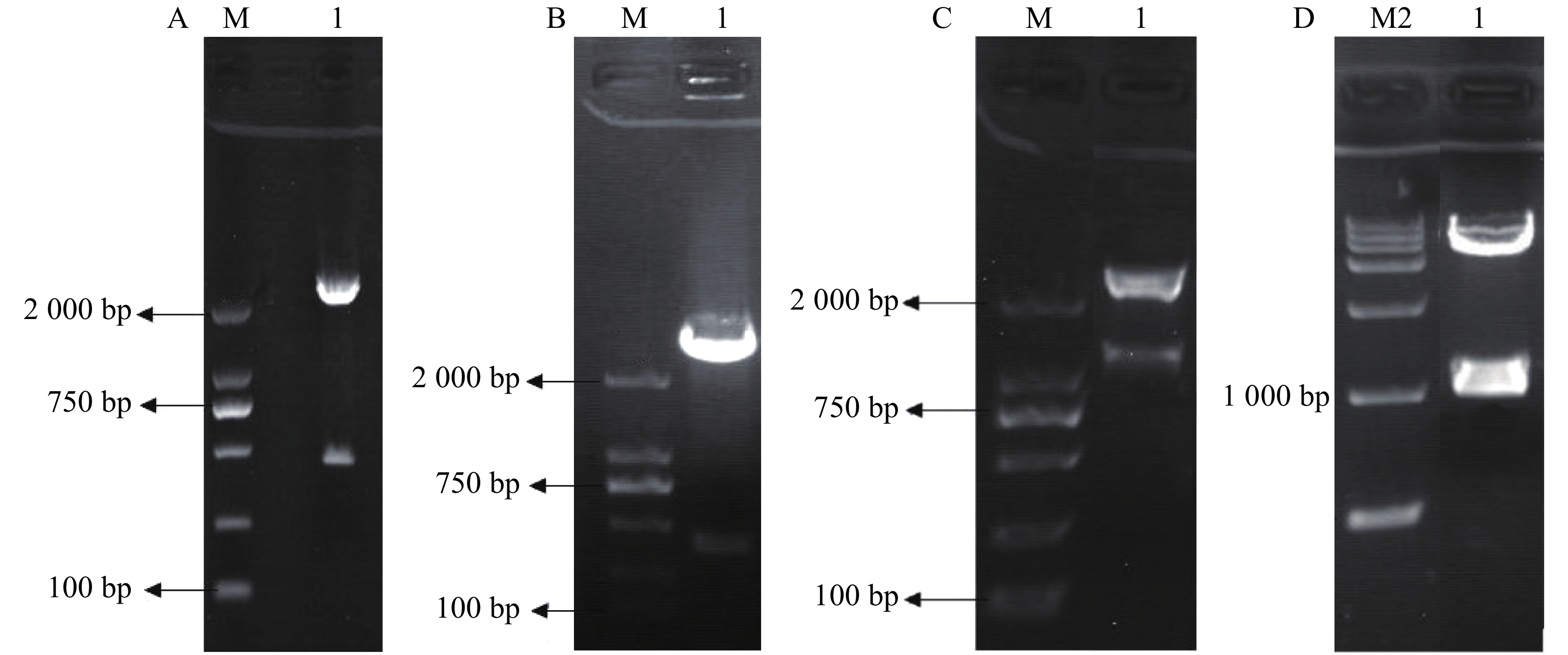
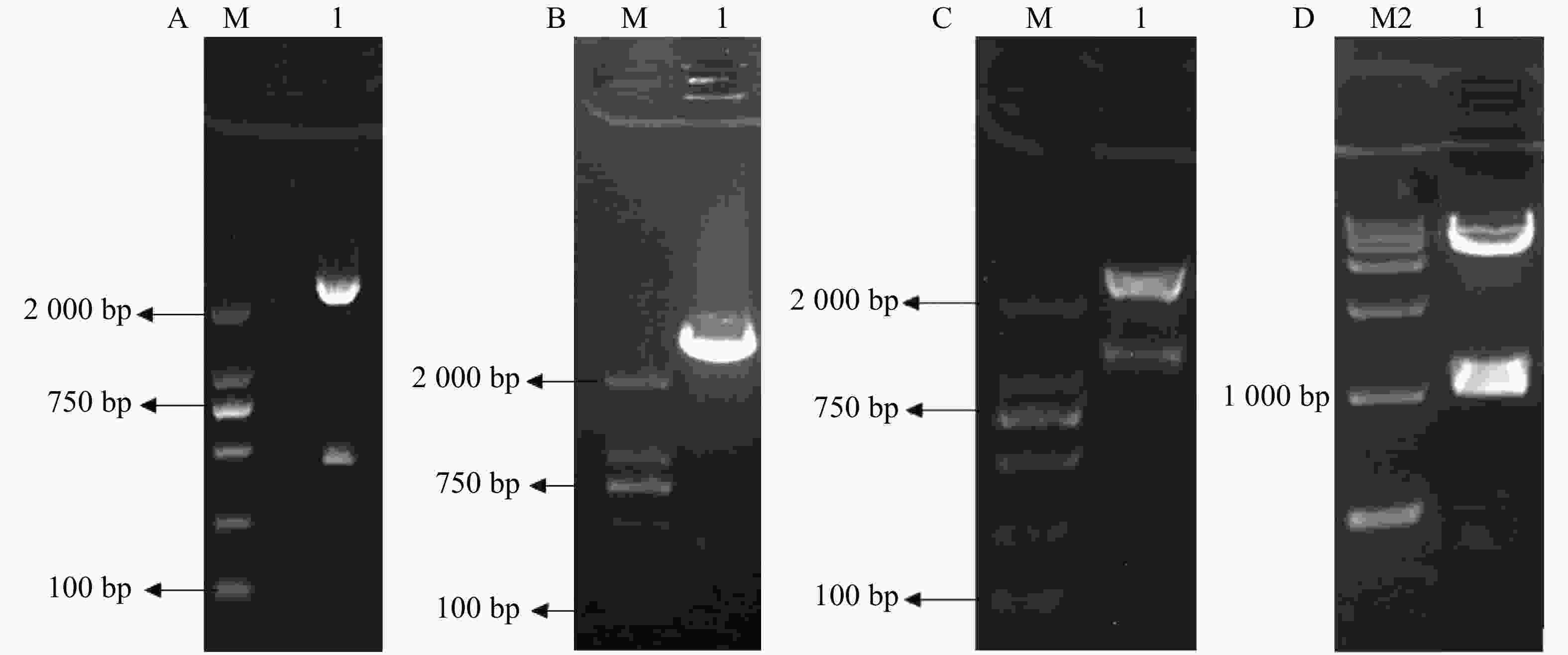
根据genebank上公布的埃及伊蚊3HKT 部分编码区序列,通过上海生工生物有限公司合成430 bp序列。产物连接到pMD18-T载体上,转化大肠杆菌DH5α,挑取单菌落,以上海生工工程有限公司质粒小量试剂盒提取质粒,随后进行酶切分析,得到载体pMD-3HKT。以Hind Ⅲ和BamH Ⅰ分别酶切质粒T282和pMD-3HKT,回收3HKT正向片段,与T282连接,转化大肠杆菌,以BamH Ⅰ和Hind Ⅲ酶切鉴定重组质粒。以SalⅠ和XbaⅠ酶切上述重组质粒,并与反向3HKT片段连接,转化大肠杆菌,SalⅠ和XbaⅠ酶切鉴定,得到含3HKT回文序列的中间载体,并克隆进pMaa7IR/XIR中,得到干涉载体pMaa7IR/3HKTIR。
-
将1 µg重组质粒pMaa7IR/3HKT IR、500 µL藻液、50 μL PEG8000、400 mg玻璃珠在涡旋振荡器上振荡共混30 s。将混合液体转移到10 mL离心管,温培养12 h,然后将细胞均匀涂布在添加色氨酸和巴龙霉素的TAP培养基上培养5~6 d,待藻落长出后,进行后续检测和伊蚊的喂食实验[7]。
-
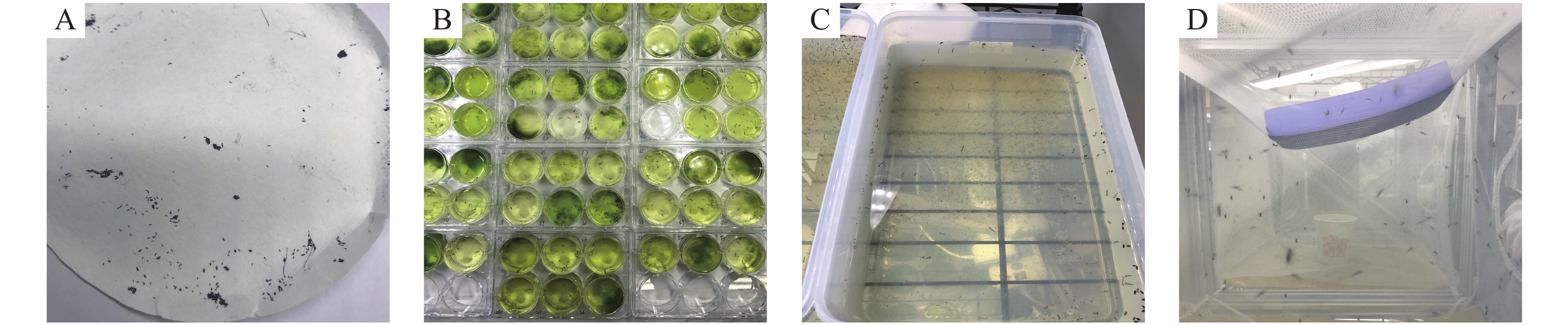
在温度26 ℃湿度75%的培养箱内将清水加入大培养皿中,添加少量饲料,将产有蚊卵的滤纸放置水中,光照2 h后,就可以观察到孵化的幼虫。幼虫成蛹后,将蛹吸出放入伊蚊成蚊饲养笼中,待成蚊羽化后以蔗糖水喂食成蚊。将Maa7IR/3HKT IR转基因工程藻株在50 mL TAP培养液中振荡培养到对数生长中期,每天取出5 mL喂食伊蚊幼虫。记录幼虫的长度、存活数量等。
-
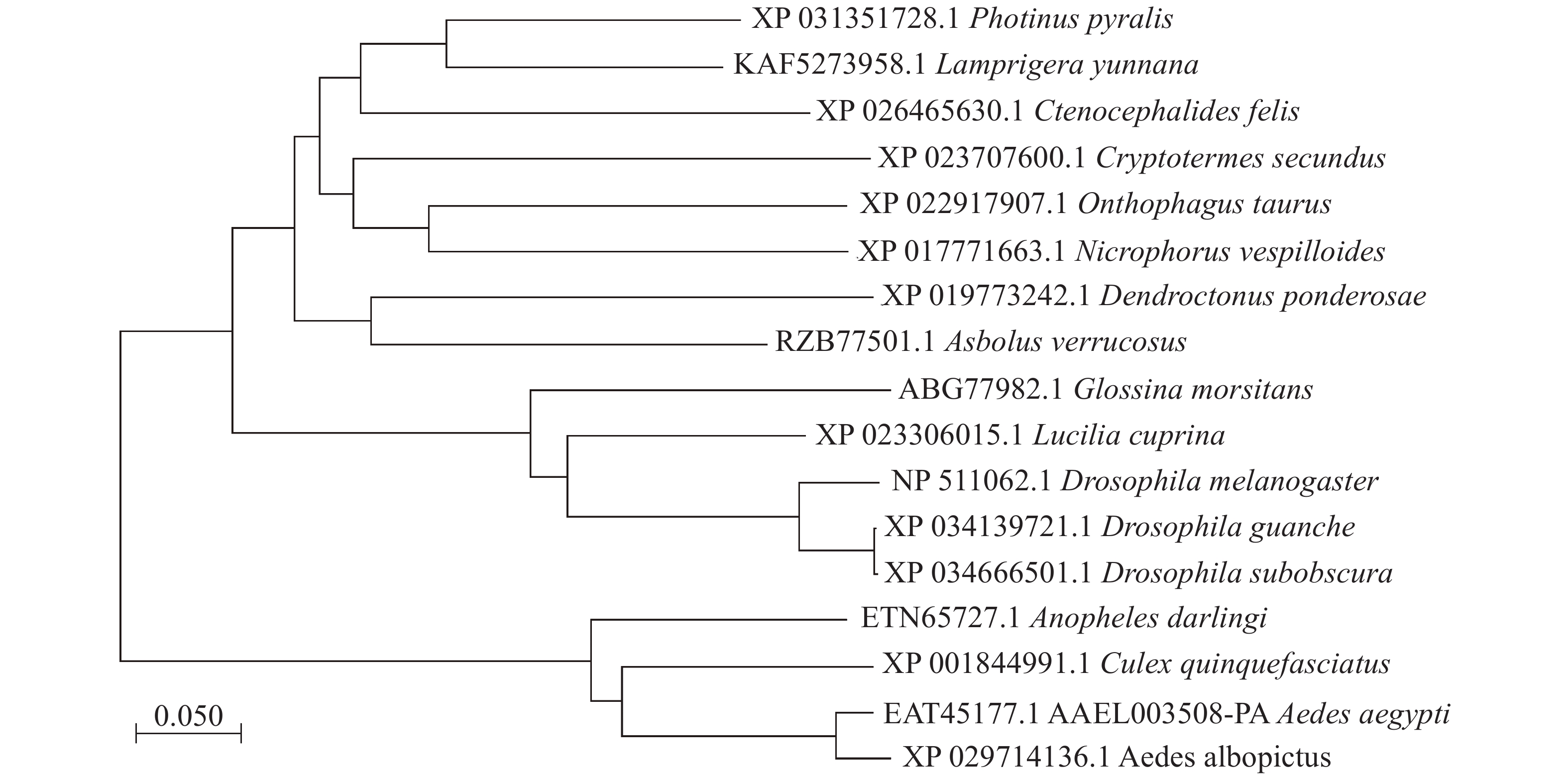
本实验的埃及伊蚊3HKT基因全长1 167 bp(GenBank登录号:XM_021849682, 蛋白EAT45177.1, AAEL003508-PA),其开放阅读框为1 203 bp,编码400个氨基酸残基,5′端非编码区长度为455 bp,3′端非编码区长度为143 bp。3HKT含有AGAT(丙氨酸乙醛转氨酶)家族保守区序列。聚类分析显示[8-9]:埃及伊蚊3HKT与白纹伊蚊(Aedes albopictus)、致倦库蚊(Culex quinquefasciatus)、达氏按蚊(Anopheles darlingi)3HKT遗传距离较近,其次是果蝇,距离较远的是萤火虫(Photinus pyralis)和扁萤虫(Lamprigera yunnana)(图1)。
3HKT RNAi正向和反向片段的获得:根据GenBank上埃及伊蚊3HKT部分编码区序列通过上海生工生物有限公司合成干涉片段,克隆进pMD18-T后,得到pMD-3HKT。测序结果表明,克隆的3HKT基因片段与埃及伊蚊3HKT基因(GenBank序列号: XM_021849682)序列同源性为100%。通过HindⅢ、BamHⅠ双酶切,将此469 bp 3HKT基因正向片段从质粒pMD-3HKT上切下来,得到3HKT基因正向片段。同样以SalⅠ、XbaⅠ将反向片段从pMD-3HKT切下来(图2-A,B)。将正向片段与质粒T282连接,获得重组质粒。在此基础上连接反向片段,得到含有正向和反向重复片段约1 200 bp的中间载体T282-3HKT (图2-C)。在此基础上,将中间载体T282-3HKT中约1 100 bp的重复序列克隆进pMaa7 IR/XIR,得到RNA表达载体pMaa7 IR/3HKTIR (图2-D)。
-
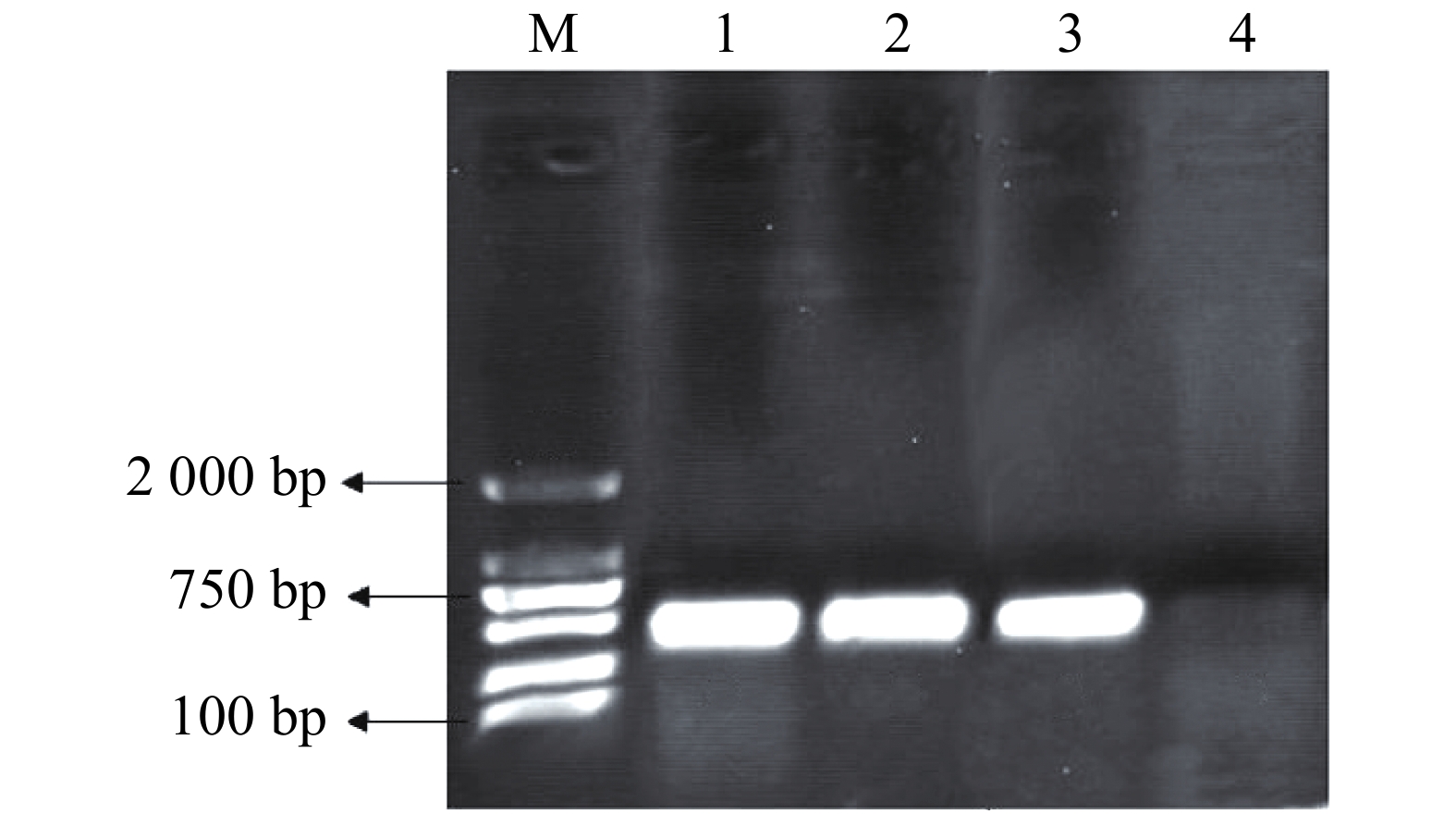
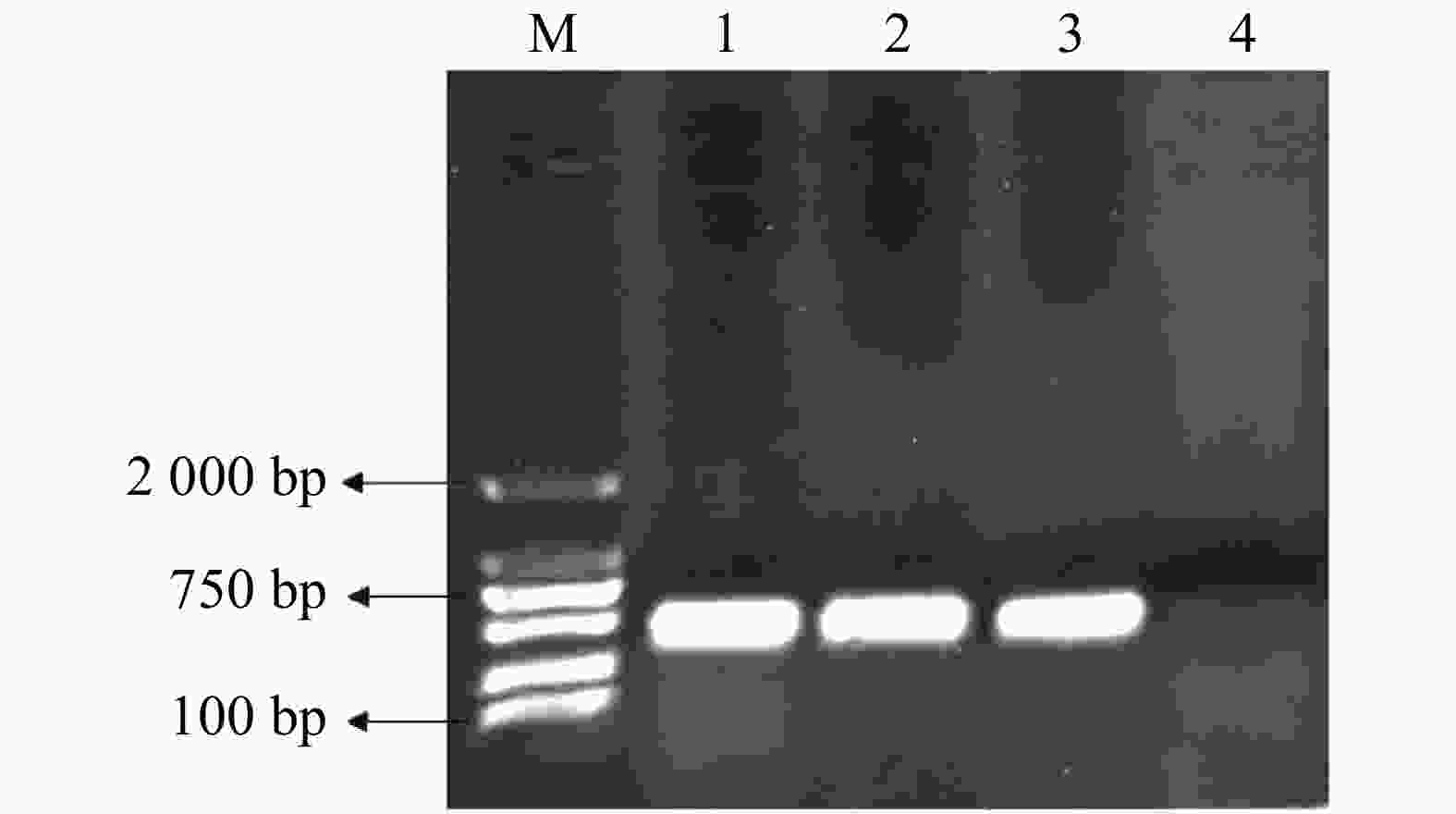
pMaa7IR/3HKTIR RNAi表达载体转化莱茵衣藻后,转基因藻株涂布于抗性TAP培养基平板上进行筛选,共得到85株3HKT RNAi转基因藻株。提取转基因藻株DNA,并对转基因藻株进行PCR扩增,鉴定是否含有插入片段,结果(图3)表明,85株3HKT RNAi转化藻株中36株呈阳性。
-
对照组幼虫喂食饲料(鼠粮)、清水、非转基因衣藻CC425、转pMaa7IR/XIR衣藻;处理组幼虫喂食转pMaa7IR/3HKTIR 衣藻(每组10只幼虫,重复3次试验),化蛹后移入蚊笼,以10%糖水喂养成蚊(图4)。在相同喂食条件下,与喂食饲料、清水、非转基因衣藻CC425、转pMaa7IR/XIR载体衣藻(Maa7)对照组相比,喂食转pMaa7IR/3HKTIR衣藻藻株3HKT-1、3HKT-2、3HKT-5、3HKT-6、3HKT-8、3HKT-11、3HKT-16、3HKT-19的伊蚊幼虫在第2天开始有幼虫死亡。死亡率分别是(30±2)%,(80±5)%,(90±4)%,100%,(80±3)%,(80±3)%,(40±2)%,(70±3)%。喂食饲料、清水、非转基因藻株CC425和转空白载体pMaa7IR/XIR藻株的死亡率为0%。喂食转基因藻株完全死亡的时间分别是:3HKT-1 第3天,3HKT-2 第5天,3HKT-5第4天,3HKT-6第2天,3HKT-8第4天,3HKT-11第7天,3HKT-16第57天,3HKT-19第4天。喂食转空白载体pMaa7IR/XIR的藻株Maa7-2的死亡率为(20±2)%(表1)。结果表明,3HKT RNAi转基因藻株对伊蚊有较强的致死作用。
时间
Time清水
Water鼠粮
FodderCC425 Maa7 3HKT-1 3HKT-2 3HKT-5 3HKT-6 3HKT-8 3HKT-11 3HKT-16 3HKT-19 第1天 0 0 0 0 0 0 0 0 0 0 0 0 第2天 0 0 0 0 (30±2)% (80±5)% (90±4)% 100% (80±3)% (80±3)% (40±2)% (70±3)% 第3天 0 0 0 0 100% (80±5)% (90±4)% 100% (90±5)% (80±3)% (60±4)% (90±4)% 第4天 0 0 0 0 100% (90±6)% 100% 100% 100% (80±3)% (60±4)% (90±4)% 第5天 0 0 0 (20±2)% 100% 100% 100% 100% 100% (80±3)% (70±5)% 100% 第6天 0 0 0 (20±2)% 100% 100% 100% 100% 100% (80±3)% (80±5)% 100% 第7天 0 0 0 (20±2)% 100% 100% 100% 100% 100% 100% 100% 100% 第8天 0 0 0 (20±2)% 100% 100% 100% 100% 100% 100% 100% 100% 第9天 0 0 0 (20±2)% 100% 100% 100% 100% 100% 100% 100% 100% 第10天 0 0 0 (20±2)% 100% 100% 100% 100% 100% 100% 100% 100% 注:CC425:以无转基因的衣藻CC425藻株喂养的伊蚊;Maa7:用pMaa7IR/XIR转基因藻株喂养的伊蚊 3HKT-1~3HKT-19:以pMaa7IR/3HKTIR转基因藻株喂养的伊蚊。
Note: CC425: Aedes aegypti fed with the non-transgenic Chlamydomonas CC425; Maa7: Aedes aegypti fed with pMaa7IR/XIR transgenic algae strain; 3HKT-1~3HKT-19: Aedes aegypti fed with the pMaa7IR/3HKTIR transgenic algae strain.Table 1. Mortality rate of Aedes aegypti fed with the pMaa7IR / 3HKTIR transgenic algae strain
-
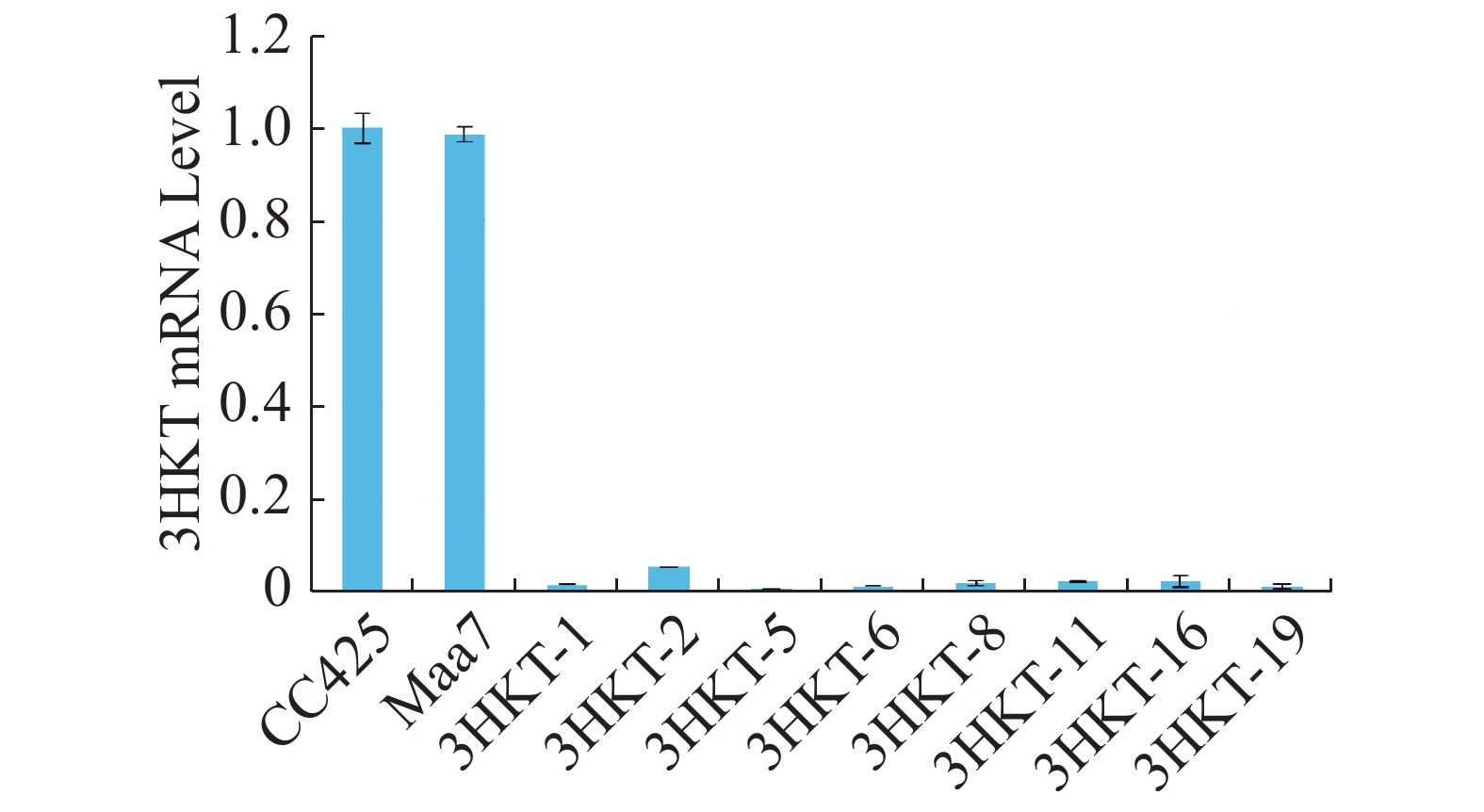
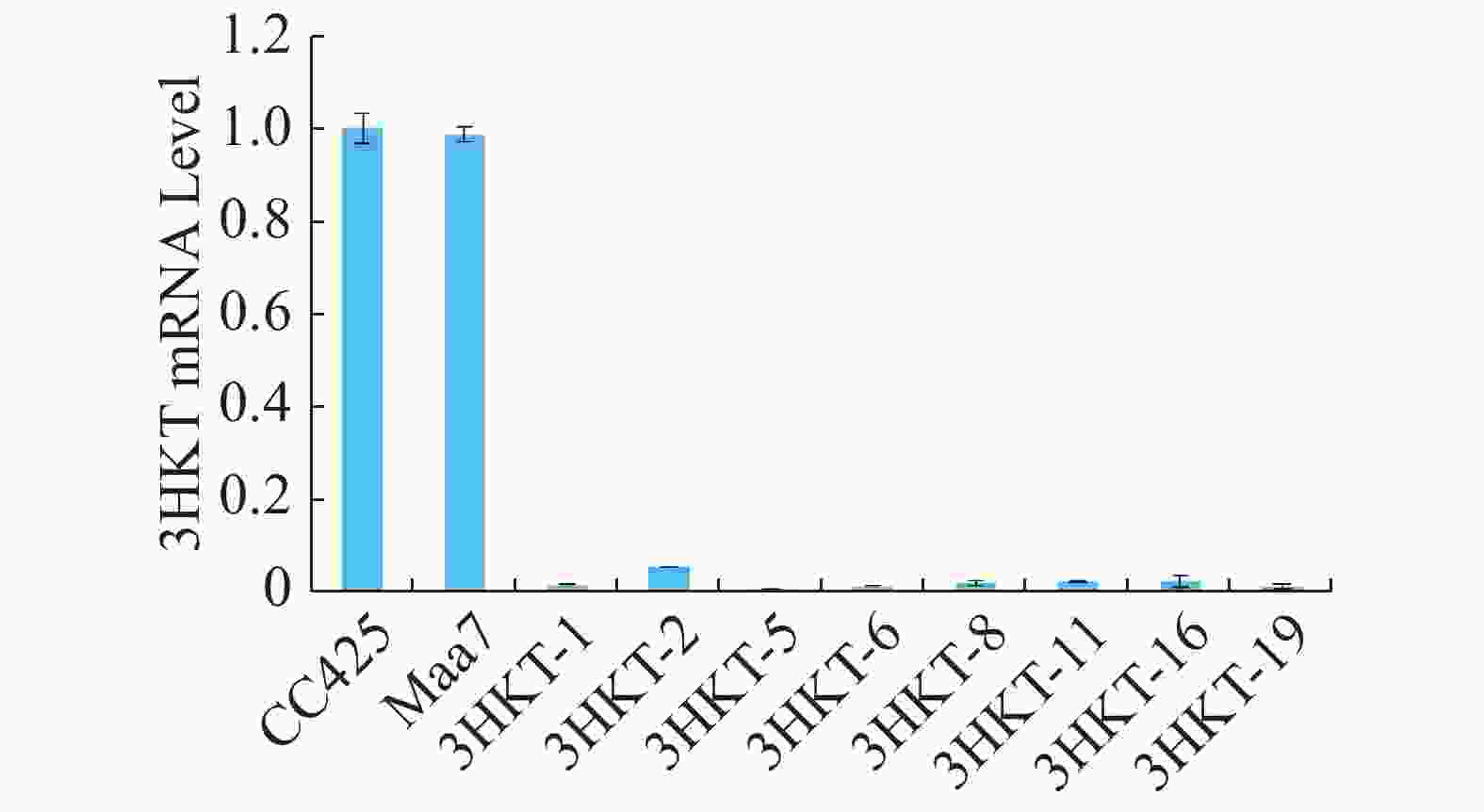
为了检测埃及伊蚊在喂食转基因工程藻后其体内3HKT基因表达是否降低,在相同的饲养条件下,在幼虫孵育的第4天,以饲喂莱茵衣藻CC425伊蚊为对照,检测饲喂pMaa7IR/3HKTIR转基因工程藻株伊蚊幼虫3HKT表达变化情况。Real time PCR检测结果(图5)表明,饲喂pMaa7IR/3HKTIR转基因工程藻株(3HKT-1、3HKT-2、3HKT-5、3HKT-6、3HKT-8、3HKT-11、3HKT-16、3HKT-19)伊蚊幼虫和饲喂CC425对照相比,它们的3HKT基因表达量有很明显的降低。饲喂3HKT-5伊蚊表达量下降最多,与对照相比下降了99.25%;其次是饲喂3HKT-19和3HKT-6伊蚊,它们的RNA表达量较对照分别下降98.66%和98.64%。结果表明,实验所选取的工程藻株均能有效沉默伊蚊体内的3HKT基因。
-
近年来,蚊媒传病严重危害全世界人类的生命健康,成为巨大的公共卫生危害,其中,伊蚊在疾病传播扮演中起十分重要的作用,例如寨卡病毒病、登革热、基孔肯雅病、黄热病等都是由伊蚊传播的。因此,需要一种有效的手段来防控伊蚊,从而达到控制疾病传播的目的。
化学杀虫剂为目前蚊虫控制的主要工具,但是,对化学杀虫剂的严重依赖又造成了很多问题,如蚊虫抗药性问题、杀虫剂对环境和人类健康造成的影响。RNAi干涉是近年生化分子生物学领域引入的一种防治害虫的新手段。昆虫具有运行RNAi机制的基本成分,在不同的昆虫组目中有许多成功的RNAi实例[10]。由于RNAi能够下调昆虫的靶基因,因此提出了将其作为控制昆虫种群的新工具[11-12]。在蚊虫研究方面,RNAi已被广泛应用于蚊子基因功能和蚊子与病原体相互作用的研究[13]。在利用RNAi干涉防治蚊虫方面,目前主要采用注射、浸泡、纳米颗粒和微生物等几种方法将dsRNA传递到蚊子幼虫中[13-15]。RNAi分子(dsRNA、miRNA、shRNA)可以通过浸泡、注射、纳米颗粒包裹或渗透处理(脱水化和再水化)进入蚊子幼虫细胞[16-22]。另一方面,利用微生物(细菌、酵母、藻类)传递dsRNA、miRNA、shRNA也有成功案例[5,23-24];Kumar等[5]利用按蚊3HKT RNAi转基因衣藻控制冈比亚按蚊(Anopheles gambiae),转基因藻株致死率平均为33%左右,最高致死率为53%;本研究转基因藻株对伊蚊的致死率为100%。产生不同效果的原因主要是针对的物种差异,以及选取的RNAi沉默片段的差异。
本研究通过向埃及伊蚊幼虫天然食物衣藻转化伊蚊3HKT RNAi表达载体,结果表明,pMaa7IR/3HKTIR转基因藻株对伊蚊幼虫的成活率、化蛹时间和数量、成虫羽化时间和数量均存在较大的影响。3HKT RNAi转基因藻株对伊蚊有一定的致死作用。由于微藻是蚊子幼虫天然食物,在自然界广泛存在。目前,小球藻、螺旋藻、衣藻等绿藻门微藻均可进行工厂化大规模生产,相对于转基因伊蚊和沃尔巴克氏体细菌感染伊蚊,生产成本低廉、技术成熟,可以长期大规模投放在封闭区域,形成优势藻种,逐步消除该地区的伊蚊,为生物杀虫阻断登革热、寨卡病毒病、黄热病等恶性传染病的流行提供新的思路。
Construction of RNAi Vector of 3HKT Gene and Its Lethal Effect on Aedes aegypti
DOI: 10.15886/j.cnki.rdswxb.2021.03.012
- Received Date: 2020-09-01
- Rev Recd Date: 2021-02-19
- Available Online: 2021-09-02
- Publish Date: 2021-10-11
-
Key words:
- Aedes aegypti /
- Chlamydomonas reinharditii /
- 3HKT gene /
- RNAi
Abstract: Mosquito-borne infectious diseases are seriously threatening the lives and health of humans all over the world. Among them, Aedes aegypti is a vector insect that spreads dengue fever, Zika virus disease, yellow fever, and chikungunya. Therefore, the control of Aedes aegypti plays an important role in the control and prevention of the aforementioned infectious diseases. In this context, an RNAi expression vector pMaa7IR/3HKTIR of the Aedes aegypti 3HKT gene was constructed and transformed to Chlamydomonas reinhardtii by the glass bead method. The transgenic algal strains were then used to feed Aedes larvae. The results showed the 3HKT RNAi transgenic algae was lethal to A. aegypti larvae. Microalgae are natural food for mosquito larvae and they are widespread in nature. Microalgae, such as Chlorella, Chlamydomonas, etc. can be produced at a large scale in the factory with low cost and placed into closed areas to form dominant algae species. This study provides a new idea for biological mosquito control to prevent the spread of dengue fever, zika virus disease, and other infectious diseases transmitted by mosquitos.
| Citation: | FEI Xiaowen, ZHANG Yang, LI Yajun, DENG Xiaodong. Construction of RNAi Vector of 3HKT Gene and Its Lethal Effect on Aedes aegypti[J]. Journal of Tropical Biology, 2021, 12(3): 356-362. doi: 10.15886/j.cnki.rdswxb.2021.03.012 |






 DownLoad:
DownLoad: