-
香蕉枯萎病又称巴拿马病、黄叶病,是一种典型的导致维管束系统毁灭的土壤真菌病害,首次于1904年在美国夏威夷被发现[1-2],是由古巴专化型的尖孢镰刀菌(Fusarium oxysopoyum f. sp. Cubence)引起[3],在香蕉的整个生育期内都会出现。尖孢镰刀菌会破坏维管束结构、堵塞木质部导管,从而引起假茎开裂、叶片发黄等症状,最终植株枯萎死亡。一百多年来,香蕉土传枯萎病一直是全球香蕉产业最大的障碍,在许多国家和地区均有报道,并造成严重的危害[4]。研究表明,长期的香蕉连作使土壤酸度增加,病原菌积累,土壤微生物群落失衡[5-7]。目前,对香蕉连作障碍采用的措施主要有:选育抗病品种、化学药剂、施用生物有机肥和轮作[8-11]。但是,香蕉是无性繁殖,选育抗病品种非常困难;化学药剂对土壤和农作物污染严重;生物肥料的防治效果还有待提升;轮作不利于保持香蕉的经济效益[12]。因此,现在急需一种有效的防治香蕉枯萎病的方法[13]。近年来,利用套作来调控土壤微生物群落的报道逐渐增多。研究表明,玉米与花生套作能有效地改变土壤微生物的群落组成和优势物种的种类[14]。张蒙蒙等[15]的研究结果表明,桑树与紫花苜宿套作能改善土壤真菌的多样性,增加真菌的丰富度。香蕉与韭葱套作能有效地减少土壤中总镰刀菌的数量[16],套作豆类和谷类作物可以提高豆科固氮能力[17],芥菜套作豆科作物增加了产量稳定性、养分有效性和水分利用效率[18]。目前,关于香蕉行间套作的研究不多,尤其是套作豆科绿肥的研究较少。在黄瓜园中套作白三叶草(Trifolium repens L),可显著降低黄瓜枯萎病发病率[19]。因此,笔者以白三叶草为套作作物,以不套种为对照,研究套作白三叶草对连作蕉园香蕉枯萎病的发病率以及土壤微生物的多样性、结构和组成的影响,旨在为套作防控香蕉枯萎病和改善蕉园土壤微生物生境提供依据。
-
本试验于2018年9月至2019年6月在海南省澄迈县香蕉基地(19°N,109°E)进行。该地区属热带季风气候,年平均气温23.8 ℃,降水量1786.1 mm,且热雨同季,台风影响较弱;试验田土壤为砖红壤(连续单作香蕉8年)。土壤基本化学性质:pH 5.53,碱解氮93.47 mg·kg−1,铵态氮14.19 mg·kg−1,硝态氮10.81 mg·kg−1,速效磷96.57 mg·kg−1,速效钾672 mg·kg−1,有机质3.64 g·kg−1。试验田所种香蕉品种为巴西蕉(Mimosa nana Lour.),1株留1个吸芽。供试绿肥为白三叶草(Trifolium repens L)。
-
2018年9月将白三叶草进行地面撒播。设置处理如下:单作香蕉(MB)、香蕉套作白三叶草(IB)。每个处理3次重复,随机分布,并设置保护行。每个重复小区种植60棵香蕉,宽窄行种植,宽行行距3 m,窄行行距2 m,香蕉株距2 m。绿肥在香蕉宽行间撒播,白三叶草行距30 cm,播种量为6 kg·hm−2,为常规播种量。水分管理:苗期和果实成熟期使田间持水量保持在75%左右,快速生长期、抽蕾期和果实生长期使田间持水量保持在85%左右。施肥管理:底肥,每株施腐熟的羊粪有机肥5 kg,同时添加磷肥0.2 kg,均匀拌入施肥穴;前期追肥(前3个月),每15~20 d追施1次肥,每株每次淋施15 g尿素和15 g硫酸钾复合肥(15-15-15);中期追肥(快速生长期至抽蕾前),每15~20 d追施1次肥,每株每次淋施15 g尿素、60 g硫酸钾复合肥和160 g硫酸钾;后期追肥(抽蕾期至采收期),每25~30 d追施1次肥,每株每次淋施50 g尿素、80 g硫酸钾复合肥和110 g硫酸钾。
-
在田间试验结束时采集土壤样品,各重复小区选取5株健康香蕉,在香蕉滴水线以内用土钻随机取4个土样,收集5~20 cm的表层土壤,然后将这5株香蕉的土样混合为1个土壤样品。每个处理采集3个重复,共15株香蕉。取土后过2 mm筛,用四分法取约100 g放入超低温冰箱,用来提取DNA,用于高通量测序;取约300 g土风干后测定化学性质。
在种植中,自出现典型香蕉枯萎病症状时开始统计发病率。当香蕉植株出现黄叶、假茎开裂并呈褐色、新叶较小等典型的症状,即统计为发病植株,每7 d调查1次,直至试验结束。香蕉枯萎病的发病率按下式计算:
用pH计法测定土壤pH值(m水∶V土=1∶2.5),油浴法测定土壤有机质,扩散碱解法测定土壤碱解氮,0.03 mol·L−1氟化铵−盐酸(0.025 mol·L−1)浸提−钼锑抗比色法测定土壤速效磷,1 mol·L−1中性乙酸铵溶液浸提−火焰光度计法测定土壤速效钾。2 mol·L−1氯化钾溶液浸提−紫外分光光度计法测硝态氮,2 mol·L−1氯化钾溶液浸提−靛酚蓝比色法测铵态氮。具体方法详见《土壤农化分析》[20]。
将每个重复的土壤样品提取2次DNA,取约0.8 g混合土壤样品,用MOBIO的土壤DNA提取试剂盒(PowerSoil DNA Isolation Kit)提取土壤DNA。每个处理共提取6次DNA。提取好的DNA用核酸定量仪(NanoDrop 2000,Thermo Scientific,USA)测定其核酸浓度后于−20 ℃冰箱保存。每个重复分别吸取20 μL样品送至北京百迈客生物科技有限公司进行测序。细菌16S rRNA (V3+V4)区域采用引物:5′- ACTCCTACGGGAGGCAGCA-3′和5′-GGACTACHVGGGTWTCTAAT-3′,真菌ITS1区域采用引物:5′-CTTGGTCATTTAGAGGAAGTAA-3′和5′-GCTGCGTTCTTCATCGATGC-3′。
-
测序返回的原始序列用USEARCH 11按标准流程分析。用97%相似性的稀疏曲线来判断测序深度的合理性。Mothur软件用于Alpha及Beta多样性分析。基于贝叶斯距离(Bray-distance)进行各处理间的主坐标分析(Principal Coordinates Analysis),用于比较各处理土壤中细菌和真菌的群落结构差异。
采用Microsoft Excel 2017软件进行数据处理与绘图,采用IBM SPSS Statistics 20统计分析软件,通过独立样本t检验进行数据比较,检验处理间的差异显著性(P<0.05)。
-
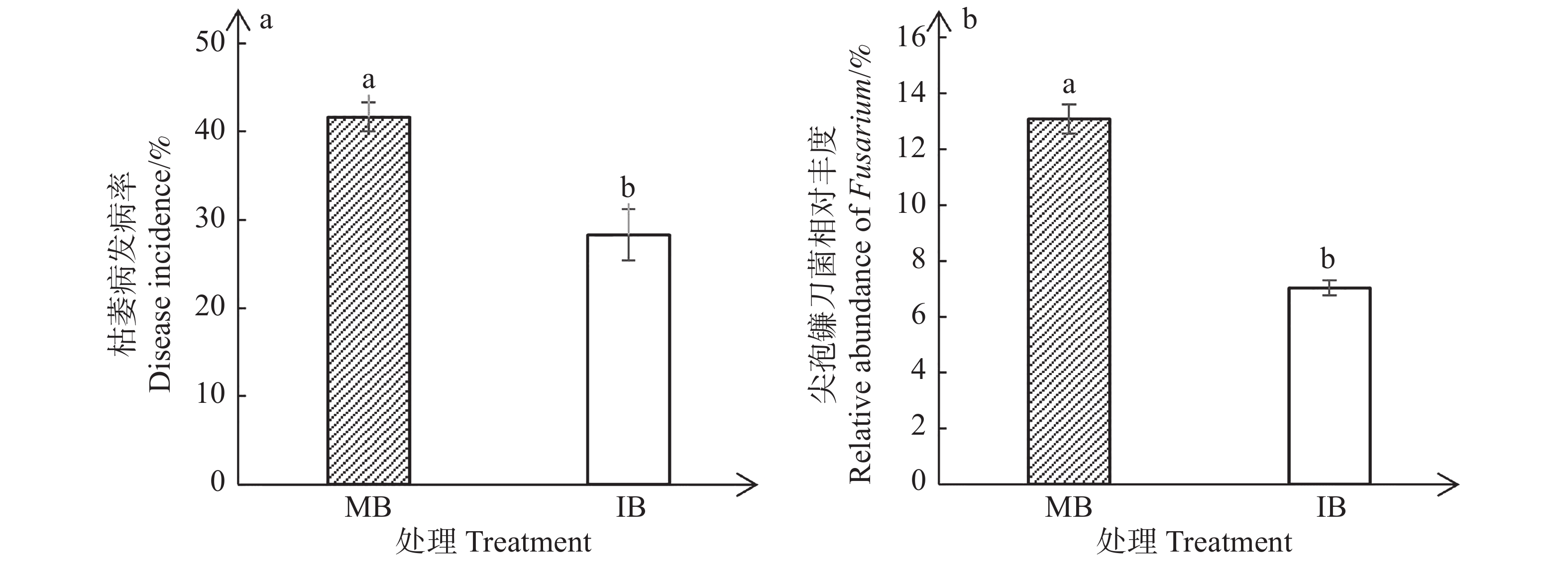
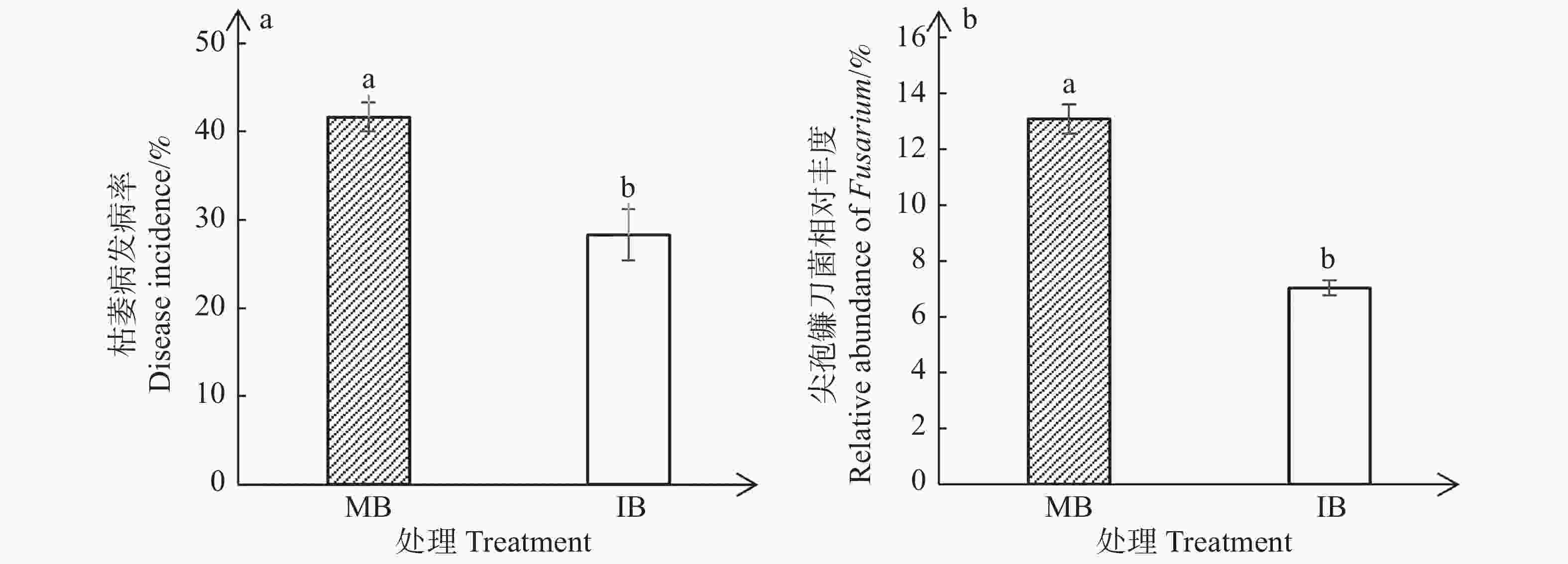
与单作香蕉处理相比,套作白三叶草处理显著降低了香蕉枯萎病的发病率(图1-a)和尖孢镰刀菌的相对丰度(图1-b)。单作香蕉处理和套作白三叶草处理的香蕉枯萎病发病率分别为41.67%和28.33%,尖孢镰刀菌相对丰度分别为13.10%和7.04%。相比于单作香蕉处理,香蕉套作白三叶草处理枯萎病发病率降低了13.34%,尖孢镰刀菌相对丰度减少了6.06%。
-
由表1可知,与单作香蕉处理(MB)相比,香蕉套作白三叶草处理(IB)土壤的速效磷含量、有机质含量、铵态氮含量和硝态氮含量均降低(P<0.05),pH值和速效钾含量增加(P<0.05)。
处理
TreatmentspH 碱解氮/
(mg·kg−1)
Alkali-hydrolyzable
nitrogen铵态氮/
(mg·kg−1)
NH4+-N硝态氮/
(mg·kg−1)
NO3−-N速效磷/
(mg·kg−1)
Available
phosphorus速效钾/
(mg·kg−1)
Available
potassium有机质/
(g·kg−1)
Organic
matterMB 5.78±0.06b 84.91±4.09a 6.75±1.05a 7.95±0.56a 90.32±3.09a 668.16±48.14b 3.08±0.03a IB 6.32±0.05a 78.87±4.13a 4.07±1.61b 5.62±0.29b 72.83±1.50b 675.37±34.11a 2.23±0.07b 注:MB:单作香蕉;IB:香蕉套作白三叶草。同列不同小写字母表示处理间在0.05水平差异显著,下同。
Note: MB: Banana monoculture; IB: Intercropping of banana with white clover. Different lowercase letters in the same column indicate a significant difference between treatments at 0.05 level, similarly hereinafter.Table 1. Effects of intercropping of banana with white clover on soil chemical properties
-
从表2可知,相比于单作香蕉处理,套作白三叶草处理土壤真菌的丰富度和多样性均显著增加。细菌的Chao、Ace和Shannon指数在处理间的差异不显著,而Invsimpson指数显著高于单作香蕉处理。
处理 Treatments 群落丰富度 Community richness 群落多样性 Community diversity Chao Ace Shannon Invsimpson 细菌 Bacteria MB 1232.56±6.62a 1217.03±6.77a 6.37±0.00a 283.37±2.38b IB 1249.79±5.70a 1235.56±4.75a 6.35±0.01a 289.00±3.67a 真菌 Fungi MB 503.18±6.07b 500.06±6.15b 4.43±0.02b 28.04±1.14b IB 586.47±7.29a 577.99±7.67a 4.52±0.03a 34.96±1.57a Table 2. Soil microbial alpha diversity indexes in different treatments
-
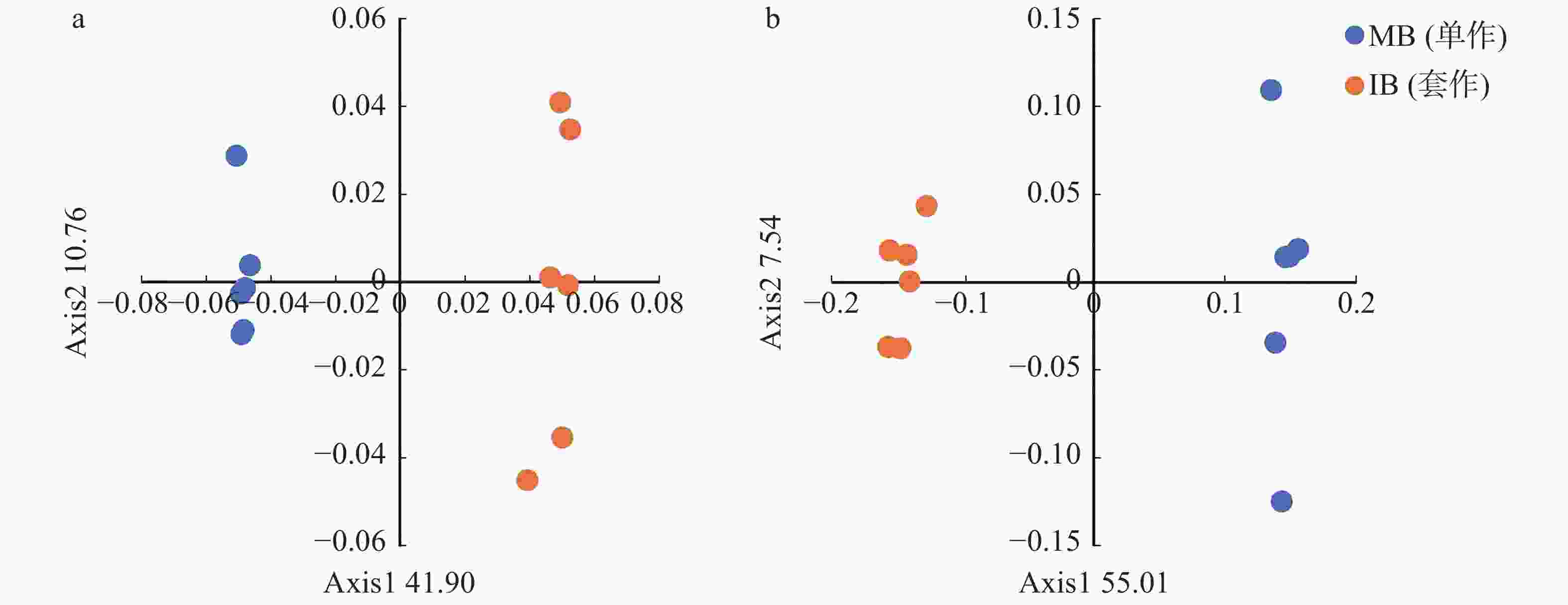
基于Bray-curits距离的主坐标分析(图2)结果表明,单作香蕉和套作白三叶草处理的细菌群落结构差异显著,第1和第2主坐标轴分别解释了总差异的41.90%和10.76%,单作香蕉和套作白三叶草处理沿第一轴(PCoA 1)明显区分开来(图2a)。由图2b可以看出,单作香蕉和套作白三叶草处理的真菌群落沿第一轴(PCoA 1)明显区分开,第一和第二主坐标轴分别解释了总差异的55.01%和7.54%。
-
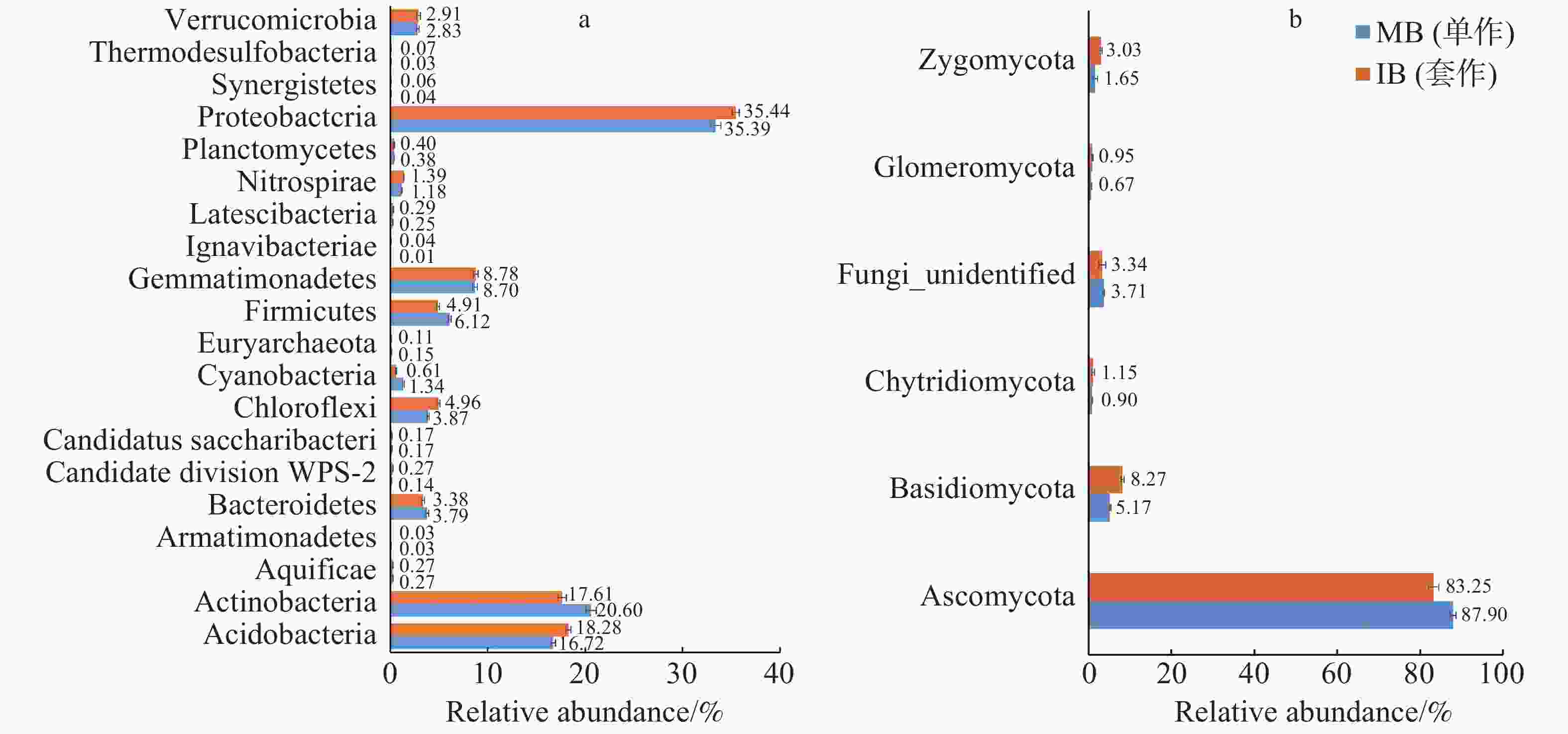
2个处理土壤微生物门水平组成的结果如图3所示。由图3-a可以看出,套作白三叶草(IB)和单作香蕉(MB)的细菌优势菌门均为变形菌门(Proteobacteria)。与单作香蕉处理相比,套作白三叶草土壤的变形菌门(Proteobacteria)、酸杆菌门(Acidobacteria)、绿弯菌门(Chloroflexi)和硝化螺旋菌门(Nitrospirae)相对丰度显著增加;放线菌门(Actinobacteria)、拟杆菌门(Bacteroidetes)和厚壁菌门(Firmicutes)相对丰度显著降低。由图3-b可以看出,各处理真菌优势门为子囊菌门(Ascomycota),与单作香蕉相比,套作白三叶草土壤的担子菌门(Basidiomycota)、球囊菌门(Glomeromycota)和接合菌门(Zygomycota)相对丰度增加;子囊菌门(Ascomycota)相对丰度降低。
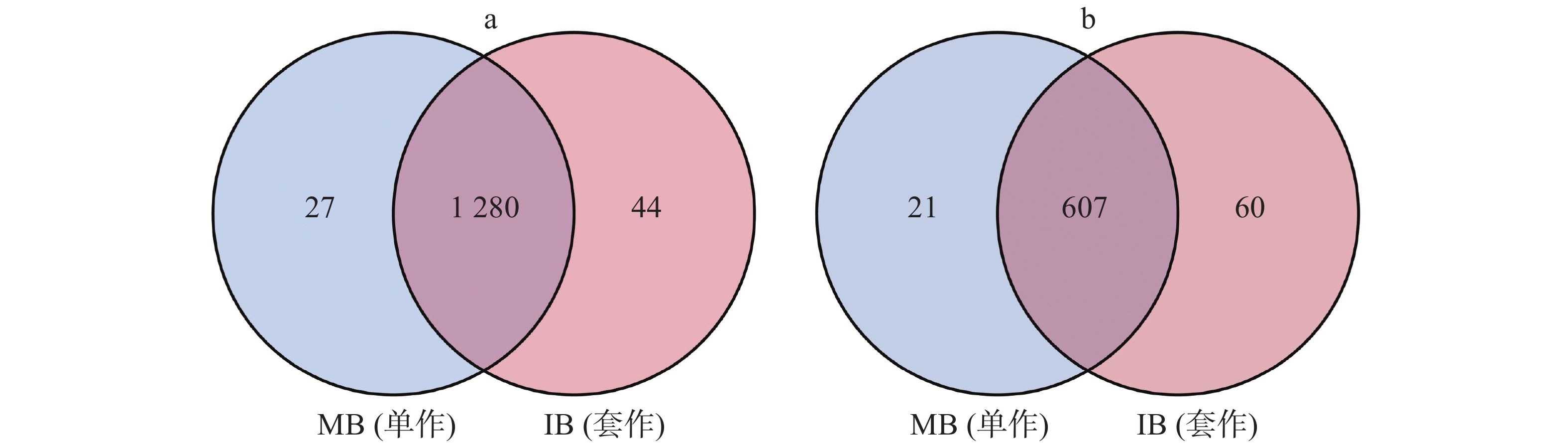
用Venn图来表示细菌、真菌在2个处理中共有OTU的数量,并计算其在总OTU里面所占的比例。单作香蕉(MB)土壤中,细菌和真菌OTU的数量分别为1307个和628个。套作白三叶草(IB)土壤中有1324个细菌保留OTU,667个真菌保留OTU。2个处理中共有的细菌和真菌OTU分别占总OTU数量的94.74%和88.22%(图4)。
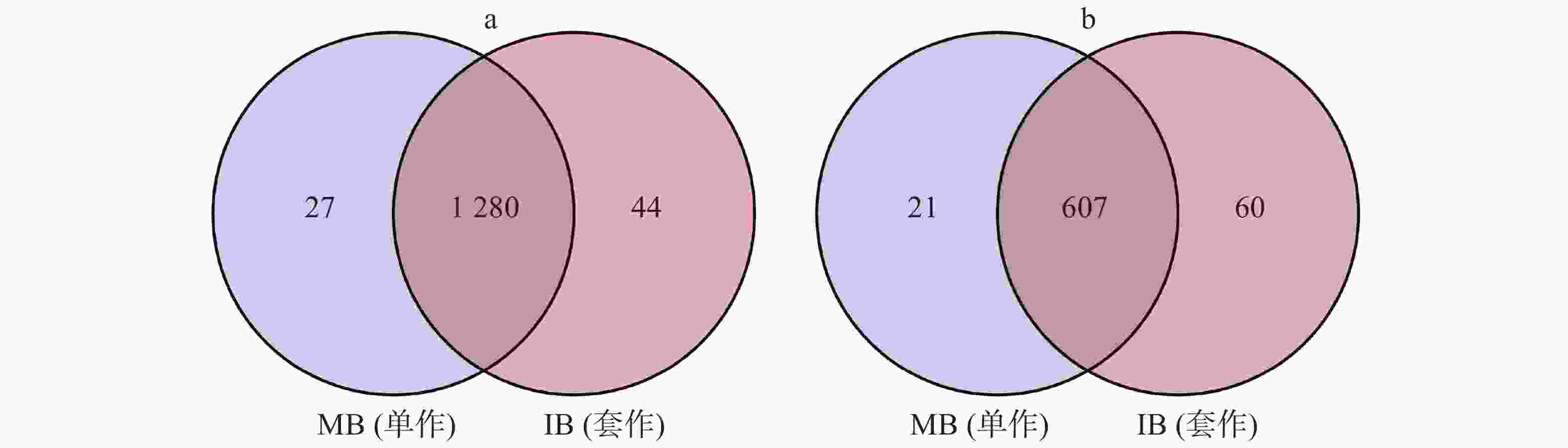
Figure 4. The venn diagram of bacterial (a) and fungal (b) OTU distribution of soil in different treatments
2个处理土壤微生物属水平组成的结果如图5所示。由图5-a可以看出,与单作香蕉处理相比,套作白三叶草处理土壤细菌的Desulfovirgula属、Gp16、Gp6、Kofleria属、硝化螺菌属(Nitrospira)、红游动菌属(Rhodoplanes)、Spartobacteria和Syntrophorhabdus属相对丰度增加,Bacillus属、Gaiella属、Gp1、Gp3、Gp4和鞘氨醇单胞菌(Sphingomonas)相对丰度降低。
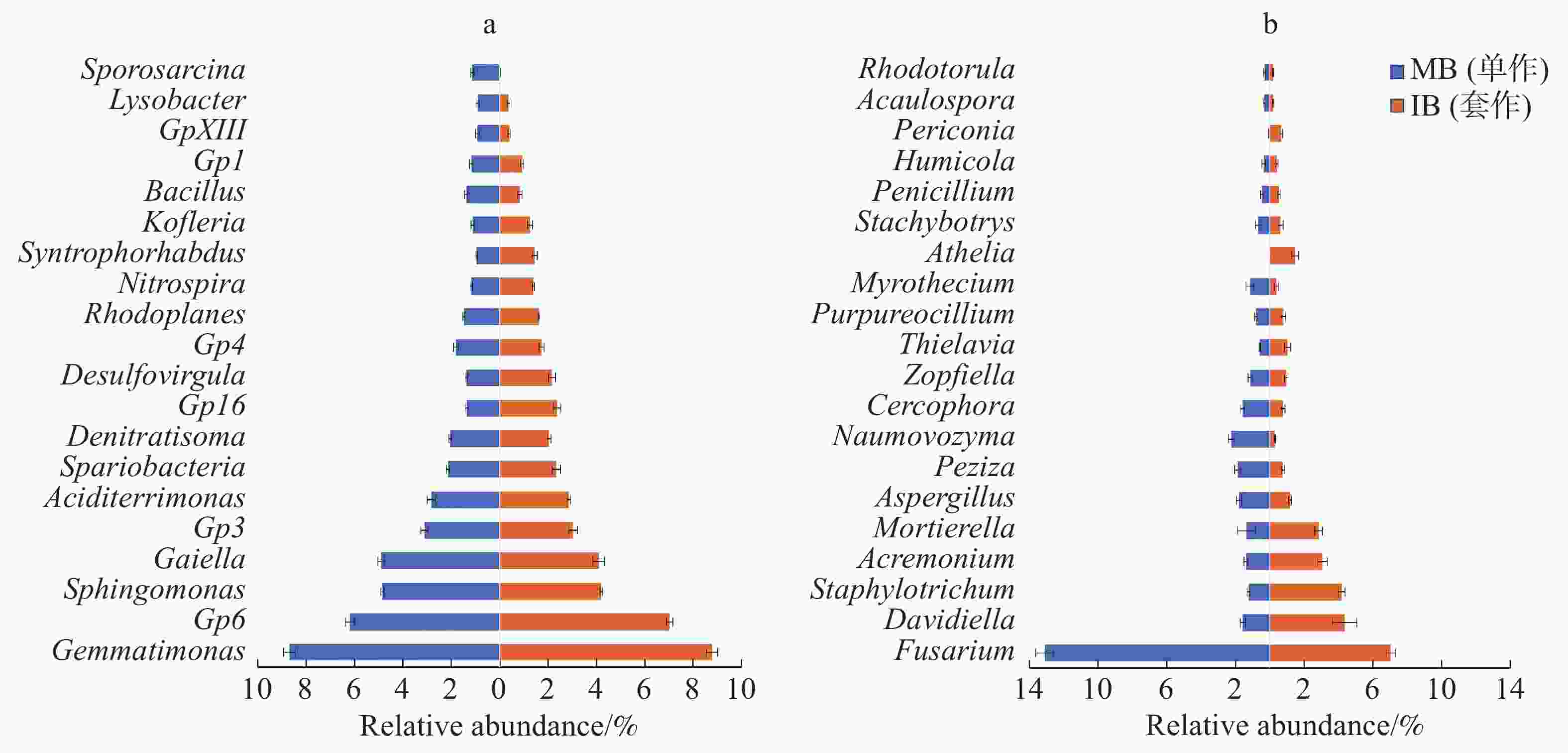
Figure 5. Relative abundance of the top 20 classified bacteria (a) and fungi (b) in the soil under different treatments
由图5-b可以看出,相比于单作香蕉处理,套作白三叶草处理土壤真菌的Davidiella属、圆孢霉属(Staphylotrichum)、支顶孢属(Acremonium)、被孢霉属(Mortierella)、梭孢壳属(Thielavia)、阿太菌属(Athelia)和黑团孢属(Periconia)相对丰度增加。而镰刀菌属(Fusarium)、曲霉属(Aspergillus)、盘菌属(Peziza)、Naumovozyma属、Cercophora属和漆斑菌属(Myrothecium)相对丰度降低。
相对丰度较高的前20个细菌属和真菌属,分别与尖孢镰刀菌做皮尔森相关性分析,筛选出具有显著负相关关系的属(表3)。其中,细菌属的GP6、GP16、兔芽孢菌属(Spartobacteria_genera_incertae_sedis)、Desulfovirgula菌、红游动菌属(Rhodoplanes)、硝化螺菌属(Nitrospira)、Syntrophorhabdus菌和Kofleria菌与尖孢镰刀菌(Fusarium)呈负相关关系(P<0.05)。真菌中,Davidiella菌、圆孢霉属(Staphylotrichum)、支顶孢属(Acremonium)、被孢霉属(Mortierella)、梭孢壳属(Thielavia)、阿太菌属(Athelia)和黑团孢属(Periconia)与尖孢镰刀菌(Fusarium)呈负相关关系(P<0.05)。
细菌 Bacteria 真菌 Fungi 属水平
Genus level平均相对丰度
Average relative abundance /%与尖孢镰刀菌相关性
Pearson correlation
with F.oxysporum属水平
Genus level平均相对丰度
Average relative abundance /%与尖孢镰刀菌相关性
Pearson correlation
with F.oxysporumGp6 6.61 −0.950** Davidiella 2.97 −0.933** Spartobacteria_genera_
incertae_sedis2.24 −0.679* Staphylotrichum 2.71 −0.989** Gp16 1.86 −0.964** Acremonium 2.23 −0.968** Desulfovirgula 1.77 −0.952** Mortierella 2.10 −0.910** Rhodoplanes 1.55 −0.913** Thielavia 0.81 −0.877** Nitrospira 1.28 −0.936** Athelia 0.74 −0.975** Syntrophorhabdus 1.20 −0.952** Periconia 0.36 −0.970** Kofleria 1.19 −0.716** Table 3. Pearson correlation between the relative abundance of genera and Fusarium
由属水平丰度图(图5)可以看出,GP16、Davidiella菌、圆孢霉属(Staphylotrichum)、支顶孢属(Acremonium)、被孢霉属(Mortierella)和阿太菌属(Athelia)明显在香蕉套作白三叶草处理的土壤中富集,且与尖孢镰刀菌的数量呈负相关关系(P<0.05)。
-
长期连作种植和不合理的施肥方式导致蕉园地发生严重的连作障碍,土壤板结、土壤容重下降、土壤微生物群落失衡,进而加剧了土传病原菌的迅速繁殖[21]。病原菌的数量与香蕉枯萎病的发病率具有显著正相关关系[22-23]。改善蕉园土壤微生物区系,降低尖孢镰刀菌的数量是防控香蕉枯萎病的有效手段。本研究结果表明,套作白三叶草处理的枯萎病发病率和尖孢镰刀菌相对丰度均显著低于单作香蕉处理。因此,套作白三叶草对尖孢镰刀菌有一定的抑制效果,能在一定程度上有效地防控香蕉枯萎病。
与单作香蕉处理相比,套作白三叶草处理显著提高了土壤pH值和速效钾含量(P<0.05),这与杨劲明等[24]的研究结果一致。很多报道认为,土壤肥力水平很大程度上影响土壤微生物的群落结构和组成,从而影响香蕉土传枯萎病的发生[25-26]。本试验结果中,较高的土壤pH值和土壤速效钾含量可能是抑制香蕉枯萎病的关键肥力因子。土壤pH值往往与枯萎病发病率呈负相关关系,这已被很多研究证实[27-28]。廖明莉等[29]利用4种豆科绿肥与小麦间作,发现4个绿肥品种均能显著提高土壤中P2O5的含量,提高效应达40.0%~50.9%。除此之外,植物与土壤微生物之间通过植物根系建立起密切联系[30]。套作作物作用于土壤,其根系占据生态位,通过根系与土壤之间的物质交换改变了土壤微生物生活的根际微环境,进而影响了土壤微生物群落,甚至有些作物的根系分泌物中的化感物质会抑制病原菌的生长[31]。赵梦丽等[32]通过研究拟南芥根系分泌物对土壤微生物群落组成和功能的影响时指出,根系分泌物可以改变土壤微生物群落结构,增加微生物中与养分循环相关的功能基因的多样性。在本试验中,Alpha多样性分析结果表明,套作白三叶草处理拥有更高的真菌多样性和丰富度。但是,对于细菌,只有InvSimpson指数显著高于单作香蕉处理。主坐标分析结果显示,土壤细菌和真菌群落结构均沿第一主坐标明显区分开来。这种群落的显著变化,可能是由于套作白三叶草后土壤微生物的生存环境变化所致[33]。因此,香蕉园套作白三叶草可以显著改变部分肥力因子,并且改变土壤微生物多样性和群落结构。
不同处理土壤中细菌和真菌群落组成在门水平有明显的差异。其中,高通量测序结果显示,在本试验蕉园土壤中,细菌的优势门为变形菌门(Proteobacteria)、真菌的优势门为子囊菌门(Ascomycota),且相比于单作香蕉处理,套作白三叶草处理显著增加了细菌变形菌门的丰度,降低了真菌子囊菌门的丰度。研究证明,变形菌门具有抑制枯萎病的作用[34],并且子囊菌门为大多数病原真菌所属的门,很多研究指出,真菌子囊菌门的降低是香蕉土传枯萎病发病率降低的重要特征[21,33]。因此,本试验中,变形菌门的增多和子囊菌门的降低也是导致套作白三叶草处理发病率降低的重要因素之一。韦恩分析结果显示,套作白三叶草处理的细菌和真菌都出现了很多特有的OTU,表明套作白三叶草处理明显改变了土壤微生物群落组成,并且优势属水平的丰度图表明,相比于单作香蕉处理,GP16、Davidiella等菌明显在套作白三叶草处理的土壤中富集,相关性分析也证明这些菌与尖孢镰刀菌都呈显著负相关关系(P<0.05)。有研究表明,酸杆菌门里一些属的改变和土传病害的发病率有较高的相关性[35],本试验套作处理中GP16的富集,可能是属水平上导致枯萎病病原菌数量和发病率降低的重要因素。
综上所述,在蕉园中套作白三叶草可以改变部分土壤肥力指标,改变土壤微生物的生存环境,重塑土壤微生物区系。套作白三叶草处理中病原菌相对丰度降低,土壤微生物多样性提高,土壤微生物群落结构和组成明显改变,这可能是导致香蕉枯萎病发病率降低的主要原因。本试验结果可以为蕉园套作豆科绿肥防控香蕉枯萎病提供借鉴。
Effect of Intercropping of Banana with White Clover on Banana Fusarium Wilt and Soil Microbial Community
DOI: 10.15886/j.cnki.rdswxb.2021.02.011
- Received Date: 2020-12-22
- Rev Recd Date: 2021-03-05
- Available Online: 2021-06-29
- Publish Date: 2021-06-25
-
Key words:
- high-throughput sequencing /
- banana Fusarium wilt /
- Fusarium oxysporum /
- microbial community
Abstract: An attempt was made to explore the changes of soil microbial community in continuous cropping banana orchards after banana was intercropped with white clover (Trifolium repens L) and the effect of the intercropping on the incidence of banana Fusarium wilt. Banana was intercropped with white clover in continuous cropping banana orchards, with monoculture of banana as control. The soil microbial community structure and composition among different treatments in the banana orchards were detected by using high-throughput sequencing and their differences were analyzed. Compared with the monoculture of banana, intercropping of banana with white clover reduced the disease incidence of banana Fusarium wilt by 13.34%, increased the pH value by 0.54, and increased the content of soil available potassium by 7.21 mg·kg−1. Compared with the monoculture of banana, the intercropping of banana with white clover significantly increased soil microbial diversity, and formed a unique community structure. Moreover, the intercropping of banana with white clover changed the community composition of soil microorganisms, significantly decreased the relative abundance of Fusarium oxysporum, and increased the relative abundance of GP16 and Davidiella. It is concluded that the intercropping of banana with white clover in continuous cropping banana orchards significantly reduced the number of F. oxysporum, increased soil pH and microbial diversity, changed the community structure of microorganisms, and then reduced the incidence of banana Fusarium wilt disease.
| Citation: | LIU Manyi, WANG Yutong, SUN Mingze, LI Rong, WANG Beibei. Effect of Intercropping of Banana with White Clover on Banana Fusarium Wilt and Soil Microbial Community[J]. Journal of Tropical Biology, 2021, 12(2): 219-227. doi: 10.15886/j.cnki.rdswxb.2021.02.011 |






 DownLoad:
DownLoad: