-
文心兰(Oncidium hybridum)在栽培过程中易受各种病原菌的侵害[1],如炭疽病。炭疽病主要由胶孢炭疽菌(Colletotrichum gloeosporioides)引起。在炭疽病发病初期,文心兰叶片上产生淡褐色凹陷的小斑点后变大,病斑颜色由淡褐色转成褐色最后成黑褐色,后期病斑处形成黑色小颗粒体,高湿度时可溢出粉红色黏状物的分生孢子堆,受光照影响而常呈轮纹状[2]。文心兰炭疽病影响文心兰的产量和品质及其经济价值。目前文心兰炭疽病的防治主要通过喷洒化学药剂,但此防治方法易造成农药残留和使病原菌产生耐药性等问题[3]。生物防治是利用了生物物种间的相互关系,以一种或一类生物防治另一种或另一类生物。与施用化学药剂相比,生物防治具有对环境友好、绿色、无公害且不易诱导病原菌产生耐药性、对病原菌有高度特异性等优点[4-5]。筛选新型生防菌株以及研究其抗菌物质已成为目前的研究热点。芽孢杆菌属是应用较广泛的生防菌,能有效防控病害(如炭疽病、褐斑病、茎腐病等)[6-7]。目前关于生防菌在文心兰上应用的研究较少,其拮抗机制尚不明确。本研究从健康文心兰叶片分离出1株具有潜在广谱拮抗活性的内生菌菌株WB75。笔者研究WB75对文心兰主要致病菌的广谱性防效,并通过16S rDNA、gyrA和gyrB等基因构建系统发育树确定其分类地位,以期获得能有效防控文心兰炭疽病的菌株,为文心兰炭疽病及其他多种病害的生物防治提供优良的拮抗菌株。
HTML
-
试验材料为海南博大兰花科技有限公司文心兰产业园区的文心兰“博大1号”品种;供试内生菌菌株WB75分离自健康文心兰“博大1号”植株。供试病原真菌: 胶孢炭疽菌(C. gloeosporioides);热带生炭疽菌(Colletotrichum tropicicola);鹰嘴豆枯萎镰刀菌(Fusarium delphinoides);腐皮镰孢菌(Fusarium solani);隔孢假壳科真菌(Paraconiothyrium thysanolaenae);尖孢镰刀菌(Fusarium oxysporum);画眉草弯孢菌(Curvularia eragrostidis);芒果球座菌(Guignardia mangiferae);巨座壳科真菌(Muyocopron alcornii),这些病原真菌菌株均分离自文心兰感病植株[8],并保存于海南大学热带特色林木花卉遗传与种质创新教育部重点实验室。本实验细菌的分离培养采用北京索莱宝科技有限公司生产的LB培养基;真菌的分离培养采用青岛高科技工业园海博生物技术有限公司生产的PDA培养基。
-
平板对峙法:用灭过菌的打孔器(内径5 mm)将病原菌(已活化)打成菌饼,用灭菌牙签将其挑到平板中心,将内生菌菌株WB75点接在距离平板中心约为3 cm处的四周,对照只接病原菌,将处理和对照置于28 ℃培养箱中培养,待对照组病原菌长满平板即结束实验,并计算抑菌率[9]。抑菌率为对照与处理菌落之差占对照菌落直径的百分比。
发酵液的制作:将2~3块内生菌菌株WB75菌饼放入200 mL LB液体培养基中,封好口后放在28 ℃ 180 r·min−1 摇床上培养7 d,将发酵液10 000 r·min−1 离心5 min,用5层灭菌纱布包住漏斗过滤离心后的发酵液3次。过滤后的发酵液一部分用0.22 μm无菌微孔滤膜过滤获得无菌发酵液;另一部分用高压灭菌锅121 ℃灭菌30 min,得到高温处理后的发酵液(高温发酵液)。处理为发酵液(无菌发酵液或高温发酵液)与PDA培养基的混合液体(体积比为1∶5),将2种含发酵液的培养基分别倒板,对照仅为PDA培养基。实验重复3次,将打成菌饼的病原菌放在培养皿的中心,待对照组病原菌长满平板,测量实验组和对照组菌落的直径,计算抑菌率。
-
验证内生菌的致病性:将内生菌菌株进行活化,再用打孔器(内径5 mm)打成菌饼;取长势一致的文心兰无菌组培幼苗,接种在改良DE培养基中,每瓶3株;处理组为在培养7 d且无污染的组培苗中间放入1个内生菌菌饼,对照组则放无菌的PDA菌饼;5个重复,用封口膜封口后放在培养箱中培养;培养条件:光照/黑暗=14 h/10 h、光强40 μmol·m−2·s−1、温度约25 ℃、相对湿度约75%。记录苗的株高、根数、净重等指标,定期观察菌苗共生状况,记录是否致病或致死[10]。使用针刺法[11]接种细菌验证其在文心兰植株上的致病性。实验采用博大兰花科技有限公司提供的8月龄文心兰盆栽苗,依次用75%酒精和无菌水消毒清洗叶片,晾干后在叶片正面采用一次性无菌针灸针轻微刺伤,每处伤口进行数次针刺(所占面积小于菌饼底面积),1片叶子上处理6~8处伤口,之后将菌饼贴于伤口之上,并将湿润的无菌滤纸铺在接种的叶片上保湿,对照接种相同大小的无菌PDA菌饼,处理设置3个重复。所有文心兰植株在28 ℃,相对湿度约60%的条件下培养,观察并记录叶片是否发病。
-
采用对峙培养法[12],与热带生炭疽菌C.tropicicola、鹰嘴豆枯萎镰刀菌F.delphinoides、腐皮镰孢菌F. solani、隔孢假壳科真菌P. thysanolaenae、尖孢镰刀菌F. oxysporum、画眉草弯孢菌C.eragrostidis、芒果球座菌G. mangiferae、巨座壳科真菌M. alcornii 共12株病原真菌进行对峙培养,每个处理重复3次,方法与1.2相同。
-
先将活化内生菌菌株(LB 平板),转入50 mL LB液体培养基中,在摇床(28℃ 150 r·min−1)上摇培24 h,取少量菌液稀释后进行革兰氏染色,菌液培养3~4 d后进行芽孢染色。内生菌菌株在LB平板上培养3~4 d后观察菌落形态等特征并拍照。
-
内生菌菌株生理生化鉴定用《常见细菌系统鉴定手册》[13]中的方法。
-
用细菌DNA 提取试剂盒提取内生菌菌株基因组DNA,分别用细菌 16S rDNA 通用引物[14]、gyrA[15]和gyrB基因[16]引物(表1)进行PCR扩增。PCR扩增产物经1%琼脂糖凝胶电泳检测后交由华大基因有限公司测序,测序结果上传至GenBank数据库,以获得序列登录号[17]。所得序列在NCBI上的 BLAST 进行相似性分析,系统发育树(Bootstrap 1000)用MEGA7.0构建(Neighbor-Joining法)。
引物名称
Primer碱基序列
Bases sequence16S通用引物 27F(5′-AGAGTTTGATCCTGGCTCAG-3′)、1492R (5′-GGTTACCTTGTTACGACTT-3′) gyrA p-gyrA-f (5′-CAGTCAGGAAATGCGTACGTCCTT-3′)、p-gyrA-r (5′-CAAGGTAATGCTCCAGGCATTGCT-3′) gyrB UP1S: (5′-GAAGTCATCATGACCGTTCTGCA -3′)、UP2rS: (5′-AGCAGGGTACGGATGTGCGAGCC-3′) Table 1. Primer sequence
-
数据整理、绘画分别采用Excel 2019和Origin 9.1软件处理。
1.1. 试验材料
1.2. 拮抗文心兰炭疽病菌株的抑菌率
1.3. 内生菌致病性验证
1.4. 内生菌菌株抑菌广谱性的测定
1.5. 内生菌菌株的分类鉴定
1.5.1. 形态鉴定
1.5.2. 生理生化鉴定
1.5.3. 分子生物学鉴定
1.6. 数据统计分析
-
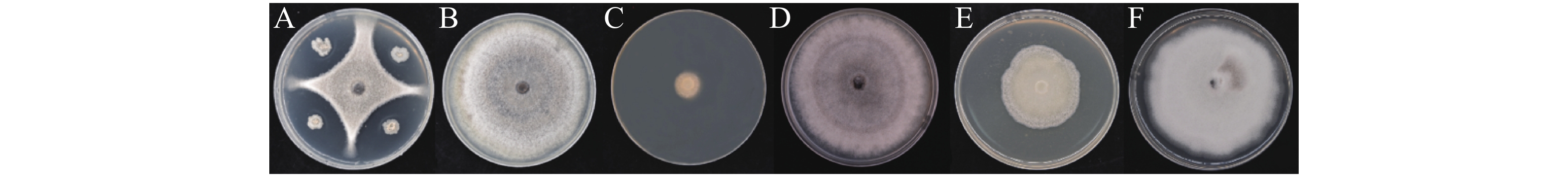
前期分离得到内生菌株WB75,以文心兰炭疽病为指示菌,进行平板对峙实验的抑菌率为(58.79±0.46)%(图1-A),并对菌株WB75的发酵液进行抑菌测定,发酵液原液的抑制率为(77.69±3.79)%,高温发酵液的抑制率下降至(41.26±4.08)%,呈现大幅下降现象,说明高温对菌株WB75发酵液中的抑菌活性物质产生较大影响。与对照组相比,实验组胶孢炭疽菌菌丝均表现出了明显被抑制的现象(图1-C、E),暗示发酵液中含有丰富的抑菌活性物质,抑制了菌丝生长。
-
将分离获得的菌株WB75进行致病性测定,7 d 后观察发现,刺伤接种与对照组接无菌PDA菌饼的伤口均无病斑产生。使用菌苗共生DE培养基,在组培苗中共生培养2个月,植株未出现病症现象。结果说明,菌株WB75对植物无致病性作用。
-
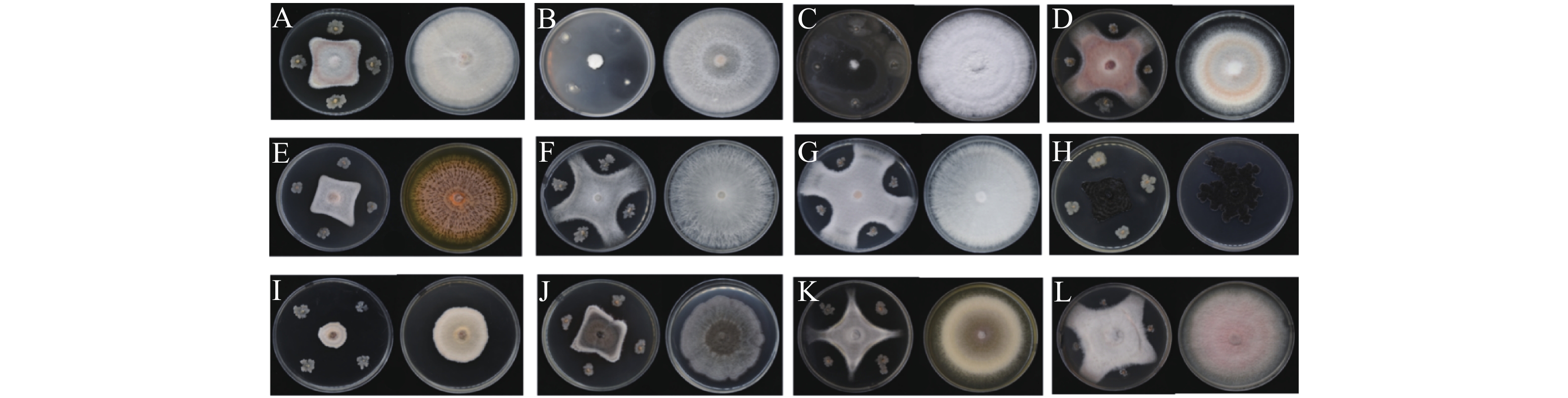
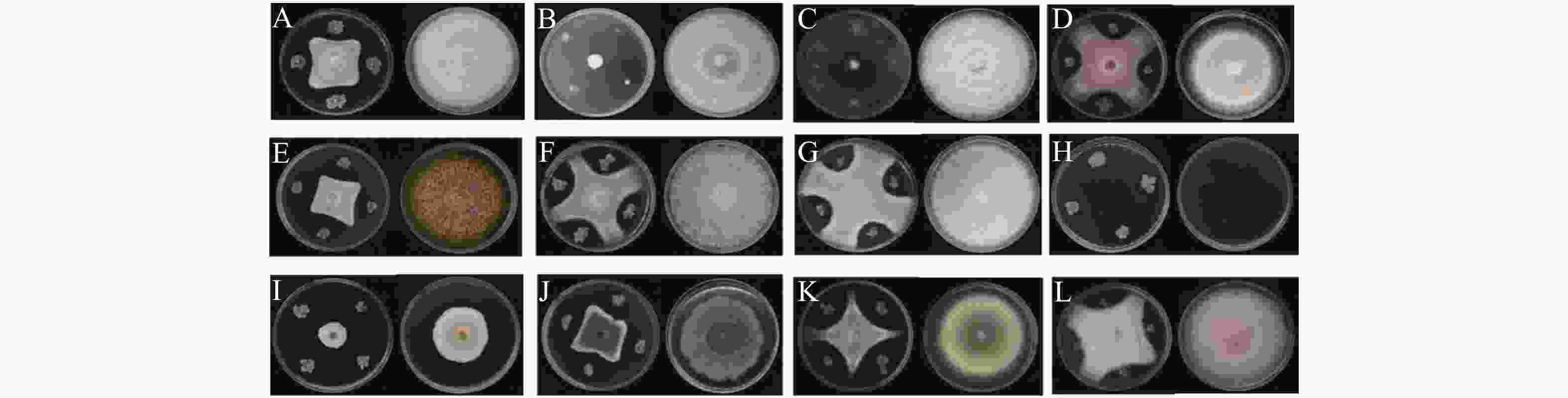
菌株WB75对不同病原菌的拮抗性表现如图2 所示。菌株WB75对不同病原菌的抑制率(表2)分析结果表明,WB75对分离获得的8种(热带生炭疽菌、 鹰嘴豆枯萎镰刀菌、腐皮镰孢菌、隔孢假壳科真菌、尖孢镰刀菌、画眉草弯孢菌、芒果球座菌、巨座壳科真菌)12株文心兰病菌均有抑制作用,其中对F. solani 的2株病原菌(WG1和WB23))抑制率超过80%,对WG1抑制作用最强,抑制率达到了91.53%;其次对WB23抑制率也达到82.79%,对7株病原真菌(W38、WB1、WB11、WB19、WB35、WB6和WG2)的抑制率超过50%。对W47、WG3和WG4的抑制效果差一些。
供试菌株
All strains菌落直径/cm
Colony diameter抑制率/%
Inhibition rate病原菌
Pathogen拮抗菌
Antagonistic endophyteW38 WB75 3.76±0.16 54.72±1.94 CK 8.30±0 0 W47 WB75 4.27±0.13 44.83±1.63 CK 7.73±0.53 0 WB1 WB75 3.98±0.30 52.58±3.64 CK 8.40±0 0 WB11 WB75 3.67±0.08 53.29±1.02 CK 7.85±0.13 0 WB19 WB75 3.23±0.05 57.08±0.69 CK 7.53±0.84 0 WB23 WB75 1.42±0.13 82.79±1.53 CK 8.23±0.03 0 WB35 WB75 2.43±0.64 52.29±12.61 CK 5.10±0.75 0 WB6 WB75 3.24±0.14 53.69±1.97 CK 6.98±0.06 0 WG1 WB75 0.67±0.04 91.53±9.06 CK 7.87±0.57 0 WG2 WB75 2.16±0.17 55.77±3.49 CK 4.88±0.89 0 WG3 WB75 3.08±0.19 45.77±3.33 CK 5.67±0.19 0 WG4 WB75 5.05±0.05 38.42±0.61 CK 8.17±0.29 0 Table 2. Inhibition rates of the endophyte strain WB75 against different pathogens
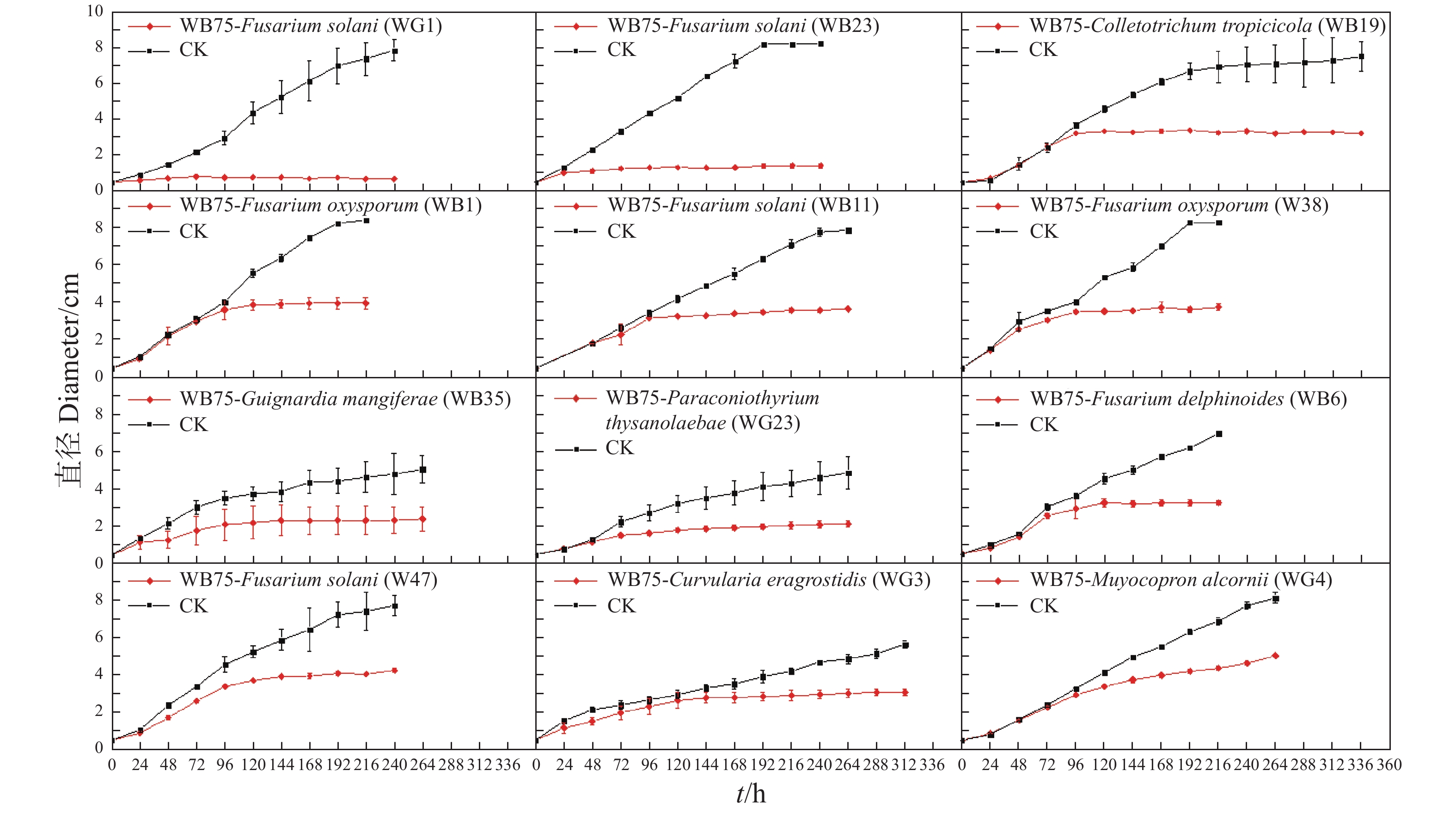
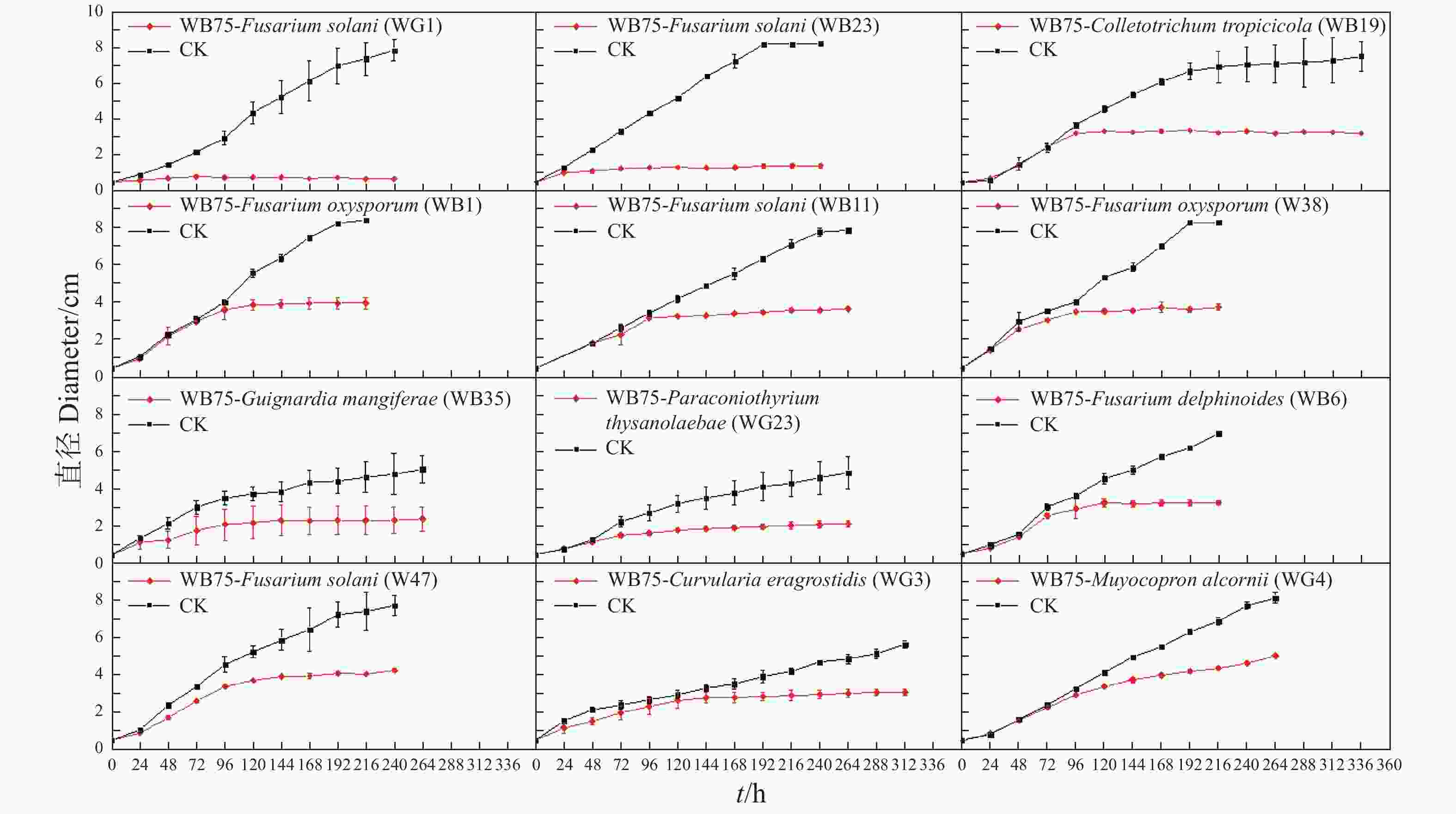
利用十字交叉法每天测量病原菌直径,绘制生长曲线(图3)。发现菌株WB75对F. solani (WG1)和F. solani(WB23)的抑制作用在48~72 h达到峰值,对C. tropicicola(WB19)的抑制作用在72~96 h达到峰值,对F. oxysporum(WB1)、F. solani(WB11)、F. oxysporum(W38)的抑制作用在96~120 h达到峰值,对G. mangiferae (WB35)、P. thysanolaenae(WG2)、F. delphinoides(WB6)和F. solani(W47)的抑制作用在120~144 h达到峰值,病原菌将不再生长,而对照组仍无限生长。对C. eragrostidis(WG3)的抑制作用在144~168 h达到峰值,而对M. alcornii (WG4)的抑制效果相对较差,在144 h后增速下降,但相对于对照组,M. alcornii(WG4)在菌株WB75的影响下,仍有一定的抑制效果。试验结果说明相同属的一些菌株具有一些相似的性质。
-
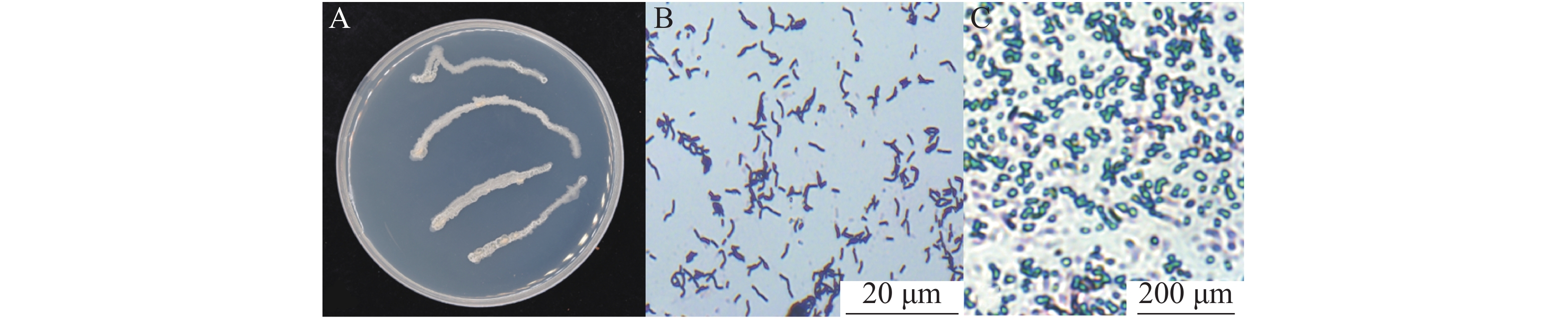
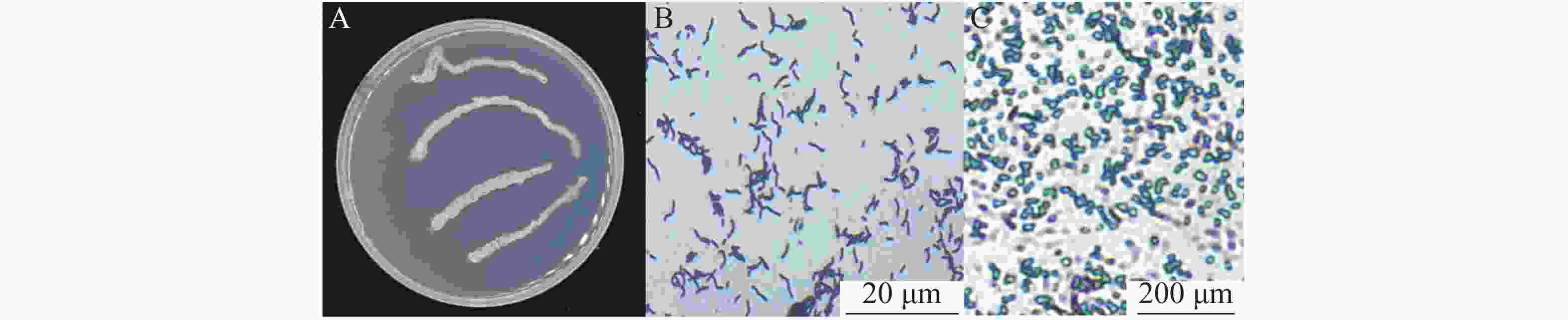
在LB固体培养基上,菌株WB75呈乳白色,表面有皱褶,菌落粗糙且边缘略显不规则(图4-A)。菌株WB75是革兰氏阳性细菌,细胞呈短杆状,单个或成对,极易成链状(图4-B),菌株WB75形态、革兰氏染色、芽孢染色等显示芽孢属特征(图4-C)。结合该菌株的生理生化特征(表3),参照《常见细菌系统鉴定手册》,判定该菌株为解淀粉芽孢杆菌。
生理生化指标 Biolog 结果 Result 接触酶试验 Catalase test + V-P 试验 Voges-Proskauertest + 明胶液化 Hydrolysis of gelatin + 甲基红试验 Methyl red test − 革兰氏染色 Gram stain + 木糖醇 xylitol − 硝酸盐还原 Nitrate reduction + 赤藓糖醇 Erythritol sugar alcohol − 色氨酸脱氨酶 Tryptophan eaminase − 纤维素分解 Cellulose decomposition − 柠檬酸盐利用 Citrate utilization + 淀粉水解 Starch hydrolysis + 注:“+”和“−”分别表示阳性和阴性。
Note: “+” and “−” mean positive and negative.Table 3. Physio-biochemical characteristics of endophyte strain WB75
-
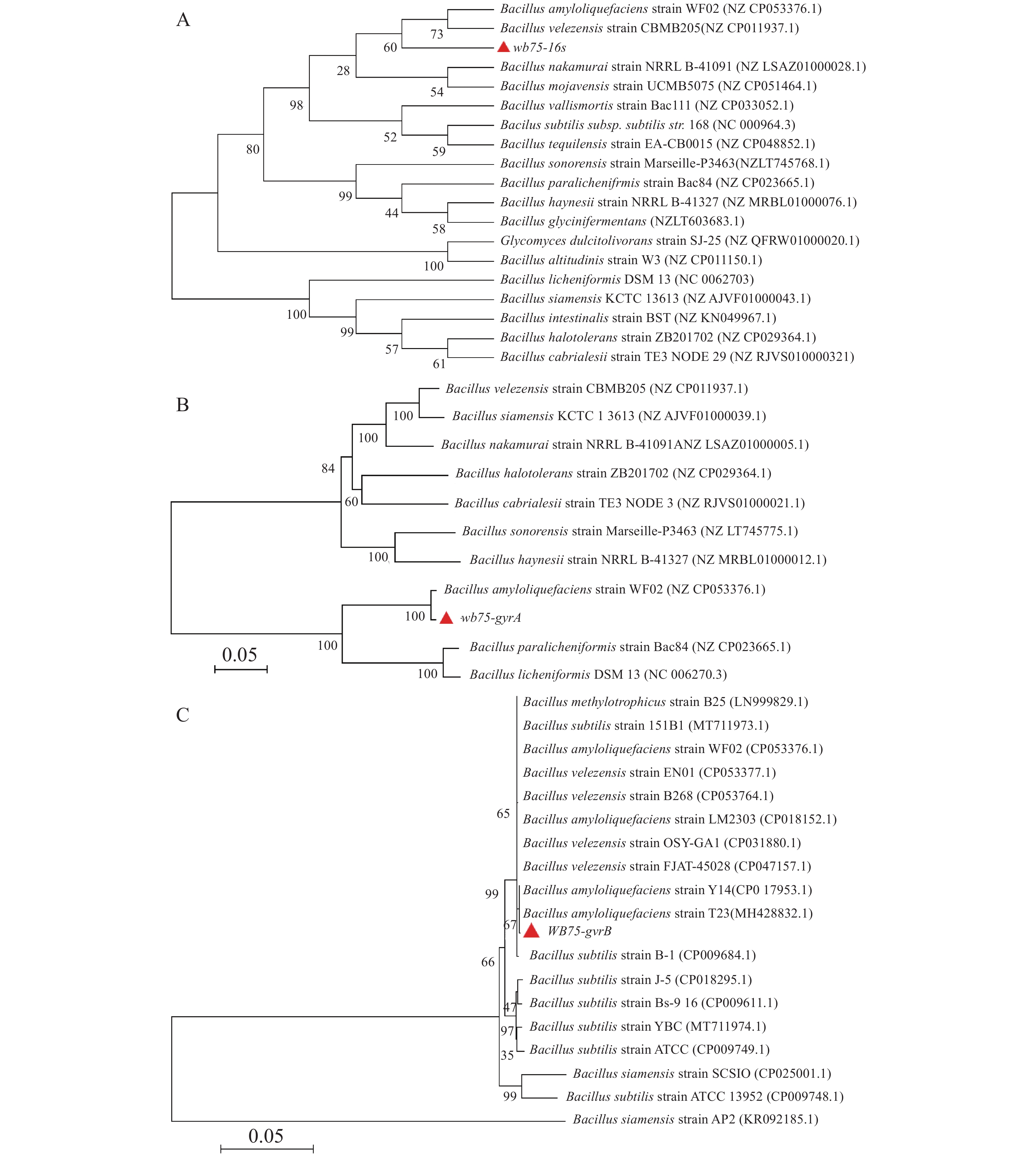
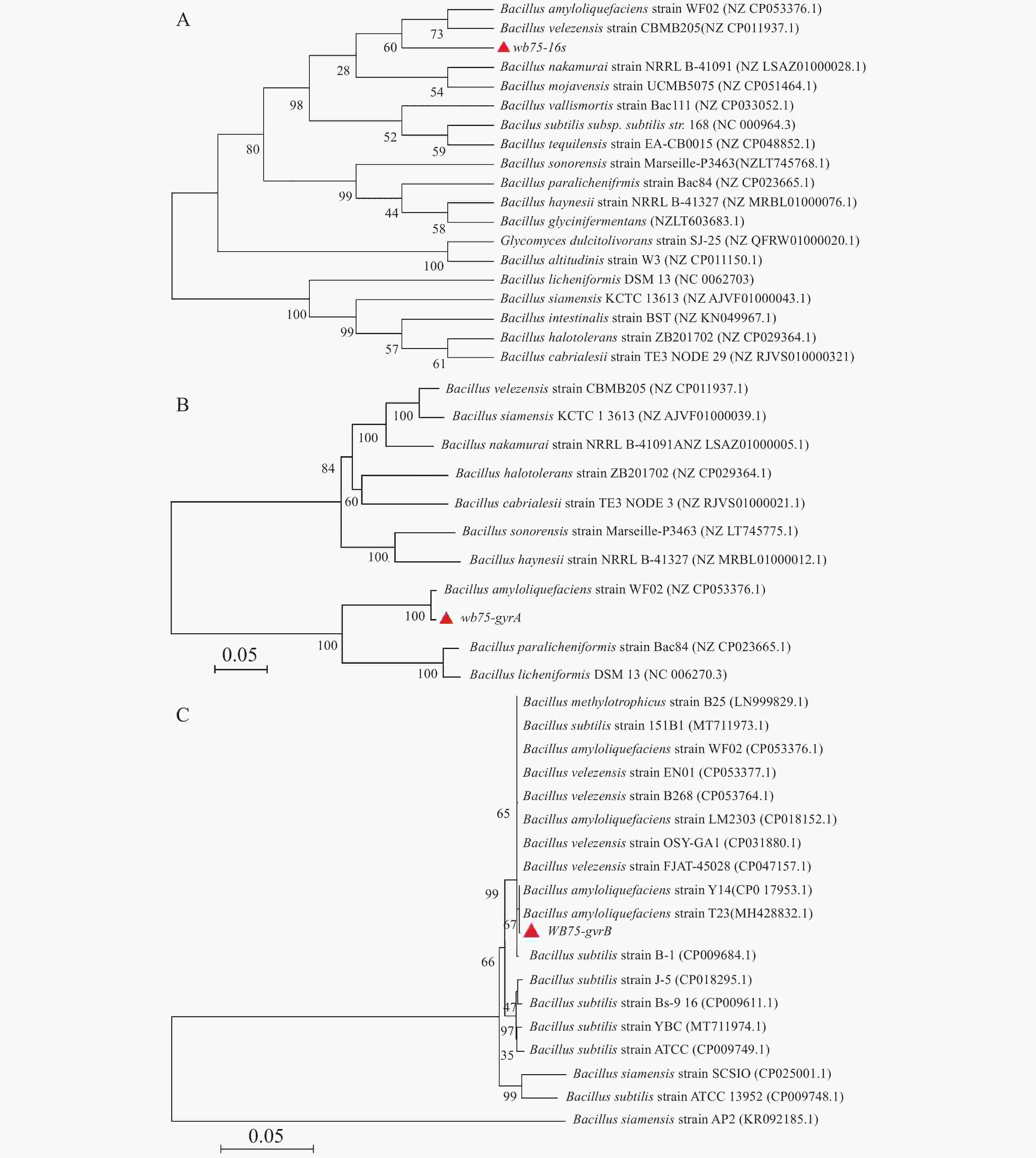
对菌株WB75的16S rDNA进行PCR扩增,得到长1 422 bp的PCR扩增产物。经过与NCBI的GenBank数据库比对,其序列与解淀粉芽孢杆菌Bacillus amyloliquefaciens (NZCP053376.1)和贝莱斯芽孢杆菌Bacillus velezensis(NZCP011937.1)相似性较高(图5-A)。菌株WB75的16S rDNA核苷酸序列已提交GenBank,登录号为MW238381。用菌株WB75 gyrA基因引物测定 gyrA基因序列,获得 943 bp 的基因片段,在NCBI上比对分析以后,发现其与解淀粉芽孢杆菌Bacillus amyloliquefaciens(NZCP053376.1)菌株 gyrA基因的一致性达到 99%以上(图5-B)。系统发育树结果(图5-B)显示,菌株WB75 与解淀粉芽孢杆菌(Bacillus amyloliquefaciens)(NZCP053376.1)分支较近,支持率较高。用菌株WB75 的gyrB基因得到序列为 1145 bp 的基因片段,与B. amyloliquefaciens(CP017953.1)和解淀粉芽孢杆菌(Bacillus amyloliquefaciens)(MH428832.1)以67%的支持率聚成同一分支(图5-C),综合3个基因的序列比对结果分析,说明菌株WB75是解淀粉芽孢杆菌。
2.1. 拮抗菌的抑菌率
2.2. 内生菌菌株的无致病力测定
2.3. 内生菌抑菌广谱性测定
2.4. 内生菌菌株的分类鉴定
2.4.1. 内生菌菌株形态和生理生化特征
2.4.2. 内生菌菌株WB75的16S rDNA、gyrA、gyrB基因序列分析
-
菌株 WB75对炭疽菌属和镰刀菌属等13株病原真菌有38.42%~91.53%的抑制作用,对F. solani(WG1)腐皮镰孢菌抑制作用最强,抑制率达到91.53%。通过形态观察、生理生化特性以及分子生物学手段,菌株 WB75被鉴定为解淀粉芽孢杆菌。
层出镰刀菌引起兰花叶斑病[18],尖孢镰孢菌引起墨兰Cymbidium sinense茎腐病和建兰Cymbidium ensifolium茎腐病[19],引起蝴蝶兰Phalaenopsis aphrodite茎腐病[20],胶孢炭疽菌是墨兰、大花蕙兰Cymbidium hybrid 和铁皮石斛Dendrobium officinale 炭疽病的病原菌[21]。本研究发现菌株WB75对以上镰刀菌属和炭疽菌属均具有较好的拮抗效果。周平兰等[22]研究发现芽孢杆菌属能拮抗兰花炭疽病病原菌。许文江等[23]研究发现内生枯草杆菌 FJAT-9986 能防治蝴蝶兰叶基腐病菌。王士燕等[24]研究发现解淀粉芽孢杆菌拮抗胶孢炭疽菌、尖孢镰孢菌和腐皮镰孢菌3种兰花病原真菌。本研究中,菌株WB75发酵液对病原真菌有很好的抑制效果,说明发酵液中存在抑菌活性物质,但菌株发酵液经过121℃高温处理后抑制效果显著下降,说明发酵液中的抑菌活性物质不耐高温,但121℃高温处理后的菌株WB75发酵液仍能溶解病原真菌菌丝,可较好地抑制病原真菌。
芽孢杆菌属是世界公认的生防菌,被广泛应用于植物病害防治[25]。有研究表明解淀粉芽孢杆菌主要通过产生蛋白酶等生防相关酶和含有抗菌肽合成基因[26]、几丁质酶及嗜铁素[27]等进行抑菌。解淀粉芽孢杆菌的广谱抑菌性已有报道,能有效防治大白菜软腐病菌和 8 种病原真菌[28],能有效抑制黄瓜枯萎病并促进黄瓜植株生长[27]。芽孢杆菌的内生芽胞,具有耐热、耐干燥和抑菌能力强等优点,且其生产工艺简单,存储期较长,是一种开发潜力很高的生防菌[29]。
本研究仅确定菌株WB75为解淀粉芽孢杆菌,且初判断其具有一定生防潜力,但对其拮抗机理的研究不够深入,对菌株发酵液含有的抑菌物质的具体成分及其抑菌机理尚不明确,这些均有待进一步研究。菌株WB75对病原菌的室内或田间防效试验也有待进一步研究。
致谢:海南大学生态与环境学院丁琼老师、植物保护学院刘铜老师对本实验给予了指导与帮助,一并致谢!






 DownLoad:
DownLoad: