-
Megalurothrips usitatus is a globally significant pest posing severe threats to cowpea quality and safety[1]. It has been officially classified as a pest in Category I Crop Disease and Pest according to Document No.654 issued by the Ministry of Agriculture and Rural Affairs of China in 2023. This thrips species exhibits a short developmental cycle with multiple generations per growing season, enabling continuous infestation throughout all the growth stages of cowpea[2]. Its rasping-sucking mouthparts and ovipositor cause direct damage through feeding and egg-laying activities on floral organs, fruits, and tender meristems, resulting in characteristic symptoms including apical and basal necrosis of pods, growth retardation, leaf curling and wrinkling, and premature floral abscission, ultimately leading to substantial yield losses and quality degradation[3]. The pest's diminutive size (1–2 mm body length, with females typically larger than males), slender morphology, and cryptic behavior within floral structures collectively contribute to significant challenges in effective pest management.
Vision-based physical control methods have been demonstrated as an efficient, eco-friendly, and cost-effective strategy for pest management, achieving notable success in controlling aphids, Laodelphax striatellus, Bemisia tabaci, and Bactrocera dorsalis[4-7]. Regarding thrips control, Tang et al. verified blue sticky traps as highly effective against the common blossom thrips (Megalurothrips usitatus)[8]. Further studies by Wang et al. and Jin et al. revealed that ultraviolet (UV)-blocking greenhouse films significantly reduced populations of Thrips palmi and M. usitatus on netted melon (Cucumis melo var. reticulatus) and cowpea (Vigna unguiculata)[9-10]. Field trials by Wu et al. confirmed the superior efficacy of blue traps against the western flower thrips (Frankliniella occidentalis)[11]. Moreover, Mi et al. reported that 450 nm blue light and blue sticky traps exhibited optimal control effects on Thrips tabaci Recent findings by Ning et al. highlighted strong phototactic behavior of M. usitatus toward UV-A and white light under laboratory conditions[12-13].
The rapid advancement of RNA interference (RNAi) technology in pest management has provided innovative solutions for agricultural production and ecological balance. Insect RNAi operates through exogenous double-stranded RNA (dsRNA)-triggered sequence-specific degradation of homologous mRNA, leading to targeted gene silencing that disrupts normal insect growth, development, reproduction, and behavioral patterns. In 1998, Fire et al. first demonstrated this phenomenon by successfully interfering with endogenous gene expression in Caenorhabditis elegans through microinjection of exogenous dsRNA[14]. Subsequently, RNAi technology has been progressively applied to agricultural pest control. Singh et al. achieved effective downregulation of SNF7 and AQP gene expression in T. tabaci through feeding delivery of target-specific dsSNF7 and dsAQP[15]. Similarly, Wang et al. successfully suppressed GABA receptor expression in F. occidentalis using oral administration[16]. More recently, Huang et al. employed dsRNA injection to silence visual perception-related genes in Zeugodacus cucurbitae, specifically targeting long-wavelength-sensitive opsins (dsZcRh1, dsZcRh2, dsZcRh6) and ultraviolet-sensitive opsins (dsZcRh3, dsZcRh4)[17]. Nevertheless, current scientific literature reveals a notable research gap regarding RNAi-mediated manipulation of visual genes in M. usitatus, with no RNAi-based studies being reported to date.
Based on the application of RNA interference (RNAi) technology in studying visual mechanisms of M. usitatus, previous studies have identified two long-wavelength-sensitive opsin genes (Rh-1 and Rh-2) associated with visual perception in this pest species[18-20]. To elucidate the potential behavioral regulation mechanisms mediated by these opsin genes, we conducted RNAi-mediated silencing targeting the opsin genes and subsequently validated their spectral sensitivity responses to green light wavelengths. This investigation aims to establish fundamental evidence for developing visual disruption strategies targeting M. usitatus, thereby providing critical theoretical support for the innovative development of phototactic behavior-regulating agents in pest management systems.
HTML
-
The adults of M. usitatus were collected from cowpea (Vigna unguiculata) crops at Batou Experimental Base (18°23′ N, 109°10′ E) in Yazhou District, Sanya City, Hainan Province, China, during November to December 2023. Test insects were maintained under controlled laboratory conditions at the temperature of (26±1) °C and the relative humidity of (70±5)%, with a photoperiod of 14 h light: 10 h dark (14L:10D) hours. After 24-hour acclimatization the adults were fed on fresh cowpea pods, and healthy and active adults exhibiting normal locomotor behavior were selected for subsequent experimental procedures.
-
The reagents included TRIzol reagent (Thermo Fisher Scientific, USA), PrimeScript™ RT Reagent Kit with gDNA Eraser (TaKaRa, Japan), ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech, Nanjing, China), T7 High Yield RNA Transcription Kit (Vazyme Biotech, Nanjing, China), eosin (Servicebio, Wuhan, China), hematoxylin (Servicebio, Wuhan, China), hydrochloric acid-ethanol solution (Servicebio, Wuhan, China), universal tissue fixative (Servicebio, Wuhan, China), and eco-friendly dewaxing clearing agent (Servicebio, Wuhan, China), with all other chemicals being of domestic analytical grade. The instruments and equipment employed in this study comprised an SZ61 stereomicroscope (Olympus, Japan), AriaMx Real-Time PCR System (Agilent Technologies, USA), fluorescence microscope (Agilent Technologies, USA), rotary microtome (LEICA, Germany), DK320S constant-temperature water bath (Shanghai Jinghong Experimental Equipment, China), SPX-160H biochemical incubator (Xiamen Guoyi Scientific Instruments, China), Micro Drop UV-Vis spectrophotometer (BIO-DL, USA), along with ancillary materials including Parafilm sealing membrane (Thermo Fisher Scientific, USA), rice paper (Shanghai M&G Stationery, China), glass tubes (50 mm height, 38 mm OD, 35 mm ID; Donghai Shixuan Quartz, China), entomological pins (Shenzhen Huayang Biotechnology, China), and a green LED monochromatic light source (520 nm wavelength, 3 W power; Zhongshan Guzhen Youweigu Lighting, China).
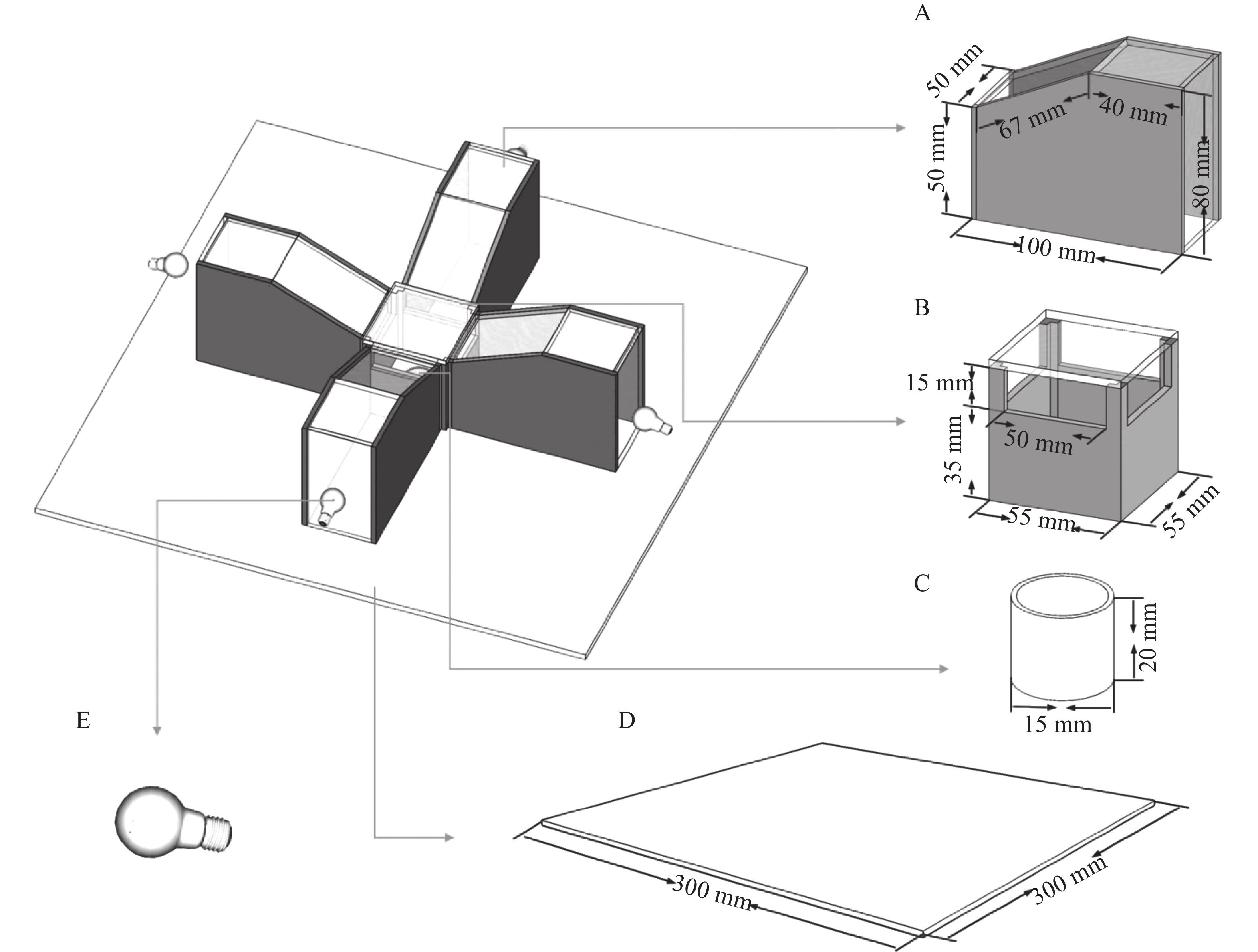
The behavioral assay apparatus was custom-built. The entire setup was constructed using transparent and opaque acrylic panels (6 mm thickness), with the dark-colored components in the diagram representing opaque acrylic panels and the light-colored sections denoting transparent ones. The apparatus consisted of five integrated components: a phototactic chamber (A), a response chamber (B), an insect collector (C), a base plate (D), and a light source (E). Detailed parameters of the experimental setup are illustrated in Fig. 1. The dark box featured a cubic structure (30 cm edge length) with 10 mm-thick walls and a hollowed-out base. It was assembled from black polystyrene foam panels to eliminate interference from external light sources.
-
Select 10–15 individuals each of first-instar nymphs, second-instar nymphs, prepupae, pseudopupae, and female/male adults of M. usitatus, and place them into pre-prepared 1.5 mL centrifuge tubes. Subsequently, position the tubes in a –20 °C freezer for 2–3 minutes to immobilize the test insects. Lay a cowpea leaf flat on the stage of an SZ61 stereomicroscope (Japan), placing insects from one developmental stage at a time above the leaf surface. During photography, use insect pins to adjust specimen orientation and positioning. For imaging the egg stage, position a female specimen at the center of a glass slide, apply 20 μL of deionized water to its central area, and carefully dissect the abdominal region using insect pins until eggs become observable.
-
Based on the previously obtained opsin genes Rh-1 (GenBank accession number PP154173), Rh-2 (GenBank accession number PP154174), and the enhanced green fluorescent protein (EGFP) gene (GenBank accession number AAB02572) from M. usitatus, dsRNA targeting fragments were designed. The β-actin gene (GenBank accession number KX108734) was selected as the internal reference. Primers were synthesized by Tsingke Biotechnology Co., Ltd., Guangzhou Branch (Table 1).
Gene Primer sequence (5′–3′) Amplicon length/bp Purpose Rh-1 F: CTTCGTCTACTTTCTGCCTCTT
R: TTCATCTTCTTGGCTTGCTC108 RT-qPCR Rh-2 F: CGCTCCGTACTCCGTCAAAC
R: CACCCATAACCCACGTCTCG120 β-actin F: CTTCGTCTACTTTCTGCCTCTT
R: TTCATCTTCTTGGCTTGCTC125 dsRh-1 F: TAATACGACTCACTATAGGGACAGTGGTAGACCAGGTGCC
R: TAATACGACTCACTATAGGGTGTTTGTCATAGGAGCAGCG427 RNAi dsRh-2 F: TAATACGACTCACTATAGGGGCTACAACGAGACGTGGGTT
R: TAATACGACTCACTATAGGGAGACGATGACTTTCAGCGGT363 dsEGFP F: TAATACGACTCACTATAGGGCAGTGCTTCAGCCGCTAC
R: TAATACGACTCACTATAGGGTGTTCTGCTGGTAGTGGTCG352 Note: The underlined part represents the T7 promoter sequence. Table 1. The information of primers
-
dsRNA was synthesized using the T7 High Yield RNA Transcription Kit (Vazyme Biotech Co., Ltd., Cat# TR101-01, China), and purified via phenol/chloroform extraction. To assess the quality of synthesized dsEGFP, dsRh-1, and dsRh-2, 2 µL of purified dsRNA samples were analyzed by gel electrophoresis, while 1 µL of each sample was subjected to spectrophotometric absorbance measurement. Purified dsRNA was stored at −80 °C for subsequent experiments.
-
During the experiment, a double-opening glass tube (height: 50 mm; outer diameter: 38 mm; inner diameter: 35 mm) was prepared as the feeding apparatus. One end of the tube was sealed with a parafilm membrane stretched to its thinnest layer. Fifty adult individuals of M. usitatus (female-to-male ratio approximately 3:1 or higher) were introduced into the tube, and the opposite end was covered with tissue rice paper to maintain ventilation, with the paper-covered end oriented downward. A 180 μL aliquot of dsRNA solution was pipetted onto the parafilm-sealed end, followed by an additional parafilm layer to prevent contamination and evaporation. The apparatus was incubated in a controlled environment chamber at the temperature of (26±1) °C, the relative humidity of (70±5)%, and a 14L: 10D photoperiod. Each dsRNA concentration was tested with three biological replicates, each containing 50 insects. Post-treatment samples were collected, flash-frozen in liquid nitrogen, and stored at −80 °C for subsequent analysis.
Real-time quantitative PCR (qPCR) methodology: Total RNA was extracted from samples using TRIzol reagent (Thermo Fisher Scientific, Cat# 15596026, USA). cDNA synthesis was performed following the protocol of the PrimeScript™ RT Reagent Kit with gDNA Eraser (TaKaRa, Cat# RR047A, Japan), and the synthesized cDNA was stored at −20 °C. RT-qPCR amplification was conducted using the ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Cat# Q711-02, China) according to the manufacturer’s instructions. Each sample was analyzed with three technical replicates. The relative expression levels of target genes were calculated using the 2−△△Ct method [21].
-
Preparation and administration of dsRNA solutions: Purified dsEGFP, dsRh1, and dsRh2 were diluted with RNase-free water to appropriate concentrations and mixed with 20% honey solution (prepared in RNase-free water) at a 1:1 ratio to generate dsRNA solutions at final concentrations of 1, 10, 100, 500, and
1000 μg·mL−1. These solutions were orally delivered to M. usitatus adults for 24 h. All other procedures followed the methodology outlined in Section 2.6.dsEGFP, dsRh-1, and dsRh-2 were mixed with 20% honey solution (prepared in RNase-free water) at a 1:1 ratio to generate dsRNA solutions at a final concentration of 100 μg·mL−1. Adult M. usitatus were fed on the dsRNA solutions (dsEGFP, dsRh-1, and dsRh-2) for 24, 48, 72, and 96 h, with fresh dsRNA solution replenished every 24 h. All other experimental procedures were consistent with the methods described in Section 2.6.
-
Adult M. usitatus were fed on dsRNA solutions (dsEGFP, dsRh-1, and dsRh-2) at a concentration of 100 μg·mL−1. Mortality was recorded at 24, 48, 72, and 96 h after treatment, with fresh dsRNA solution replenished every 24 h. The experiment included a treatment group (100 insects) and a control group (100 insects), totaling 400 insects. All other procedures followed the methodology described in Section 2.6.
-
The experiment was conducted under dark indoor conditions during daytime. Adult M. usitatus were fed on dsEGFP, dsRh-1, or dsRh-2 at a concentration of 100 μg·mL−1 along with fresh cowpea leaves for 72 h prior to evaluating their behavioral response to a green LED light source (wavelength: 520 nm). All ambient light sources in the laboratory were turned off to eliminate interference. Using an aspirator, adult M. usitatus were transferred into the insect introduction chamber (C) of the phototactic apparatus, which was then positioned at the center of the base plate (D). The phototactic chamber (A) and response chamber (B) were assembled onto the base plate (D), ensuring the introduction chamber remained centered within the response chamber. A monochromatic light source was affixed to the rear of the phototactic chamber (80 mm length × 50 mm width) and enclosed within a lightproof box to block external light. The light source (E) was activated for 10 min, after which the number of insects migrating into the illuminated phototactic chamber was recorded. The assay included five biological replicates, each comprising 30 insects. Following each trial, the apparatus was disinfected with 75% ethanol. Phototactic rate was calculated as: (Number of responsive insects/ Total number of introduced insects) × 100%.
-
Cross-regulatory expression analysis of opsin genes: Adult M. usitatus were fed on dsEGFP, dsRh-1, or dsRh-2 solutions at 100 μg·mL−1 for 72 h, with fresh dsRNA solution replenished every 24 h. All other experimental procedures were consistent with the methodology described in Section 2.6.
-
Statistical analyses were performed using GraphPad Prism version 9.5.1 (GraphPad Software, Inc., USA). Student’s t-test was employed to evaluate significant differences between two groups, while Tukey’s honestly significant difference (HSD) test was applied for comparisons among three or more groups; survival data were analyzed by Kaplan-Meier method with Breslow-Wilcoxon test.
1.1. Insects
1.2. Reagents and Apparatus
1.3. Observation on the Developmental Duration of M. usitatus
1.4. Opsin Gene Primers for M. usitatus
1.5. dsRNA Synthesis and Detection
1.6. Oral Delivery of dsRNA and Real-time Quantitative PCR (qPCR) Methodology in M. usitatus
1.7. Silencing Efficiency Assessment at Various dsRNA Concentrations and Time Points
1.8. Survival Curve Analysis in M. usitatus
1.9. Phototactic Behavior Assay in M. usitatus
1.10. Cross-regulatory Expression Analysis of Opsin Genes Following RNA Interference in M. usitatus
1.11. Data Analysis
-
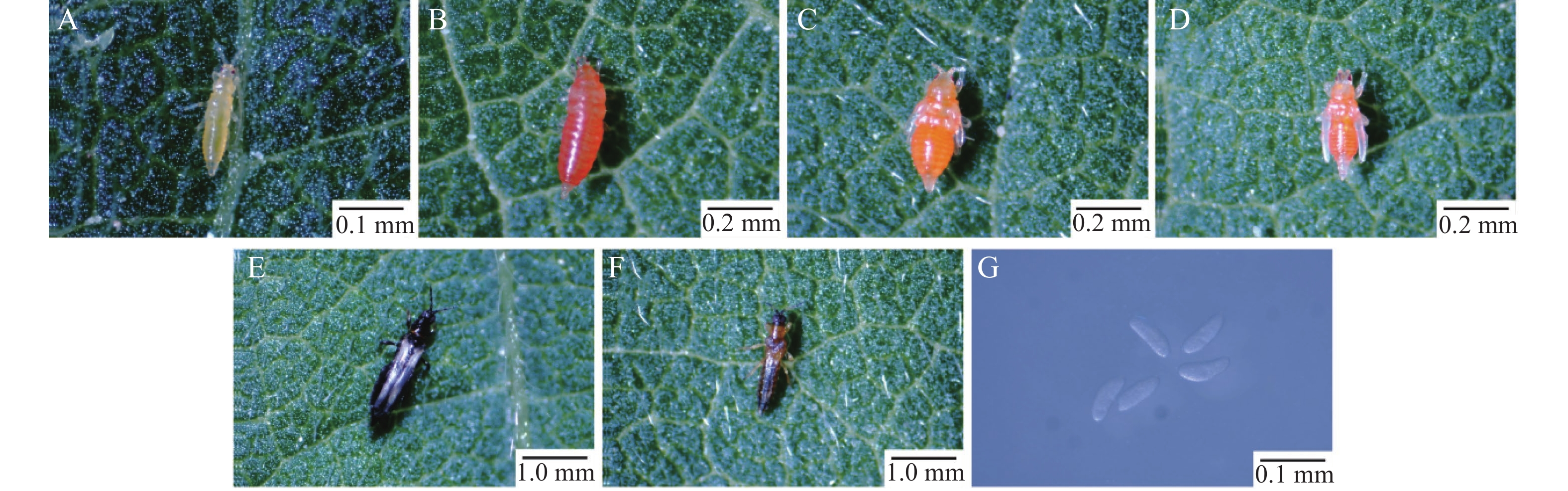
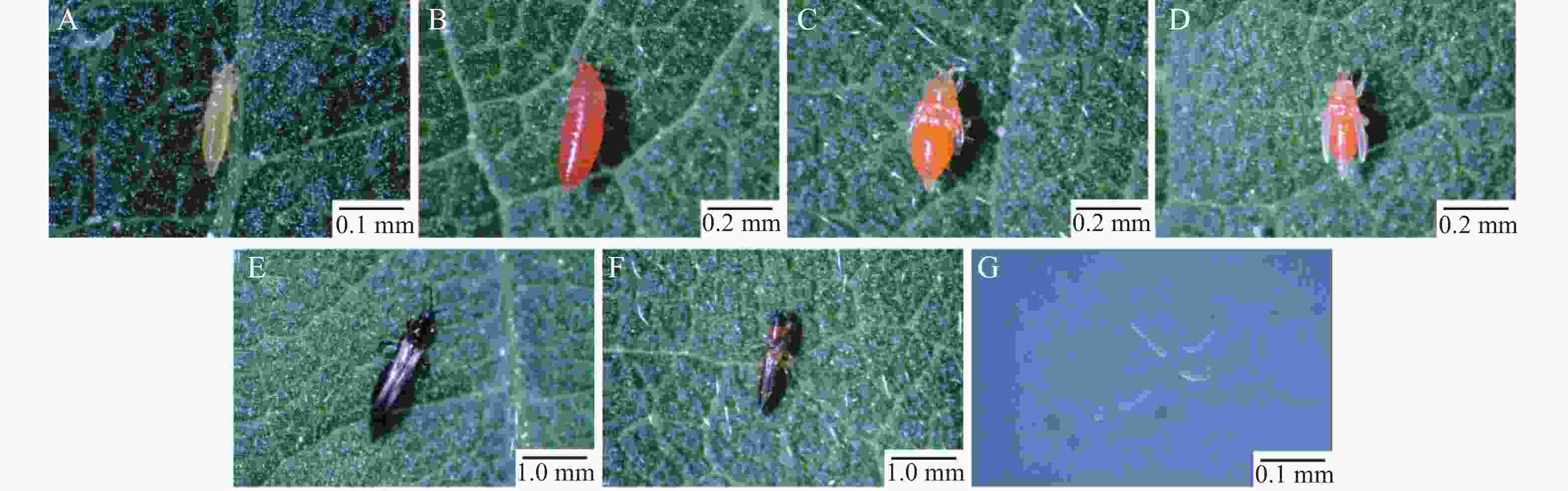
M. usitatus is a hemimetabolous insect, progressing through five developmental stages: egg, first-instar nymph, second-instar nymph, prepupa, pseudopupa, and adult (Fig. 2). The first-instar nymph measures approximately 0.5 mm in length, with a white or pale-yellow body and red punctate compound eyes (Fig. 2-A). The second-instar nymph is approximately 1 mm long, exhibiting an orange-red body and incompletely developed red punctate compound eyes (Fig. 2-B). The prepupa (1 mm in length) displays an orange-yellow body, gradually maturing compound eyes, and short, translucent white wing buds on the dorsum, with stubby, translucent antennae (Fig. 2-C). During the pseudopupal stage (1 mm in length), the orange-yellow body is close to adult morphology, featuring enlarged dorsal wing buds and antennae extending posteriorly (Fig. 2-D). Adult females (1–2 mm) are generally larger than males. Both sexes exhibit dark brown pigmentation along the anterior and posterior margins of the fringed wings and at the abdominal terminus, with creamy-white coloration elsewhere. Females possess a dark brown body, while males display a dark brown head and abdominal transverse bands, with the remaining body regions dark yellow (Fig. 2-E—F). Eggs are minute (0.1–0.2 mm), translucent, and white (Fig. 2-G).
-
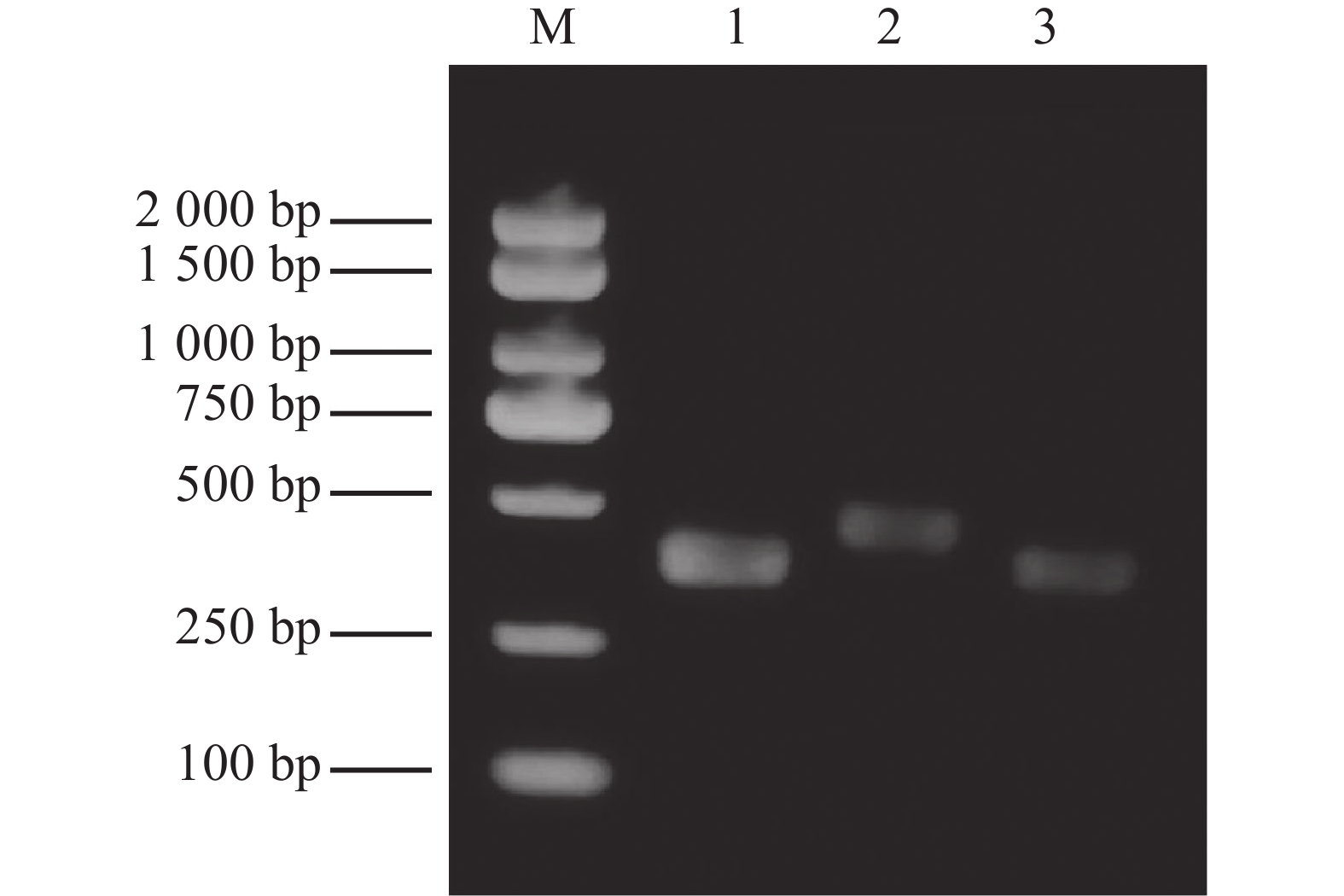
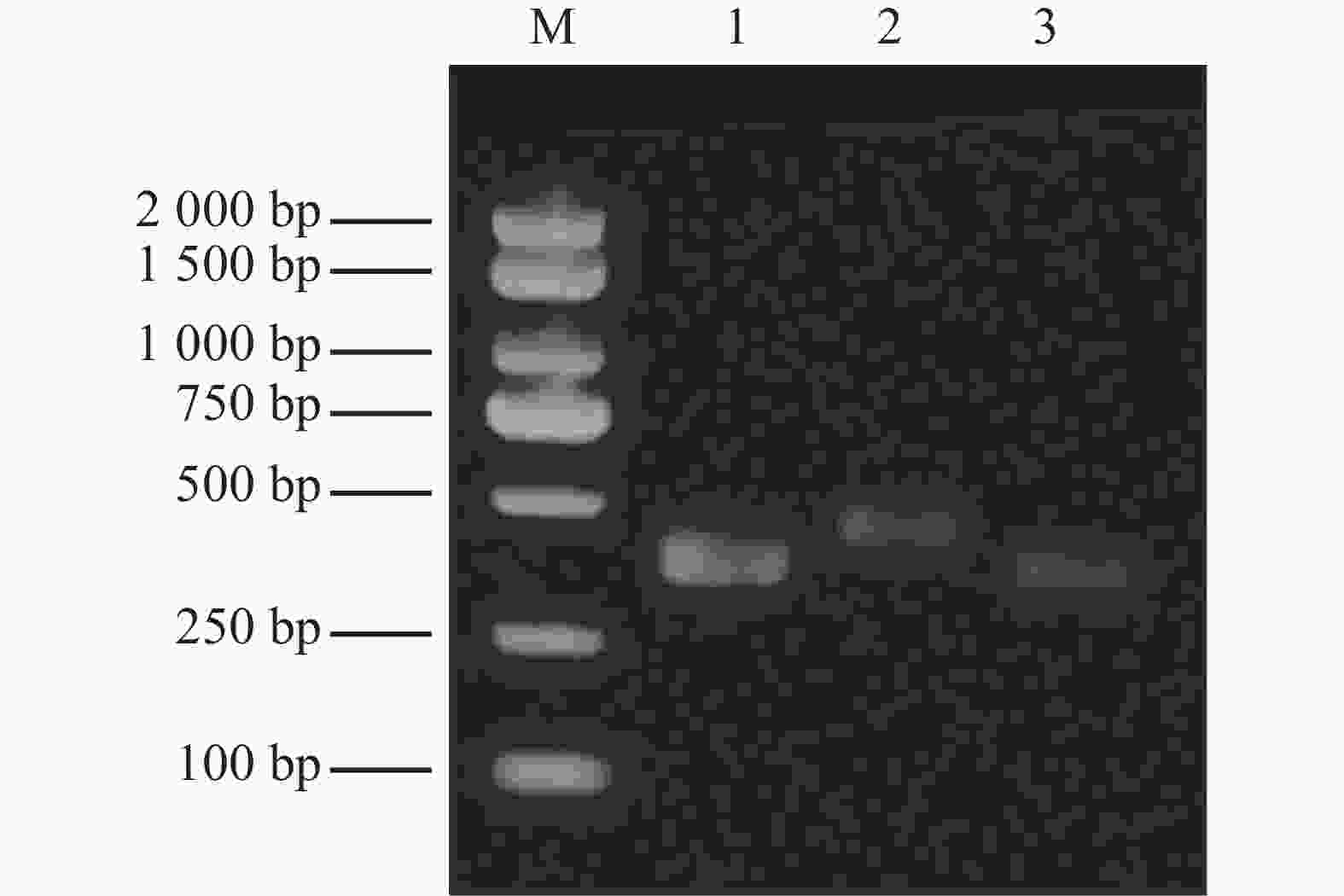
The synthesized dsEGFP, dsRh-1, and dsRh-2 exhibited single target bands with correct molecular sizes (dsEGFP: 352 bp; dsRh-1: 427 bp; dsRh-2: 363 bp). The OD260/OD280 ratios ranged between 1.9 and 2.0, confirming high-quality dsRNA suitable for subsequent experiments in M. usitatus (Fig. 3).
-
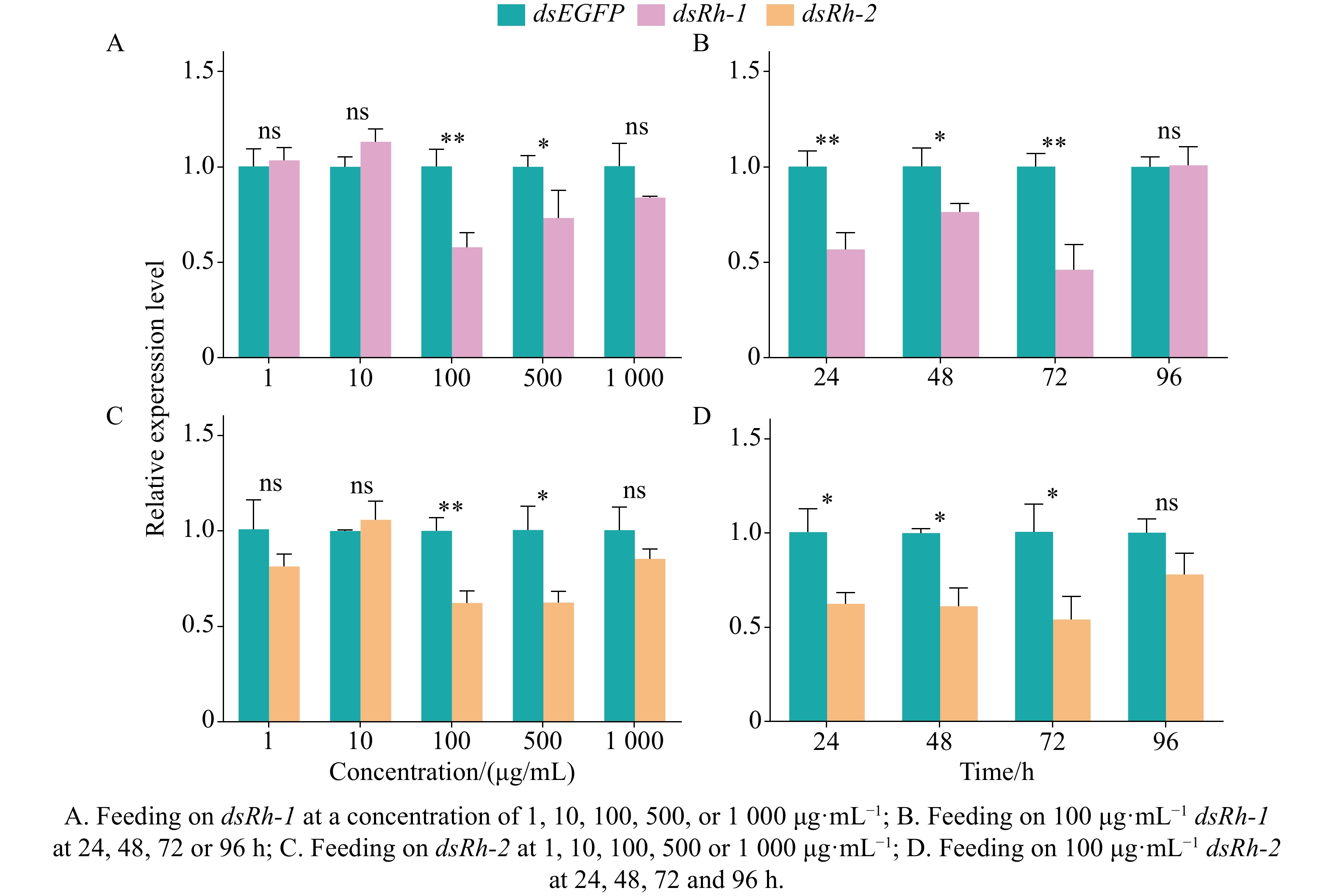
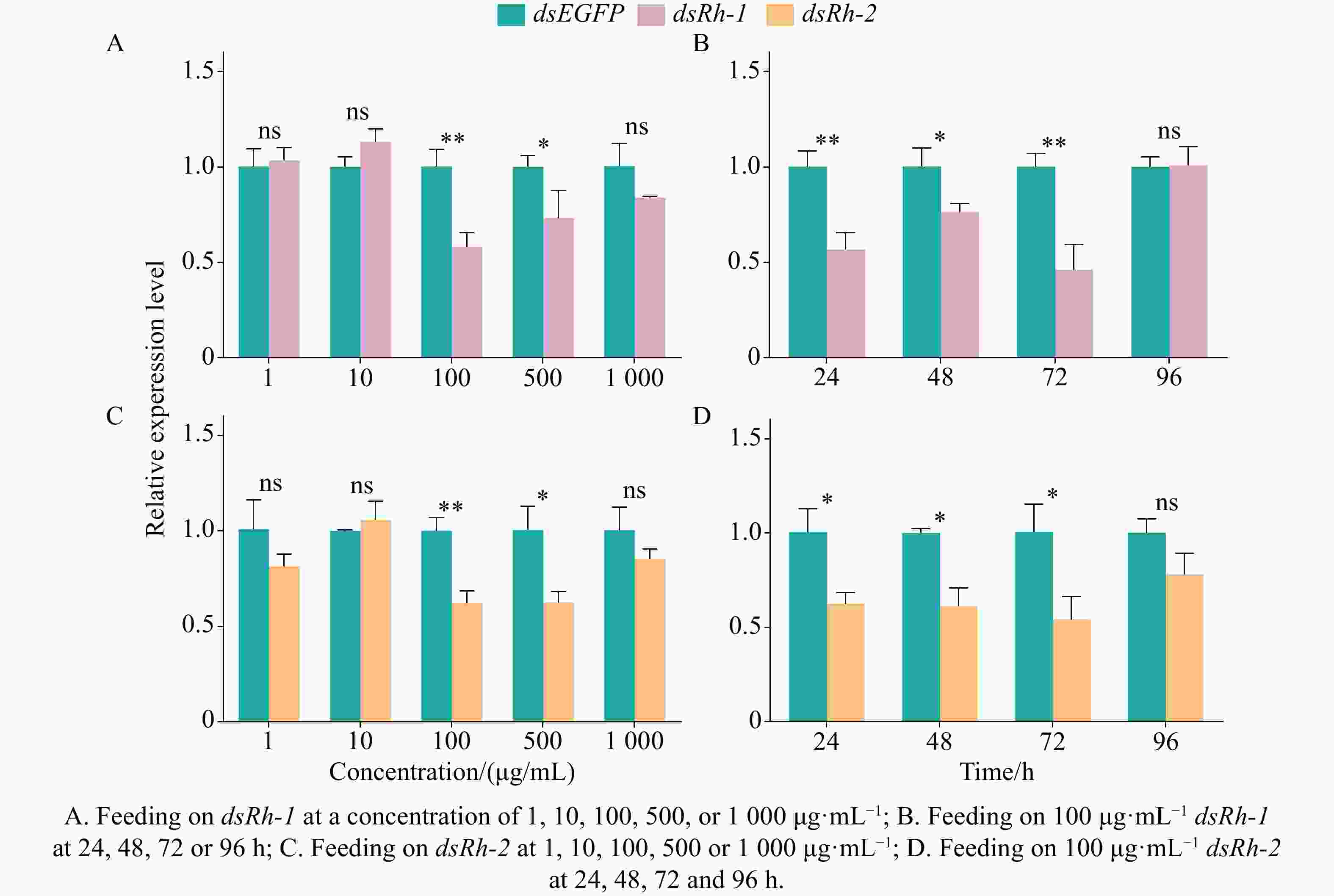
After 24 h of feeding on dsEGFP and dsRh-1 at a concentration of 1, 10, 100, 500, or
1000 μg·mL−1, significant differences (P < 0.05 or P < 0.01) were detected in the relative expression of the opsin gene Rh-1 in M. usitatus. The highest silencing efficiency (42.13%) was observed at 100 μg·mL−1, followed by 26.73% at 500 μg·mL−1. No significant changes (P > 0.05) in Rh-1 expression were observed in the groups treated with dsEGFP or dsRh-1 at 1, 10, or1000 μg·mL−1 (Fig. 4-A). When M. usitatus was fed on dsRh-1 at the optimal concentration of 100 μg·mL−1 for 24, 48, 72, or 96 h, significant temporal variations (P < 0.05 or P < 0.01) in Rh-1 expression were observed. Maximum silencing efficiency (53.87%) occurred at 72 h, followed by 43.30% at 24 h and 23.60% at 48 h. No significant difference (P > 0.05) in Rh-1 expression was detected between dsEGFP and dsRh-1 treated groups at 96 h (Fig. 4-B).After 24 h of feeding on dsEGFP and dsRh-2 at a concentration of 1, 10, 100, 500, or
1000 μg·mL−1, significant differences (P < 0.05 or P < 0.01) were observed in the relative expression of the opsin gene Rh-2 in M. usitatus. The highest silencing efficiencies were 37.70% at 100 μg·mL−1 and 37.54% at 500 μg·mL−1. In contrast, no significant changes (P > 0.05) in Rh-2 expression were detected in the groups treated with dsEGFP or dsRh-2 at 1, 10, or1000 μg·mL−1 (Fig. 4-C). When M. usitatus was fed on dsRh-2 at the optimal concentration of 100 μg·mL−1 for 24, 48, 72, or 96 h, significant temporal differences (P < 0.05) in Rh-2 expression were observed. Maximum silencing efficiency (45.82%) occurred at 72 h, followed by 38.79% at 48 h and 37.54% at 24 h. No significant difference (P > 0.05) in Rh-2 expression was detected between the dsEGFP and dsRh-2 treated groups at 96 h (Fig. 4-D) -
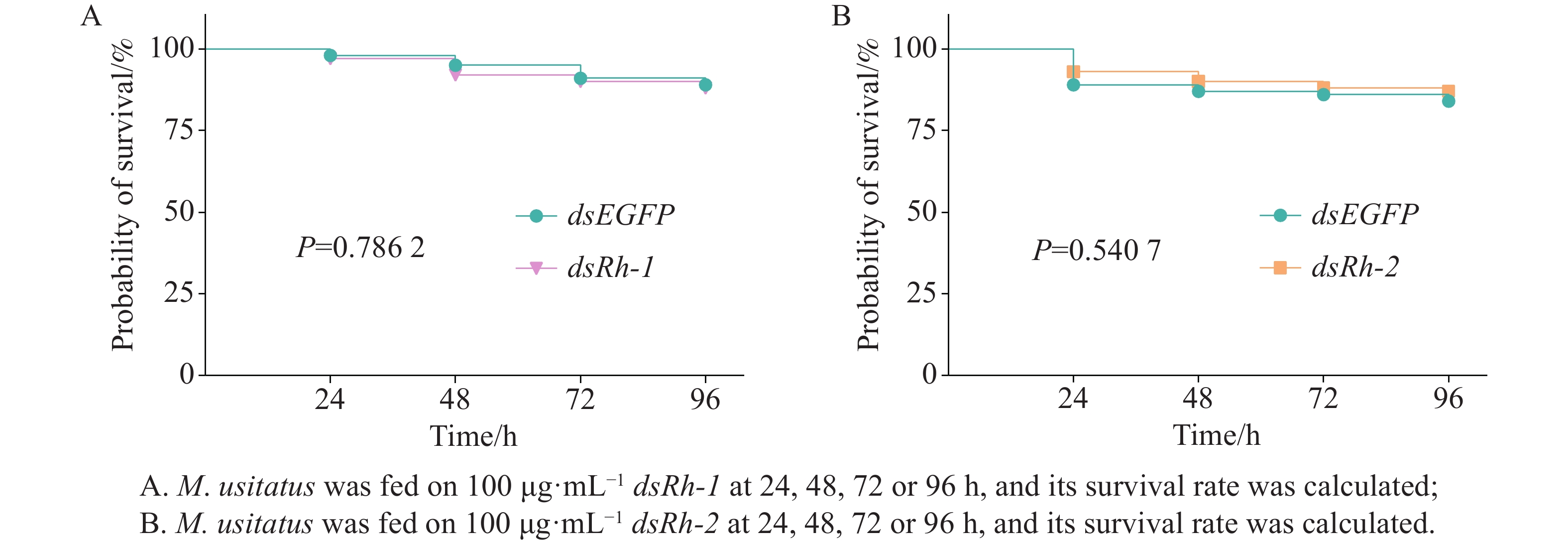
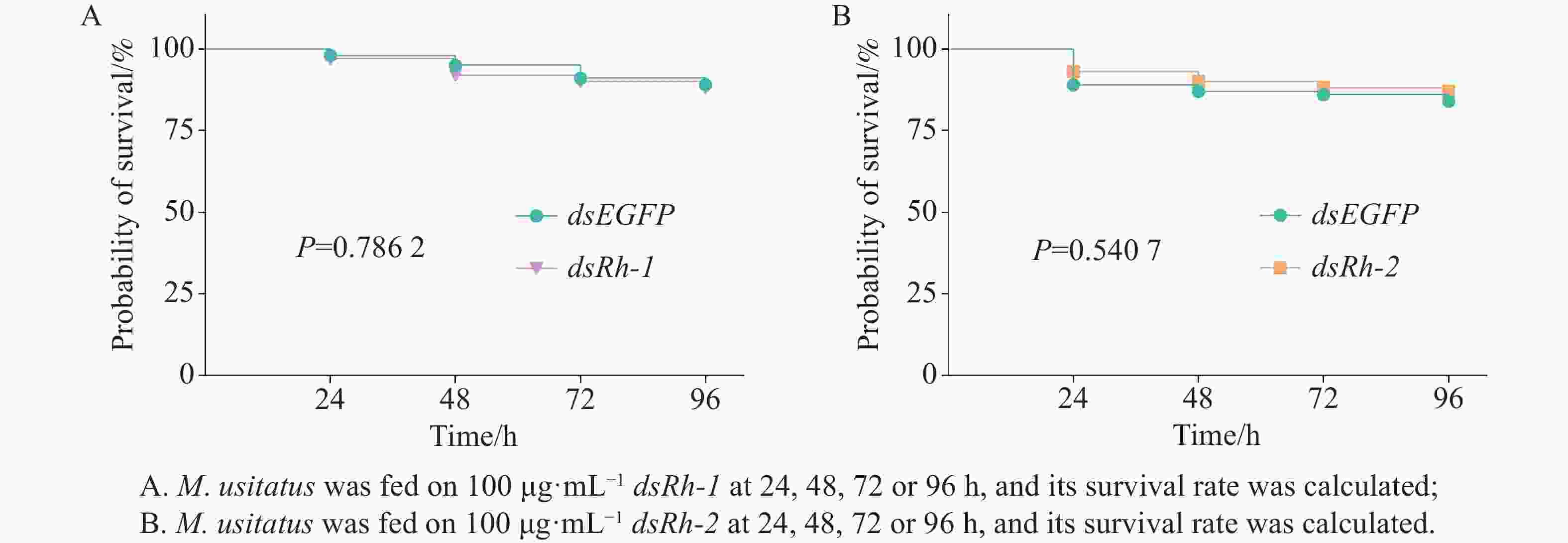
No significant differences in survival rates (P =
0.7862 ) were observed among M. usitatus adults treated with dsEGFP or dsRh-1 at a concentration of 100 μg·mL−1 for 24, 48, 72, or 96 h (Fig. 5-A). Similarly, survival rates showed no significant difference (P =0.5407 ) between groups fed on dsEGFP and dsRh-2 at a concentration of 100 μg·mL−1 over the same treatment duration (Fig. 5-B). -
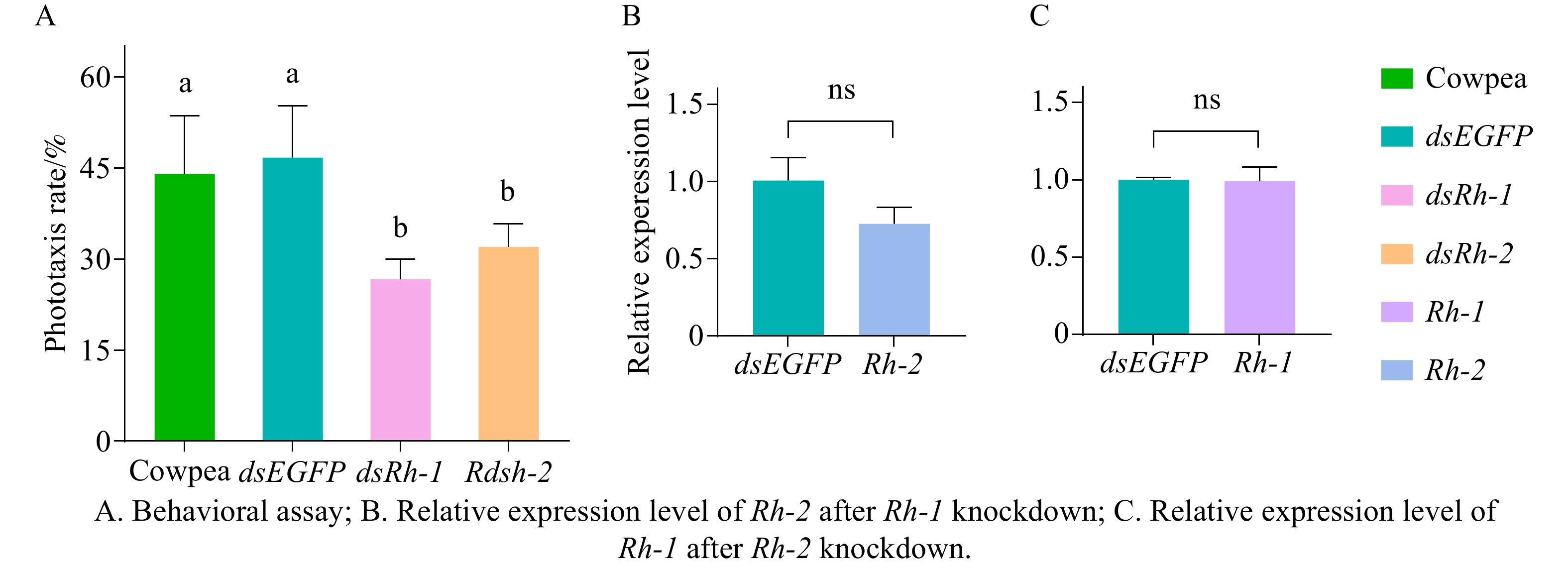
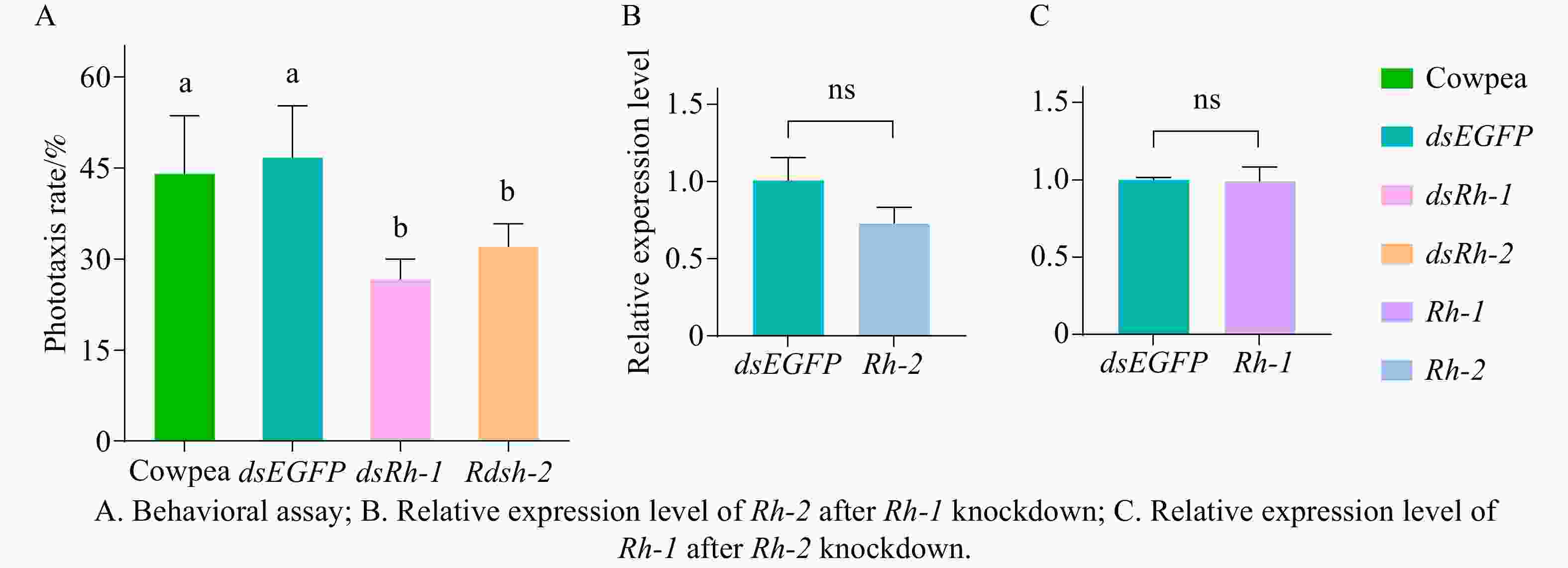
Adult M. usitatus were fed on fresh cowpea leaves or dsEGFP, dsRh-1 or dsRh-2 at a concentration of 100 μg·mL−1 for 72 h and subsequently assayed for phototactic response to a green LED light source (520 nm). No significant difference (P > 0.05) in phototactic response rate was observed between the cowpea-fed control group and the dsEGFP-treated group (Fig. 6-A). In contrast, the dsRh-1-treated group exhibited a phototactic response rate of 26.67%, significantly lower than that of both the control groups (P < 0.05, Fig. 6-A). Similarly, the dsRh-2-treated group showed a rate of 32.00%, significantly lower than that of the cowpea-fed or dsEGFP-treated groups (P < 0.05, Fig. 6-A).
After feeding M. usitatus on dsEGFP and dsRh-1 at 100 μg·mL−1 for 72 h (resulting in Rh-1 knockdown), qRT-PCR analysis revealed no significant difference in the relative expression level of the other opsin gene Rh-2 compared to the control group (P > 0.05, Fig. 6-B). Using the same method, feeding on dsRh-2 at 100 μg·mL−1 (achieving Rh-2 knockdown) similarly showed no significant difference in Rh-1 expression as against the controls (P > 0.05, Fig. 6-C).
2.1. Stage-specific Developmental Duration in M. usitatus
2.2. Quality Assessment of Synthesized dsRNA in M. usitatus
2.3. Evaluation of Silencing Efficiency in Rh-1 and Rh-2 Opsin Genes of M. usitatus
2.4. Life Table Determination of M. usitatus
2.5. Phototactic Behavior Assay and Opsin Gene Expression Analysis in M. usitatus
-
Upon reaching the adult stage, M. usitatus develops fully functional compound eyes, enabling acute visual perception. Concurrently, wing buds metamorphose into fringed wings, facilitating short-distance flight. Studies indicate that opsin genes are highly expressed in both female and male adults[19]. Field-based color trapping experiments demonstrated that blue sticky traps captured the highest number of adults, with an average of 337 individuals attracted at between 08:00–10:00[22]. Laboratory assays revealed that UV-A light exhibits stronger attraction to adults compared to white, blue, or green light[23]. Additionally, exposure to 420 nm light significantly prolongs the nymphal and pseudopupal stages while reducing eclosion rates and adult survival[23]. Broad-spectrum illumination (460–730 nm) also markedly suppresses oviposition capacity in females[24]. These findings suggest a potential correlation between adult-stage opsin gene overexpression and heightened responsiveness to color-based trapping, as well as light-mediated disruptions to developmental processes likely linked to visual gene regulation. Consequently, visual-targeted control strategies during this critical life stage are essential for effective management of M. usitatus. Innovative approaches integrating chromatic traps and photoperiod modulation could enhance eco-friendly control efficacy against this pest species.
Studies utilizing RNA interference (RNAi) to silence insect opsin genes remain relatively limited. Chen et al. successfully employed CRISPR/Cas9 to knockout the LW-opsin gene in Plutella xylostella, creating a LW-13 mutant line that showed significantly reduced phototactic responses to seven light spectra (white, ultraviolet, blue, yellow, green, red, and infrared) under 2.5 lx illumination[25]. Similarly, Li et al. achieved 44.79%, 54.81%, and 43.00% reductions in phototactic behavior towards ultraviolet, blue, and green light, respectively in Diaphorina citri through oral delivery of dsRNA targeting opsin genes Dc-UV, Dc-BW, and Dc-LW[26]. Wang et al. demonstrated that microinjection of Rh6 targeting dsRNA in Bactrocera minax adults significantly decreased their preference for green substrates and reduced oviposition on immature (green) citrus fruits compared to mature (yellow) controls[27]. Complementary findings by Liu et al. showed that injecting 6 μg of Se-lw specific dsRNA into each compound eye of Spodoptera exigua led to a 57.1% decrease in green light phototactic after 15 min exposure to green LEDs[28]. Our study corroborates these findings, demonstrating that feeding M. usitatus adults on dsRh-1 or dsRh-2 at 100 μg·mL−1 for 72 h significantly reduced their phototactic preference for green light (520 nm). This RNAi-mediated suppression of opsin genes effectively alters spectral sensitivity in this pest species. The practical application of this approach could be particularly valuable for protecting cowpea (Vigna unguiculata) crop, as the plants maintain green pigmentation throughout their growth cycle, making them potentially less attractive to opsin-silenced M. usitatus populations. In follow-up assays, M. usitatus adults fed on dsRh-1 or dsRh-2 at 100 μg·mL−1 for 24, 48, 72, or 96 h exhibited no significant differences in survival rate compared to the dsEGFP treated control group (P > 0.05), confirming that RNAi mediated opsin silencing, unlike chemical pesticides, avoids broad-spectrum toxicity and ensures agricultural safety. After feeding on dsRh-1 at 100 μg·mL−1 for 72 h, the relative expression level of opsin gene Rh-2 showed no significant difference compared to the control group (P > 0.05). After feeding on dsRh-2 using the same method, the relative expression level of Rh-1 also showed no significant difference (P > 0.05). These suggest that Rh-1 and Rh-2 may possess independent regulatory regions and exhibit autonomous expression regulation mechanisms. However, deeper functional operations of opsin genes and related synergistic effects require further verification. Wang Yaohui reported that after interfering with opsin genes in Bactrocera minax and Bactrocera dorsalis, the expression levels of the target opsin genes were significantly down-regulated, while other opsin genes showed no significant changes, consistent with the results of this study[29]. This indicates that independent expression of opsin genes likely reduces the complexity of visual signal integration, enabling each opsin gene to maintain independent spectral sensitivity, which can be validated by methods such as electrophysiology. qRT-PCR results by Liu et al. demonstrated that after knocking down the opsin gene Se-lw, the expression levels of other opsin genes increased, differing from the results of this study[28]. The discrepancy may be related to neural feedback compensation mechanisms of opsin Se-lw, insect species, and experimental methods.
This study demonstrates that RNA interference (RNAi)-mediated silencing of opsin genes in adult M. usitatus alters their spectral sensitivity. These findings provide foundational data for developing visual disruptants and phototactic-based control strategies targeting this pest species.
-
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.






 DownLoad:
DownLoad: