-
光叶巴豆(Croton laevigatus Vahl)为大戟科(Euphorbiaceae)巴豆属(Croton)植物,广泛分布于我国的广东、海南、云南等地[1],全年均可采摘,其根、叶可入药,在中医学中光叶巴豆是治疗胃痛、疟疾、骨折的传统药物[2]。现代植物化学研究表明,光叶巴豆的特征化合物为二萜类化合物,主要包括西松烷型二萜[3]、半日花烷型二萜[4-5]、克罗烷型二萜[6-7]、laevinane型二萜[6, 8]等,其中,西松烷型内酯类二萜laevigatlactones B和克罗烷型二萜laevigatbenzoate对HeLa细胞具有细胞毒活性,其IC50值分别为38.4 µmol·L−1[3]和45.4 µmol·L−1[7];克罗烷型二萜furocrotinsulolide A和3,4,15,16-diepoxy-cleroda-13, 14-diene-12,17-olide对LPS诱导RAW 264.7小鼠巨噬细胞产生NO具有抑制作用,其IC50值分别为(10.4 ± 0.8) µmol·L−1和(96.0 ± 1.0) µmol·L−1[阳性对照(13.1 ± 1.9) µmol·L−1][5];半日花烷型二萜3α-Hydroxy-ent-labda-8,12E,14-triene-18-oic acid和ent-labda-8(17),12E,14-triene-3α,18-diol对蛋白质酪氨酸磷酸酶(PTP1B)具有抑制活性,其IC50值分别为4.11和8.33 µmol·L−1[6]。因此,为进一步探究光叶巴豆的化学成分,笔者对海南产光叶巴豆枝叶的化学成分及生物活性进行研究,旨在为该植物资源的开发利用提供科学依据。
HTML
-
核磁共振波谱仪(德国Bruker AV-500);旋转蒸发仪(德国Heidolph Laborota);Sephadex LH-20(德国Merck);氘代试剂(德国Merck);分析型高效液相色谱仪(美国Agilent Technologies 1260 Infinity);半制备高效液相色谱仪(美国Agilent Technologies 1260 Infinity Ⅱ);半制备色谱柱(C18,250 mm × 10.0 mm,ID)(日本COSMOSIL公司);超纯水装置(上海博讯实业有限公司);薄层色谱硅胶板及柱色谱硅胶(青岛海洋化工厂);色谱级试剂(天津康科德公司);其他试剂均为重蒸工业试剂;顺铂(上海源叶生物科技有限公司);阿维菌素(上海源叶生物科技有限公司);人慢性髓原白血病细胞株K562、人肝癌细胞株BEL-7401、人胃癌细胞株SCG-7901、人肺癌细胞株A549和HeLa人宫颈癌细胞均购自中国科学院上海生命科学研究院细胞库;全齿复活线虫由云南大学莫明和教授提供。
光叶巴豆采自海南省昌江县,经中国热带农业科学院热带生物技术研究所黄圣卓博士鉴定为大戟科(Euphorbiaceae)巴豆属(Croton)植物光叶巴豆(Croton laevigatus)。
-
将干燥的光叶巴豆枝叶样品(4.15 kg)经φ=95%的乙醇常温浸泡提取3次,时间依次为7、5、3 d,合并提取液,过滤,减压浓缩至无醇味,得乙醇提取物。将乙醇提取物分散于水中形成悬浊液,再依次用乙酸乙酯、正丁醇进行萃取,对萃取液进行减压浓缩得到干浸膏,最终获得乙酸乙酯部位(159.0 g)、正丁醇部位(70.0 g)。
乙酸乙酯萃取物(159.0 g)经小孔吸附树脂,以甲醇/水(40%~100%,体积分数)进行梯度洗脱,经薄层色谱检测,合并相同流分后,得到14个组分(Fr.1~Fr.14)。Fr.2 (790.0 mg)经Sephadex LH-20凝胶柱色谱洗脱,洗脱剂为甲醇,得到6个亚组份(Fr.2.1~Fr.2.6),Fr.2.1 (433.7 mg)经硅胶柱色谱,以石油醚−乙酸乙酯(3∶1~2∶1,体积比)为洗脱剂,得到化合物1 (5.0 mg)、化合物2 (4.6 mg)。Fr.3 (500.0 mg)经Sephadex LH-20凝胶柱色谱洗脱,洗脱剂为甲醇,得到2个亚组份Fr.3.1和Fr.3.2,Fr.3.1 (333.1 mg)经硅胶柱色谱,以氯仿−甲醇(250∶1~50∶1,体积比)为洗脱剂,得到化合物3 (7.0 mg)。Fr.4 (4.29 g)经ODS柱色谱,以甲醇/水(40%~100%,体积分数)进行梯度洗脱,得到16个亚组份(Fr.4.1~Fr.4.16),Fr.4.3经Sephadex LH-20凝胶柱色谱洗脱,洗脱剂为甲醇,得到4个流分(Fr.4.3.1~Fr.4.3.4),Fr.4.3.3(98.1 mg)经硅胶柱色谱,以氯仿−甲醇(500∶1~400∶1,体积比)为洗脱剂,得到化合物8 (13.1 mg),Fr.4.3.4 (50.8 mg)经硅胶柱色谱,以石油醚−乙酸乙酯(6∶1~5∶1,体积比)为洗脱剂,得到化合物9(4.1 mg)、12 (1.1 mg)。Fr.4.8(53.9 mg)经硅胶柱色谱,以石油醚−乙酸乙酯(16∶1~8∶1,体积比)为洗脱剂,得到化合物4(1.7 mg)、5 (3.1 mg)。Fr.4.9 (283.6 mg)经硅胶柱色谱,以石油醚−乙酸乙酯(19∶1~16∶1,体积比)为洗脱剂,得到化合物6 (17.3 mg)。Fr.4.12(131.6 mg)经硅胶柱色谱,以石油醚−乙酸乙酯(16∶1~12∶1,体积比)为洗脱剂,得到化合物7 (4.2 mg)。Fr.5 (10.0 g)经ODS柱色谱,以甲醇/水(50%~100%,体积分数)进行梯度洗脱,得到27个亚组份(Fr.5.1-Fr.5.27),Fr.5.2 (73.5 mg)经Sephadex LH-20凝胶柱色谱洗脱,洗脱剂为甲醇,得到2个流分(Fr.5.2.1和Fr.5.2.2),Fr.5.2.1 (17.1 mg)经半制备型高效液相色谱,以C18柱为色谱柱,用甲醇/水(45∶55,体积比)为洗脱剂,得到化合物10 (2.1 mg),Fr.5.3 (252.6 mg)经Sephadex LH-20凝胶柱色谱洗脱,洗脱剂为甲醇,得到5个流分(Fr.5.3.1-Fr.5.3.5),Fr.5.3.5 (22.2 mg)经半制备型高效液相色谱,以C18柱为色谱柱,用甲醇/水(47∶53,体积比)为洗脱剂,得到化合物11 (2.4 mg)。
-
利用贝尔曼漏斗法[9]和MTT法[10]分别测定化合物1~12的全齿复活线虫(Panagrellus redivivus)致死活性和细胞毒活性。
1.1. 仪器与材料
1.2. 提取与分离
1.3. 生物活性测试
-
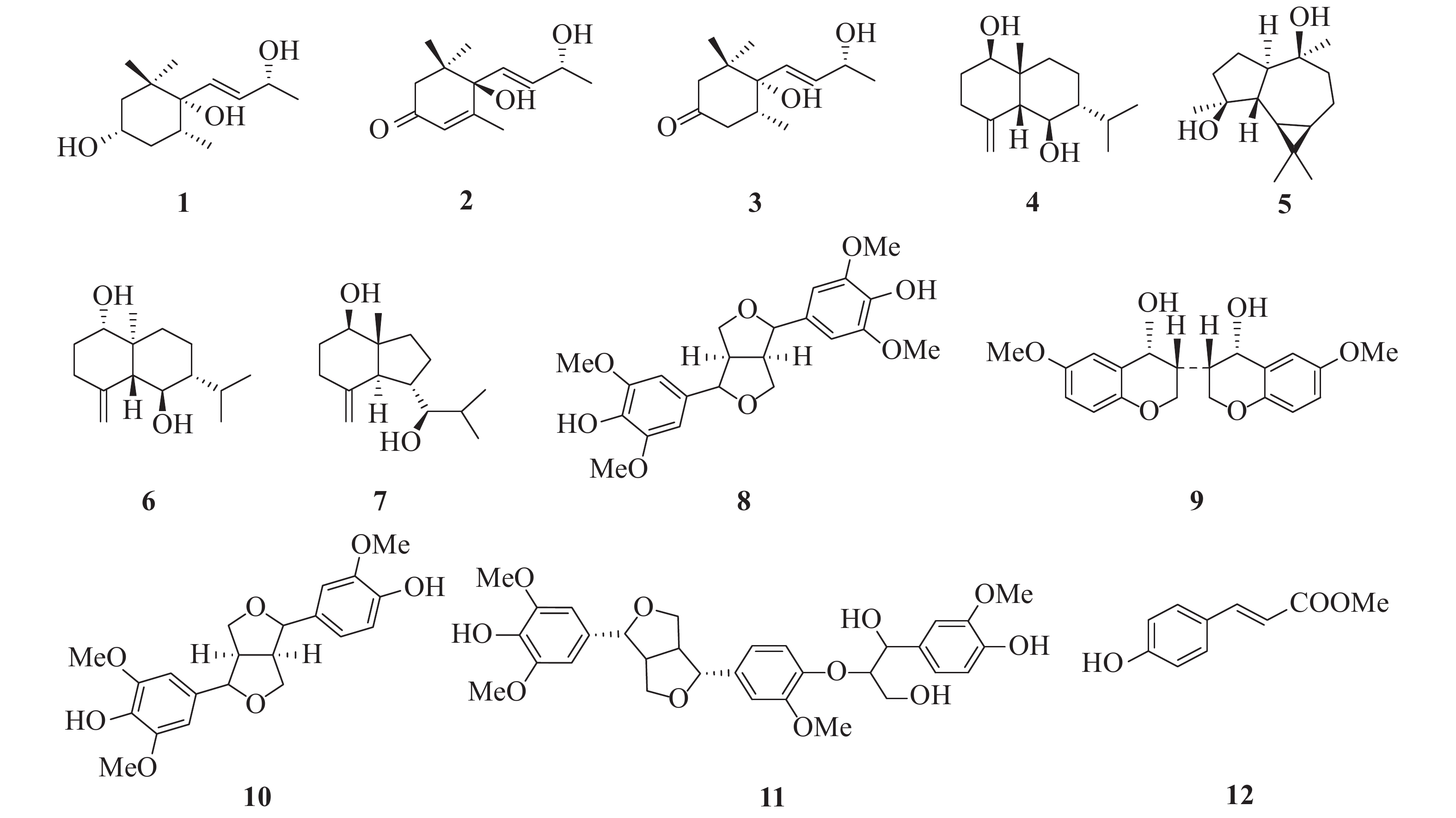
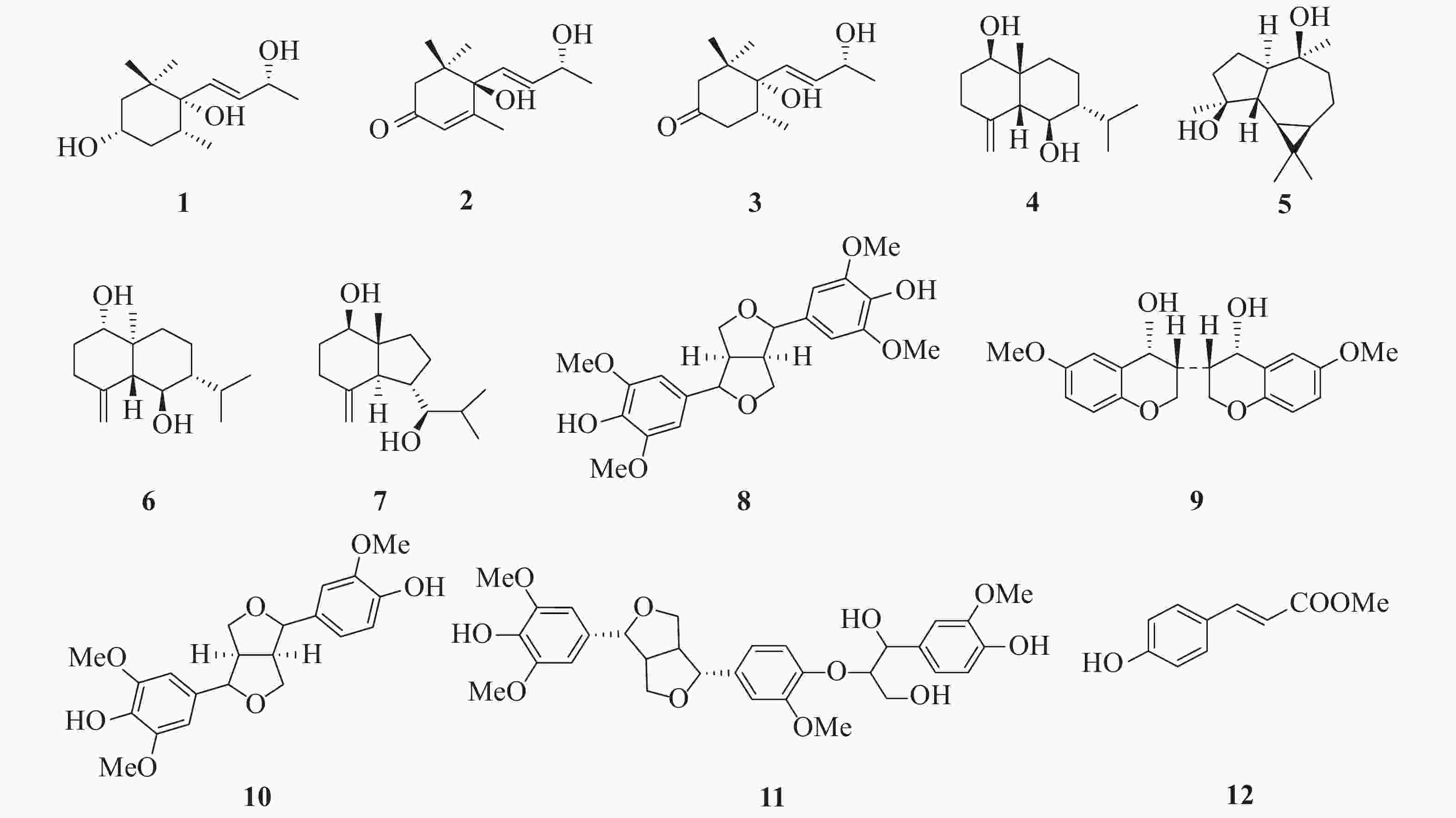
本实验从光叶巴豆中一共分离得到12个化合物,并根据1H NMR、13C NMR和ESI-MS数据鉴定了化合物1~12的结构(图1)
化合物1 无色油状物(CHCl3);ESI-MS:m/z 229 [M+H]+,分子式C13H24O3;1H NMR (CDCl3,500 MHz) δ:5.75 (1H,dd,J = 15.7,6.0 Hz,H-8),5.58 (1H,d,J = 15.7 Hz,H-7),4.39 (1H,t,J = 6.0 Hz,H-9),3.86 (1H,m,H-3),1.93 (1H,m,H-5),1.78 (1H,d,J = 12.0 Hz,H-4a),1.61 (1H,d,J = 12.0 Hz,H-2a),1.52 (1H,m,H-2b),1.35 (1H,d,J = 12.0 Hz,H-4b),1.30 (3H,d,J = 6.0 Hz,H-10),0.96 (3H,s,H-11),0.87 (3H,s,H-12),0.79 (3H,d,J = 6.8 Hz,H-13);13C NMR (CDCl3,125 MHz) δ:39.6 (C-1),45.3 (C-2),66.8 (C-3),39.3 (C-4),34.2 (C-5),77.0 (C-6),133.1 (C-7),134.5 (C-8),68.7 (C-9),24.0 (C-10),25.1 (C-11),24.7 (C-12),16.0 (C-13)。以上波谱数据与文献[11]报道的基本一致,故鉴定为(3S,5R,6S,7E,9R)-3,6-dihydroxy-5,6-dihydro-β-ionol。
化合物2 无色油状物(CHCl3);ESI-MS:m/z 225 [M+H]+,分子式C13H20O3;1H NMR (CDCl3,500 MHz) δ:5.90 (1H,m,H-7),5.85 (1H,m,H-8),5.78 (1H,s,H-4),4.40 (1H,m,H-9),2.44 (1H,d,J = 17.1 Hz,H-2a),2.23 (1H,d,J = 17.1 Hz,H-2b),1.89 (3H,s,H-13),1.29 (3H,d,J = 6.4 Hz,H-10),1.07 (3H,s,H-11),1.00 (3H,s,H-12);13C NMR (CDCl3,125 MHz) δ:41.3 (C-1),49.8 (C-2),198.2 (C-3),127.0 (C-4),163.0 (C-5),79.2 (C-6),129.0 (C-7),135.9 (C-8),68.2 (C-9),24.2 (C-10),23.0 (C-11),23.9 (C-12),19.1 (C-13)。以上波谱数据与文献[12]报道的基本一致,故鉴定为吐叶醇。
化合物3 无色油状物(CHCl3);ESI-MS:m/z 227 [M+H]+,分子式C13H22O3;1H NMR (CDCl3,500 MHz) δ:5.83 (1H,dd,J = 15.8,5.8 Hz,H-8),5.70 (1H,dd,J = 15.8,1.0 Hz,H-7),4.42 (1H,m,H-9),2.83 (1H,d,J = 13.6 Hz,H-2a),2.41 (1H,t,J = 13.0 Hz,H-4a),2.26 (1H,m,H-4b),2.19 (1H,ddd,J = 13.7,4.5,2.2 Hz,H-5),1.90 (1H,dd,J = 13.6,1.9 Hz,H-2b),1.32 (3H,d,J = 6.4 Hz,C-10),0.96 (3H,s,C-12),0.93 (3H,s,C-11),0.88 (3H,d,J = 6.4 Hz,C-13);13C NMR (CDCl3,125 MHz) δ:42.7 (C-1),51.6 (C-2),211.6 (C-3),36.6 (C-4),45.3 (C-5),77.3 (C-6),131.9 (C-7),135.3 (C-8),68.5 (C-9),24.1 (C-10),24.6 (C-11),24.5 (C-12),16.0 (C-13)。以上波谱数据与文献[13]报道的基本一致,故鉴定为4,5-dihydroblumenol A。
化合物4 无色油状物(CHCl3);ESI-MS:m/z 239 [M+H]+,分子式C15H26O2;1H NMR (CDCl3,500 MHz) δ:4.98 (1H,s,H-15b),4.84 (1H,s,H-15a),3.95 (1H,dd,J = 11.7,4.9 Hz,H-1),3.51 (1H,t,J = 10.1 Hz,H-6),2.29 (2H,m,H-3),2.21 (1H,m,H-11),2.06 (1H,dt,J = 14.0,3.2 Hz,H-9b),1.89 (1H,m,H-2a),1.84 (1H,d,J = 10.1 Hz,H-5),1.62 (1H,dd,J = 11.7,5.9 Hz,H-2b),1.47 (1H,m,H-8b),1.29 (1H,m,H-8a),1.26 (1H,m,H-7),1.05 (1H,td,J = 14.0,4.0 Hz,H-9a),0.94 (3H,d,J = 7.1 Hz,H-13),0.87 (3H,s,H-14),0.84 (3H,d,J = 7.1 Hz,H-12);13C NMR (CDCl3,125 MHz) δ:68.3 (C-1),31.2 (C-2),29.9 (C-3),145.6 (C-4),61.8 (C-5),67.3 (C-6),49.2 (C-7),18.2 (C-8),34.5 (C-9),40.2 (C-10),26.6 (C-11),16.4 (C-12),21.1 (C-13),21.5 (C-14),114.4 (C-15)。以上波谱数据与文献[14]报道的基本一致,故鉴定为5-epi-eudesma-4 (15)-ene-1β,6β-diol。
化合物5 淡黄色油状物(CHCl3);ESI-MS:m/z 239 [M+H]+,分子式C15H26O2;1H NMR (CDCl3,500 MHz) δ:1.50~1.90 (8H,m,H-1,2,3,8,9),1.25 (3H,s,H-14),1.21 (1H,m,H-5),1.17 (3H,s,H-15),1.04 (6H,s,H-12,13),0.91 (1H,m,H-8),0.64 (1H,ddd,J = 11.0,9.6,6.2 Hz,H-7),0.43 (1H,dd,J = 11.0,9.6 Hz,H-6);13C NMR (CDCl3,125 MHz) δ:56.5 (C-1),23.9 (C-2),41.3 (C-3),80.5 (C-4),48.6 (C-5),28.4 (C-6),26.8 (C-7),20.3 (C-8),44.6 (C-9),75.2 (C-10),19.7 (C-11),28.8 (C-12),16.6 (C-13),24.7 (C-14),20.5 (C-15)。以上波谱数据与文献[15]报道的基本一致,故鉴定为aromadendrane-4β,10β-diol。
化合物6 无色油状物(CHCl3);ESI-MS:m/z 239 [M+H]+,分子式C15H26O2;1H NMR (CDCl3,500 MHz) δ:5.01 (1H,s,H-15a),4.73 (1H,s,H-15b),3.70 (1H,t,J = 9.8 Hz,H-6),3.41 (1H,dd,J = 11.6,4.7 Hz,H-1),2.32 (1H,ddd,J = 13.2,5.0,2.1 Hz,H-3a),2.23 (1H,m,H-11),2.07 (1H,td,J = 13.2,5.3 Hz,H-3b),1.90 (1H,m,H-9a),1.86 (1H,m,H-2b),1.73 (1H,d,J = 9.8 Hz,H-5),1.55 (1H,m,H-2a),1.51 (1H,m,H-8b),1.29 (1H,m,H-7),1.24 (1H,m,H-8a),1.23 (1H,m,H-9b),0.93 (3H,d,J = 7.0 Hz,H-12),0.85 (3H,d,J = 7.0 Hz,H-13),0.69 (3H,s,H-14),13C NMR (CDCl3,125 MHz) δ:79.1 (C-1),32.0 (C-2),35.2 (C-3),146.3 (C-4),56.0 (C-5),67.2 (C-6),49.4 (C-7),18.2 (C-8),36.4 (C-9),41.8 (C-10),26.1 (C-11),21.2 (C-12),16.3 (C-13),11.7 (C-14),107.9 (C-15)。以上波谱数据与文献[16]报道的基本一致,故鉴定为ent-4 (15)-eudesmene-1β,6α-diol。
化合物7 淡黄色油状物(CHCl3);ESI-MS:m/z 239 [M+H]+,分子式C15H26O2;1H NMR (CDCl3,500 MHz) δ:4.95 (1H,d,J = 1.1 Hz,H-15b),4.81 (1H,d,J = 1.1 Hz,H-15a),3.59 (1H,dd,J = 11.3,4.9 Hz,H-1),3.23 (1H,dd,J = 9.8,2.4 Hz,H-7),2.33 (1H,m,H-6),2.30 (1H,m,H-3b),2.11 (1H,td,J = 13.5,5.7 Hz,H-3a),1.92 (1H,m,H-8b),1.87 (1H,m,H-2a),1.83 (1H,d,J = 10.9 Hz,H-5),1.74 (1H,m,H-9b),1.74 (1H,m,H-11),1.50 (1H,m,H-2b),1.39 (1H,m,H-9a),1.32 (1H,m,H-8a),0.99 (3H,d,J = 6.8 Hz,H-13),0.90 (3H,d,J = 6.9 Hz,H-12),0.66 (3H,s,H-14);13C NMR (CDCl3,125 MHz) δ:79.1 (C-1),32.0 (C-2),35.1 (C-3),149.1 (C-4),56.6 (C-5),39.5 (C-6),82.9 (C-7),26.2 (C-8),37.5 (C-9),49.7 (C-10),31.5 (C-11),14.9 (C-12),20.7 (C-13),12.4 (C-14),107.8 (C-15)。以上波谱数据与文献[17]报道的基本一致,故鉴定为(7R*)-opposit-4 (15)-ene-1β,7-diol。
化合物8 乳白色油状物(CHCl3);ESI-MS:m/z 419 [M+H]+,分子式C22H26O8;1H NMR (CDCl3,500 MHz) δ:6.58 (4H,s,H-2,6,2′,6′),5.53 (2H,s,OH-4,4′),4.73 (2H,d,J = 3.9 Hz,H-7,7′),4.28 (2H,dd,J = 8.8,6.5 Hz,H-9a,9′a),3.92 (2H,m,H-9b,9′b),3.89 (12H,s,OMe-3,5,3′,5′),3.09 (2H,m,H-8,8′);13C NMR (CDCl3,125 MHz) δ:54.5 (C-8,8′),86.1 (C-7,7′),71.9 (C-9,9′),132.1 (C-1,1′),102.8 (C-2,6,2′,6′),147.2 (C-3,5,3′,5′),134.3 (C-4,4′),56.5 (OMe-3,5,3′,5′)。以上波谱数据与文献[18]报道的基本一致,故鉴定为丁香脂素。
化合物9 乳白色油状物(CHCl3);ESI-MS:m/z 739 [2M+Na]+,分子式C20H20O6;1H NMR (CDCl3,500 MHz) δ:6.90 (2H,d,J = 2.1 Hz,H-5,5′),6.89 (2H,d,J = 8.1 Hz,H-8,8′),6.82 (2H,dd,J = 8.1,2.1 Hz,H-7,7′),4.74 (2H,d,J = 4.2 Hz,H-4,4′),4.24 (2H,dt,J = 9.2,6.9 Hz,H-2b,2b′),3.91 (6H,s,OMe-6,6′),3.88 (2H,dd,J = 9.2,3.6 Hz,H-2a,2a′),3.10 (2H,m,H-3,3′);13C NMR (CDCl3,125 MHz) δ:71.8 (C-2,2′),54.3 (C-3,3′),86.0 (C-4,4′),108.7 (C-5,5′),146.8 (C-6,6′),119.1 (C-7,7′),114.4 (C-8,8′),145.4 (C-9,9′),133.1 (C-10,10′),56.1 (OMe-6,6′)。以上波谱数据与文献[19]报道的基本一致,故鉴定为rel- (3R,3′S,4R,4′S)-3,3′,4,4′-tetrahydro-6,6′-dimethoxy[3,3′-bi-2H-benzopyran]-4,4′-diol。
化合物10 无色油状物(CHCl3);ESI-MS:m/z 411 [M+Na]+,分子式C21H24O7;1H NMR (CDCl3,500 MHz) δ:6.92 (2H,m,H-2′,5′),6.82 (1H,dd,J = 8.1,1.8 Hz,H-6′),6.58 (2H,s,H-2,6),5.59 (1H,s,OH-4′),5.49 (1H,s,OH-4),4.75 (1H,d,J = 4.8 Hz,H-7),4.72 (1H,d,J = 4.8 Hz,H-7′),4.26 (2H,m,H-9b,9′b),3.91 (3H,s,OMe-3′),3.90 (6H,s,OMe-3,5),3.88 (2H,m,H-9a,9′a),3.10 (2H,m,H-8,8′);13C NMR (CDCl3,125 MHz) δ:132.3 (C-1),102.8 (C-2,6),147.3 (C-3,5),134.4 (C-4),86.0 (C-7),54.3 (C-8),71.8 (C-9),133.0 (C-1′),108.7 (C-2′),146.8 (C-3′),145.4 (C-4′),114.4 (C-5′),119.1 (C-6′),86.3 (C-7′),54.6 (C-8′),72.0 (C-9′),56.5 (OMe-3,5),56.1 (OMe-3′)。以上波谱数据与文献[20]报道的基本一致,故鉴定为(+)-皮树脂醇。
化合物11 无色油状物(CHCl3);ESI-MS:m/z 583 [M-H]−,分子式C31H36O11;1H NMR (CDCl3,500 MHz) δ:6.96 (1H,d,J = 1.2 Hz,H-2″),6.90 (1H,d,J = 1.5 Hz,H-2′),6.89 (1H,d,J = 8.1 Hz,H-5′),6.85 (1H,d,J = 8.2 Hz,H-5″),6.83 (1H,dd,J = 8.1,1.5 Hz,H-6′),6.74 (1H,dd,J = 8.2,1.2 Hz,H-6″),6.63 (2H,s,H-2,6),4.99 (1H,t,J = 3.9 Hz,H-7″),4.77 (1H,d,J = 4.9 Hz,H-7′),4.75 (1H,d,J = 4.9 Hz,H-7),4.29 (2H,m,H-9a,9′a),4.12 (1H,m,H-8″),3.93 (3H,m,H-9b,9′b,9″a),3.91 (3H,s,OMe-3′),3.90 (6H,s,OMe-3,5),3.89 (3H,s,OMe-3″),3.49 (3H,m,H-9″b),3.12 (2H,m,H-8,8′);13C NMR (CDCl3,125 MHz) δ:138.0 (C-1),102.9 (C-2,6),153.6 (C-3,5),134.4 (C-4),86.2 (C-7),54.7 (C-8),72.3 (C-9),132.9 (C-1′),108.7 (C-2′),146.9 (C-3′),145.5 (C-4′),114.4 (C-5′),119.1 (C-6′),85.9 (C-7′),54.2 (C-8′),71.7 (C-9′),131.4 (C-1″),108.5 (C-2″),146.7 (C-3″),145.0 (C-4″),114.3 (C-5″),118.9 (C-6″),72.7 (C-7″),87.3 (C-8″),60.7 (C-9″),56.4 (OMe-3,5),56.1 (OMe-3′,3″)。以上波谱数据与文献[21]报道的基本一致,故鉴定为thero-ficusesquilignan A。
化合物12 淡黄色油状物(CHCl3);ESI-MS:m/z 179 [M+H]+,分子式C10H10O3;1H NMR (CDCl3,500 MHz) δ:7.64 (1H,d,J = 16.0 Hz,H-7),7.43 (2H,d,J = 8.6 Hz,H-2,6),6.84 (2H,d,J = 8.6 Hz,H-3,5),6.30 (1H,d,J = 16.0 Hz,H-8),3.79 (3H,s,OMe-9);13C NMR (CDCl3,125 MHz) δ:127.1 (C-1),130.1 (C-2,6),116.0 (C-3,5),157.7 (C-4),144.6 (C-7),115.5 (C-8),167.9 (C-9),51.7 (OMe-9)。以上波谱数据与文献[22]报道的基本一致,故鉴定为反式对羟基肉桂酸甲酯。
本研究从光叶巴豆中共分离鉴定了12个化合物,其中,包括降倍半萜类化合物3个,倍半萜类化合物4个及苯丙素类化合物5个,全齿复活线虫致死活性测试结果(表1)表明,倍半萜类化合物1~7对全齿复活线虫的致死活性相对较低或没有,而苯丙素类化合物8~12对全齿复活线虫具有较好的致死活性。所有化合物均无细胞毒活性。
组别
Group全齿复活线虫校正死亡率/%
Corrected mortality of P. redivivus组别
Group全齿复活线虫校正死亡率/%
Corrected mortality of P. redivivus1 14.40 ± 0.04 8 34.45 ± 0.02 2 — 9 52.42 ± 0.08 3 — 10 49.16 ± 0.06 4 — 11 62.91 ± 0.04 5 — 12 91.42 ± 0.01 6 7.71 ± 0.04 阳性对照 100 ± 0.00 7 33.35 ± 0.06 注:阳性对照为阿维菌素 (2.5 g·L−1);化合物质量浓度为2.5 g·L−1;“—”在活性初筛条件下未观测到抑制活性。
Note: Abamectin (2.5 g·L−1) was used as positive control; the mass concentration of the compounds was 2.5 g·L−1; “—”: No inhibitory activity.Table 1. Biological activities of compounds 1-12 against Panagrellus redivivus






 DownLoad:
DownLoad: