-
主持人:徐 冉
糖尿病是由胰岛素分泌缺陷或胰岛素作用障碍引起的高血糖为特征的代谢性疾病,是三大慢性疾病之一[1]。我国的糖尿病患者人数居世界首位,其中Ⅱ型糖尿病是糖尿病的主要类型,人数约占总患者的90%[2-4]。α-葡萄糖苷酶可以抑制糖苷酶活性和延缓餐后血糖浓度的上升,在治疗Ⅱ型糖尿病中具有显著优势[5]。目前临床应用的α-葡萄糖苷酶抑制剂药物如阿卡波糖、伏格列波糖等,其对人体肠道和肝脏均具有副作用[6]。因此,开发安全价廉的天然辅助降血糖物质具有重要意义。常用的挥发油提取方法主要有水蒸气蒸馏法、溶剂浸取法、超临界CO2萃取法等[7]。超临界CO2萃取法出油率高,设备昂贵,且成本较高;有机溶剂浸提法操作简单,但耗时长,挥发油品质不高,易有溶剂或杂质存留;而水蒸气蒸馏法成本低,操作简便,无污染,且脂溶性组分损失少,适合规模生产。石碌含笑(Michelia shiluensis, M. shiluensis)属木兰科(Magnoliaceae)含笑属(Michelia Linn)植物,也是海南特有树种和国家Ⅱ级濒危保护植物[8-9],主要分布于海南省的中部以及南部的山区,其他地区也有少量分布,是热带和亚热带地区极具发展前景的珍贵树种。精油是植物衍生的产品,通常由数十至数百种挥发性化合物组成,富含许多重要的活性成分,已经应用于化妆品、食品、医药以及生物科技等领域[10-11]。木兰科含笑属植物的精油多具有芳香气味且具有抗氧化、抑菌等生物活性,如白兰(Michelia alba)和台湾含笑(Michelia compressa)精油的抗真菌活性[12-13]。黄心夜合(Michelia martini)多个部位中的挥发油在体外和体内实验中都表现出较强的抗氧化和降血脂的活性[14]。目前,有关石碌含笑的研究主要集中在繁育栽培、种群结构等,而关于石碌含笑精油与其相关活性的研究尚未见有报道[15-16],故选用石碌含笑栽培种的花苞和叶片作为原料,用水蒸气蒸馏法提取精油,通过GC-MS分析精油中的挥发性成分,并探讨精油对α-葡萄糖苷酶的抑制作用,为石碌含笑资源高值化利用提供新思路,同时也为其在降血糖功能的深入研究提供理论依据。
HTML
-
2021年4月于海南省海口市采集长势良好,树龄超10年的石碌含笑花蕾期的新鲜花苞和叶片。该植株花期为4月下旬至5月下旬,花蕾期为4月上旬至中旬。乙醚(分析纯):四川西陇科学有限公司;二甲基亚砜(DMSO):天津富宇精细化工有限公司;磷酸氢二钠:西陇科学股份有限公司;磷酸二氢钠:西陇科学股份有限公司;α-葡萄糖苷酶、对硝基苯基-α-D-吡喃葡萄糖苷(PNPG):Sigma Chemical。
-
气相色谱-质谱联用仪(7820-5977):安捷伦公司,美国;全波长多功能酶标仪(Synergy H1):Bio-Tek,美国;电子秤(EN2062):上海民侨精密科学仪器有限公司;电热套(98-1B):天津泰斯特仪器有限公司。
-
将石碌含笑新鲜的花苞和叶片分别进行剪碎处理,将280.5 g花苞和 589.5 g叶片分别放进圆底烧瓶中加入蒸馏水浸没进行水蒸气蒸馏提取,煮沸后连续提取8 h直至精油不再增加后转移出上层精油部分,利用乙醚萃取,V乙醚∶V精油=1∶1,萃取3次,放置样品至其中的乙醚完全挥发后,称取精油质量,获得花苞精油0.30 g,叶片精油0.28 g,提取率分别为0.11%和0.05%。将精油密封避光,置于冰箱4 ℃保存。
-
气相色谱条件:采用HP-5MS 5% Phenyl Methyl Siloxane(30 m×0.25 mm×0.25 μm)弹性石英毛细管柱;升温程序:柱温50 ℃,以5 ℃·min-1升温至310 ℃,保持10 min;汽化室温度为250 ℃;载气为高纯He(99.999%);柱前压43 kPa,载气(He)流速1.0 mL·min-1;进样量1.0 μL, 进样方式采用不分流进样,溶剂延迟时间为4 min。
质谱条件:电子轰击(EI)离子源,电子能量70 eV,接口温度280 ℃,离子源温度230 ℃,四级杆温度150 ℃,调谐方式为标准调谐,电子倍增电压1 718 kV,质量扫描范围为40~800 m/z。
-
采用PNPG法对两个部位精油进行α-葡萄糖苷酶抑制活性测定[17]。配制0.1 mol·L-1, pH6.8磷酸盐缓冲溶液(PBS);利用PBS溶解α-葡萄糖苷酶配制0.2 U·mL-1α-葡萄糖苷酶溶液;配制2.5 mmol·L-1的PNPG溶液;DMSO溶解配制1.5 mmol·L-1金雀异黄酮溶液作为阳性对照,溶解样品配制初始浓度为3 g·L-1。取450 μL α-葡萄糖苷酶溶液与45 μL待测样品溶液混合摇匀,空白对照和本底对照也是加入45 μL待测样品溶液(3次重复),阴性对照为45 μL DMSO溶液,阳性对照为45 μL金雀异黄酮溶液,在96孔板反应15 min后,空白和本底对照中加入40 μL PBS溶液,阴性对照、阳性对照以及其余待测样品溶液中加入40 μL PNPG溶液,反应15 min后在405 nm波长下测定每孔的吸光度(OD)。计算公式如下:
式中,OD阴:阴性对照吸光度值; OD本底:本底的吸光度值;OD样:待测样品的吸光度值; OD空: 空白对照吸光度值。
-
所有试验重复进行3次,采用SPSS 26.0软件进行单因素分析(One-way ANOVA)和邓肯检验(Duncan’s test)并进行均值比较(P< 0.05),结果以均值±标准偏差来表示,采用Excel 2022软件对分析后的试验数据进行处理并作图。
-
按上述GC-MS条件对花苞和叶片的精油的化学成分进行分析,得到花苞和叶片的精油的总离子流色谱图(图1),用峰面积归一化法计算所鉴定化合物的相对百分含量, 两个部位成分种类及含量分析结果见表1。图1表明,在保留时间0~25 min内的离子流和峰面积差异不明显,在保留时间25 ~40 min 内,花苞挥发油的离子流强度和峰面积均较大。花苞和叶片的挥发油的峰最高强度和峰面积最大值均出现在保留时间20 min左右,其化学成分分别为β-红没药烯和愈创木醇。保留时间在15~28 min的化合物主要为倍半萜类成分。从两种挥发油中鉴定出的化合物的总相对含量分别达到99.99%和100%,较为充分地实现分析两种挥发油的目的。

Figure 1. GC-MS total ion flow diagram of the essential oil of M. shiluensis (A) Flower bud essential oil (B) Leaf essential oil
编号No. 化合物名称 Compound name 分子式Molecular formula 保留时间/minRetention time/min 相对含量/%Relative content/% 花苞Flower bud 叶片Leaf 花苞Flower bud 叶片Leaf 1 δ-Elemene δ-榄香烯 C15H24 16.432 - 0.65 - 2 β-Elemene β-榄香烯 C15H24 17.887 15.032 9.7 1.98 3 (-)-α-Santalene (-)-α-檀香烯 C15H24 18.557 - 2.12 - 4 γ-Elemene γ-榄香烯 C15H24 18.896 16.035 1.35 0.83 5 Trans-β-bergamotene 反式-β-波旁烯 C15H24 18.943 - 2.98 - 6 Guaia-6,9-diene 6,9-愈创二木烯 C15H24 19.151 - 0.58 - 7 (-)-Isoledene (-)-异喇叭烯 C15H24 19.299 - 0.8 - 8 (E)-β-Famesene (Z)-β-金合欢烯 C15H24 19.43 - 2.07 - 9 (+)-Aromandendrene (+)-香橙烯 C15H24 19.62 - 3.06 - 10 γ-Cadinene γ-杜松烯 C15H24 19.982 - 1.38 - 11 α-Curcumene α-姜黄烯 C15H24 20.13 17.257 10.27 2.02 12 (Z)-β-Farnesene (Z)-β-金合欢烯 C15H24 20.189 - 3.48 - 13 Zingiberene α-姜油烯 C15H24 20.409 17.602 2.86 2.84 14 β-Maaliene β-马榄烯 C15H24 20.587 17.708 3.41 2.52 15 β-Bisabolene β-红没药烯 C15H24 20.771 17.898 10.64 2.7 16 Butylated hydroxytoluene 2,6-二叔丁基对甲酚 C15H24O 20.836 17.952 0.72 0.44 17 β-Sesquiphellandrene β-倍半水芹烯 C15H24 21.104 18.225 3.22 1.67 18 Elemol 榄香醇 C15H26O 21.703 18.824 0.94 4.4 19 Spatulenol 桉油烯醇 C15H24O 22.403 19.436 1.37 1.71 20 Guaiol 愈创木醇 C15H26O 22.878 20.112 5.48 25.22 21 Eremophila-9,11(13)-dien-12-ol 艾里莫芬-9,11(13)-二烯-12醇 C15H24O 23.05 - 0.98 - 22 Zingiberenol 姜醇 C15H26O 23.181 21.934 2.63 0.44 23 γ-Eudesmol γ-桉叶油醇 C15H26O 23.383 20.706 0.7 3.12 24 (-)-Globulol (-)-蓝桉醇 C15H26O 23.513 19.578 3.59 1.56 25 — 23.733 - 0.65 - 26 Guaiol acetate 愈创木酚乙酸酯 C17H28O2 23.798 - 1 - 27 Aristolen 马兜铃烯 C15H24 23.935 - 1.39 - 28 β-Eudesmol β-桉叶醇 C15H26O 24.083 21.109 2.08 3.8 29 α-Eudesmol α-桉叶醇 C15H26O 24.13 21.181 3.5 2.79 30 Caryophyllene oxide 氧化石竹烯 C15H24O 24.326 25.65 2.4 0.39 31 Bulnesol 异愈创木醇 C15H26O 24.439 24.439 3 11.76 32 Trans-longipinocarveol 反式长叶香芹醇 C15H24O 25.015 - 0.9 - 33 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol6-异丙烯基-4,8a-二甲基-1,2,3,5,6,7,8,8a-八氢萘-2-醇 C15H24O 25.318 - 0.89 - 34 3-Isopropyl-6,7-dimethyltricyclo[4.4.0.0(2,8)]decane-9,10-diol3-异丙基-6,7-二甲基三环[4.4.0.0 (2,8)]癸烷-9,10-二醇 C15H26O2 25.632 - 0.81 - 35 4-(2,2,6-Trimethyl-7-oxabicyclo[4.1.0]hept-4-en-1-yl)pent-3-en-2-one4-[2,2,6-三甲基-7-氧杂二环[4.1.0]庚-1-基]-3-丁烯-2-酮 C14H20O2 25.864 - 1.01 - 36 Valerenal 缬草烯醛 C15H22O 26.232 - 0.53 - 37 Isoaromadendrene epoxide 异香橙烯环氧化物 C15H24O 27.033 - 0.63 - 38 1,7-Dimethyl-4-(1-methylethyl)-Spiro[4.5]dec-6-en-8-one1,7-二甲基-4-(1-甲基乙基) -螺[4.5]癸-6-烯-8-酮 C15H24O 28.612 - 0.63 - 39 (Z,Z,Z)-1,8,11,14-Heptadecatetraene (Z,Z,Z)-1,8,11,14-十七碳四烯 C17H28 28.73 25.893 5.59 13.54 40 Bicycloelemene 双环榄香烯 C15H24 - 13.631 - 0.89 41 Caryophyllene 石竹烯 C15H24 - 15.649 - 0.9 42 Alloaromadendrene 香树烯 C15H24 - 16.652 - 0.68 43 γ-Muurolene γ-衣兰油烯 C15H24 - 17.05 - 0.38 44 Germacrene-D 大牛儿烯-D C15H24 - 17.151 - 1.45 45 Bicyclogermacrene 双环-大根老鹳草烯 C15H24 - 17.542 - 1.46 46 (E)-3,7,11-trimethyl-1,6,10-Dodecatrien-3-ol 反式-橙花叔醇 C15H26O - 19.216 - 2.81 47 Viridiflorol 绿花白千层醇 C15H26O - 19.756 - 5.71 48 2-Ethylbutyric acid, phenethyl ester 乙酸苯乙酯 C14H20O2 - 20.949 - 0.57 49 (E)-1-(1,3-dimethyl-1,3-butadienyl)-2,2,6-trimethyl-7-oxabicyclo[4.1.0]heptane (E)-1-(1,3-二甲基-1,3-丁二烯基)-2,2,6-三甲基-7-氧杂双环[4.1.0]庚烷 C15H24O - 23.121 - 0.48 50 (-)-Spathulenol (-)-斯巴醇 C15H24O - 23.513 - 0.39 51 2,2'-Methylenebis[6-(1,1-dimethylethyl)-4-methyl-phenol2,2'-亚甲基双-(4-甲基-6-叔丁基苯酚) C23H32O2 - 35.265 - 0.56 注: “-”指未检测到该成分。Note: “-” means the component is not detected.Table 1. Composition of the essential oils from buds and leaves of M. shiluensis
石碌含笑花苞精油中共分离鉴定出39个化学成分(表1),它们的化学结构包括36个倍半萜和3个其他类型物质。花苞精油中包含多种特征香气成分及生物活性成分,其中β-红没药烯(10.64%)、α-姜黄烯(10.27%)、β-榄香烯(9.70%)、(Z,Z,Z)-1,8,11,14-十七碳四烯(5.59%)、愈创木醇(5.48%)相对含量较高。C15H24的17种同分异构体都为倍半萜烯类化合物;C15H24O的8种同分异构体有4种是倍半萜醇类,2种倍半萜氧化物类,1种倍半萜酚类、1种倍半萜酮类;C15H26O的8种同分异构体都为倍半萜醇类。石碌含笑叶片精油中共鉴定出31个化学成分(表1),主要是倍半萜醇类化合物,主要成分有愈创木醇(25.22%)、异愈创木醇(11.76%)、(Z,Z,Z)-1,8,11,14-十七碳四烯(13.54%)、[1aR-(1aα,4β,4aβ,7α,7aβ,7bα)]-十氢-1,1,4,7-四甲基-1H-环丙烷[e]天青-4-醇(5.71%)、α-桉叶油醇(3.79%)、β-桉叶油醇(3.8%)、γ-桉叶油醇(3.12%)等。叶片精油中仅有C15H24 (13种)、C15H24O (4种)、C15H26O (10种)3类同分异构体,C15H24的13种同分异构体都为倍半萜烯类化合物;C15H24O的同分异构体有2种是倍半萜醇类,1种倍半萜氧化物类,1种倍半萜酚类;C15H26O的同分异构体都为倍半萜醇类。
表1和表2所示,花苞和叶片精油中含有相同的化学成分,但各成分相对含量有所差异,两种精油共有的成分18个,愈创木醇、异愈创木醇、α-姜黄烯等主要成分含量有明显差异。两个部位精油的组成均以醇类和烯烃类为主,花苞中的烯烃类物质有18个(65.55%),β-红没药烯、α-姜黄烯以及β-榄香烯为主要成分,醇类物质有14个(27.82%),含量最高的是愈创木醇。β-榄香烯具有抑制肿瘤生长,增强脾脏和胸腺免疫功能的功效,这与其结构中的三个不饱和双键密切相关[18-19]。β-红没药烯属于姜辣素,姜辣素可以显著降低小鼠的血糖值[20]。α-姜黄烯、β-红没药烯及姜醇等存在的异戊烯基团可能对化合物提高α-葡萄糖苷酶的抑制作用具有一定的影响。Zhang等[21]通过构效关系研究发现1个额外的异戊烯基团可以提高α-葡萄糖苷酶的抑制作用。叶片精油中主要烯烃类物质有14个(33.86%),含量最高的是α-姜油烯,醇类物质有14个(64.76%),主要醇类物质是愈创木醇和异愈创木醇。愈创木醇和异愈创木醇是一类七元环偶联结构的倍半萜烯化合物,该类型的化合物多数具有乙酰胆碱酯酶抑制活性,且愈创木烷型倍半萜糖苷的C-10位羟基可能具有重要的抗炎活性[22-23]。有研究表明,愈创木醇可以抑制M2巨噬细胞而达到抗肺癌的作用[24]。
部位Part 烯烃类/%Olefins/% 醇类/%Alcohols/% 酚类/%Phenolics/% 脂类/%Lipids/% 醛类/%Aldehydes/% 酮类/%Ketones/% 其他/%Others/% 花苞 Flower bud 65.55 27.82 0.72 - 0.53 1.64 3.68 叶片 Leaf 33.86 64.76 1.00 0.57 - - 0.39 注: “-”指未检测到该类化合物。Note: “-”means that no such compound is detected.Table 2. Main compounds of the essential oils from buds and leaves of M. shiluensis
-
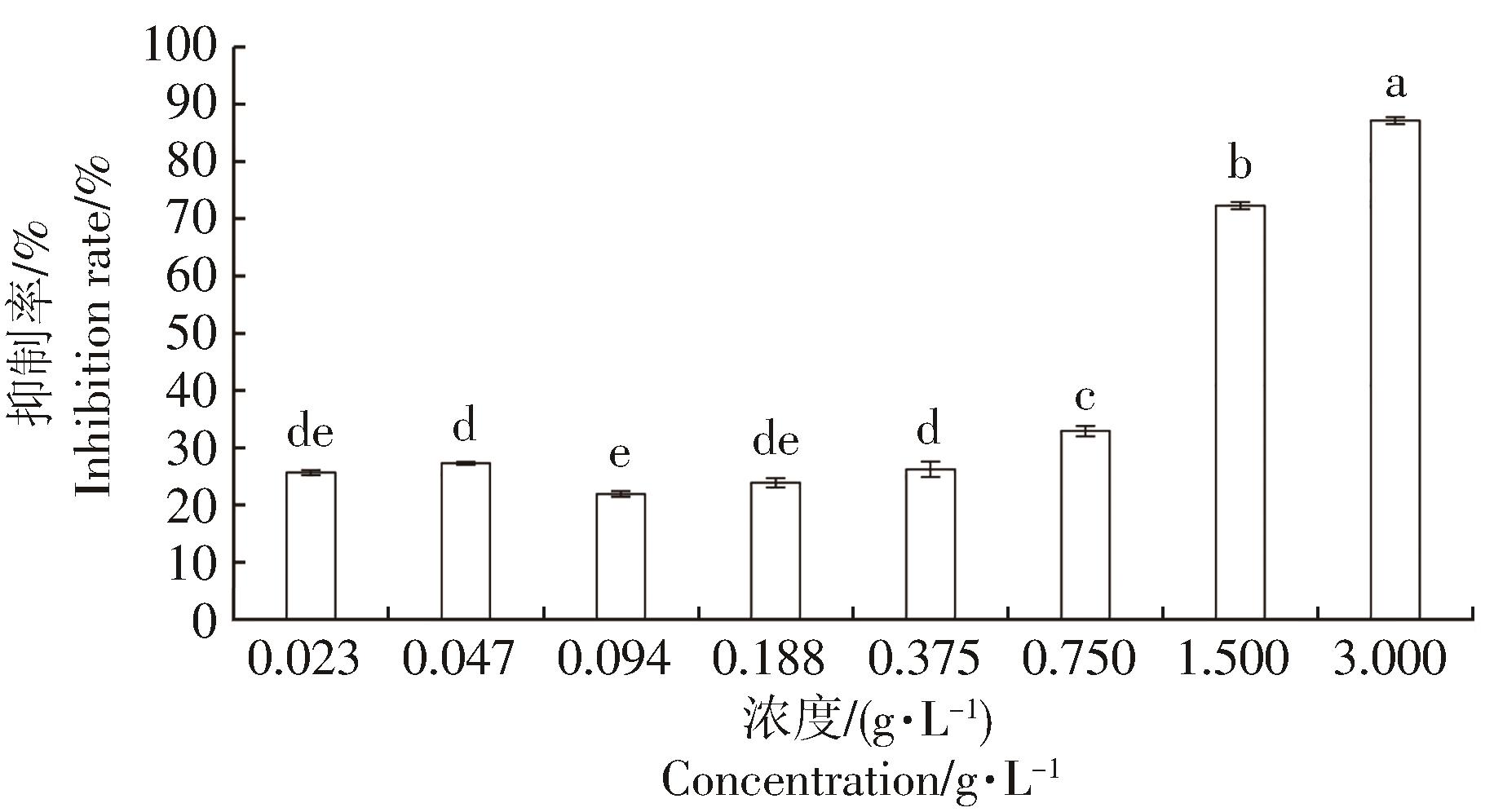
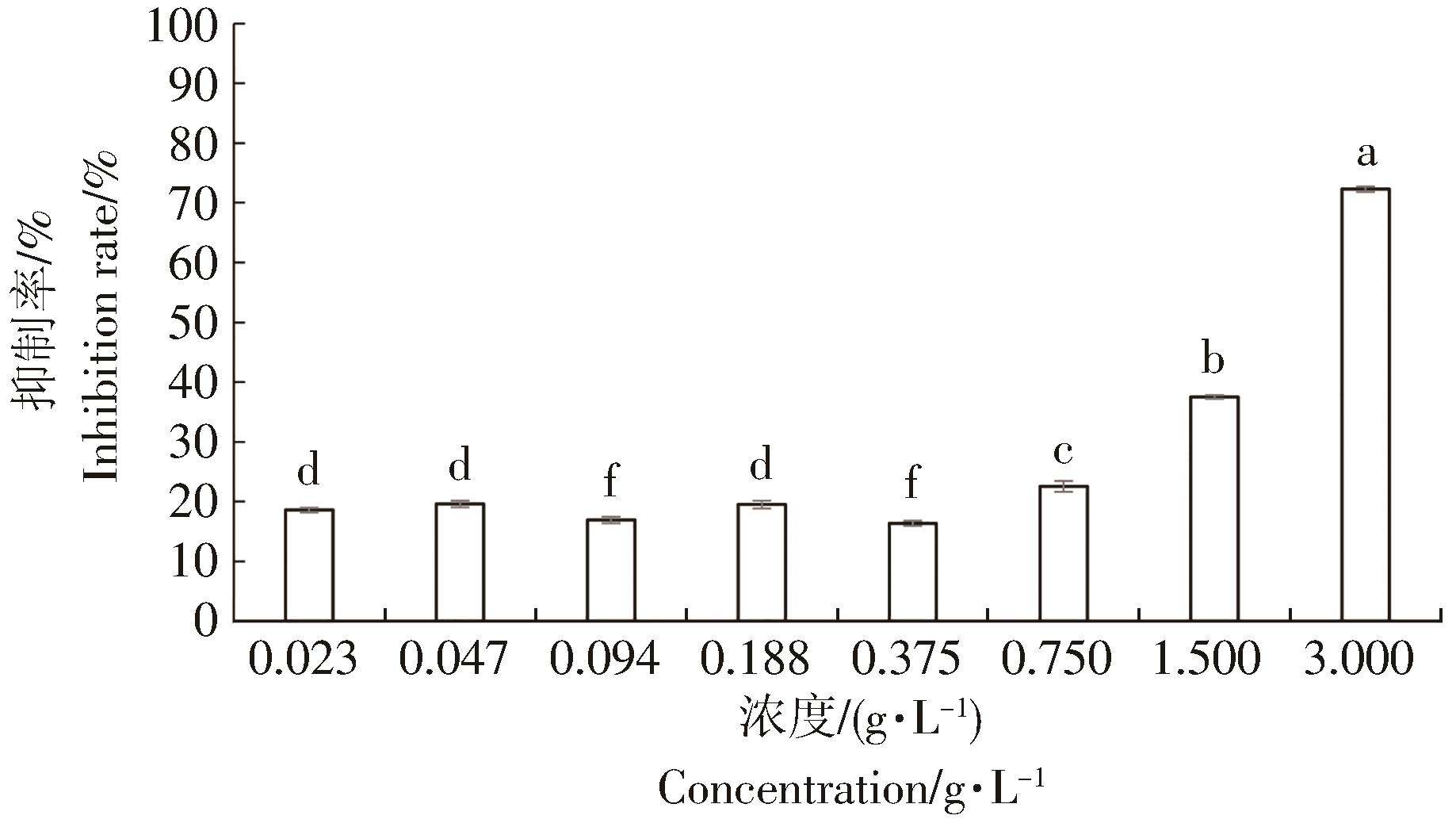
石碌含笑花苞和叶片的精油对α-葡萄糖苷酶(0.2 U·mL-1)的抑制率见图2、图3。活性测试实验结果表明,以金雀异黄酮[IC50=(20.55±2.66)μmol·L-1]为阳性对照,两种挥发油的质量浓度在0.75 g·L-1后,其对α-葡萄糖苷酶的抑制率均出现明显的增加。当浓度为3 g·L-1时,石碌含笑叶片挥发油对α-葡萄糖苷酶的抑制率达到87.13%,IC50=(0.73±0.04)g·L-1;花苞挥发油对α-葡萄糖苷酶的抑制率为72.27%,其IC50=(1.77±0.07)g·L-1。这表明石碌含笑叶片和花苞中的一些成分可能具有一定的降血糖活性,且叶片中挥发油的抑制率较高于花苞挥发油的抑制率,这种抑制作用可能是由于石碌含笑叶片挥发油中存在大量倍半萜醇类[25]。廖天柱[26]对六种含笑属植物提取物进行α-葡萄糖苷酶的IC50研究表明,醉香含笑(Michelia macclurei)的抑制活性最为显著, IC50为(0.69±0.06)g·L-1,其次是多花含笑(Michelia floribunda)[IC50=(1.33±0.12)g·L-1]、深山含笑(Michelia maudia)[IC50=(1.61±0.14)g·L-1]、乐昌含笑(Michelia chapensis)[(3.15±0.31)g·L-1]、台湾含笑(Michelia compressa)(IC50=(5.33±0.34)g·L-1)和阔瓣含笑(Michelia platypetala)[IC50=(6.26±0.43)g·L-1]。相较于乐昌含笑、阔瓣含笑和台湾含笑,石碌含笑挥发油对α-葡萄糖苷酶表现出显著的抑制作用。尽管体外抑制酶活性表现一般,但不能完全否定石碌含笑提取物在医疗美容、降血糖药物研发等领域的开发潜力和应用价值。石碌含笑挥发油的降血糖活性表现一般,也可能是与植物的生长环境、提取溶剂以及提取方式有关[27-28]。
-
α-葡萄糖苷酶将低聚糖或二糖水解为可吸收的单糖,从而促进人体对碳水化合物的吸收。当其活性被抑制时,能有效降低对碳水化合物的消化速率,从而达到降低餐后血糖的目的[29]。天然来源的α-葡萄糖苷酶抑制剂具有安全疗效、副作用小等优点,天然酶抑制剂的研发成为现在医药研究领域的研究热点[30-31]。目前尚未见有关于石碌含笑挥发油的成分分析和含笑属植物挥发油对α-葡糖苷酶抑制活性的报道。
本研究通过GC-MS对石碌含笑花苞和叶片的精油进行化学成分分析以及测定了两个部位精油的α-葡萄糖苷酶抑制活性。本研究表明,在花苞和叶片的精油的组分分析中分别得到39个和31个化学成分,两个部位精油的组成都是以倍半萜醇类和倍半萜烯烃类为主,这与文献[32]报导的相似,且醇类和烯烃类相对含量的差异是区别两种精油的重要指标。花苞的挥发油成分较为丰富,可能与组织结构里有利于释放挥发油的栅栏组织和海绵组织类型有关, 而且细胞排列方式、释放挥发油成分的分泌细胞也有影响[33]。两个部位挥发油化学组成有一定的相似性,有18种成分一致,分别占叶片精油的58.06%、花苞精油的46.15%,花苞的烯烃类物质有18种,以β-红没药烯、α-姜黄烯、α-桉叶油醇以及β-榄香烯为主要成分;醇类物质有14种,含量最高的是愈创木醇;其中β-榄香烯存在于大多数含笑属植物挥发油中[34-35]。桉叶油醇能诱导人肝癌细胞的凋亡,对肝癌细胞具有抗增殖活性;可利用桉叶油醇抑制肿瘤血管的生成和肿瘤的增殖达到治疗的效果;桉叶油醇是未来治疗胆管癌的潜在药物[36-38]。β-榄香烯和姜烯作为植物精油中常见的主要成分,已被报道具有一定的抗癌活性[39-41]。β-榄香烯能抑制肺癌细胞生长,为治疗肺癌细胞提供了一种新的方法[42]。汪洪武等[43]的研究表明含笑花的主要成分为倍半萜醇类和倍半萜烯烃类物质,主要包括β-榄香烯、愈创木醇等成分,其中大牻牛儿烯B、长蠕孢吉码烯、丁香烯和β-榄香烯等构成了含笑特有香味。叶片主要烯烃类物质有14种,主要醇类物质有14种,主要醇类物质是愈木创醇和异愈创木醇。愈创木醇能够直接作用于寄生虫,同时对于非小细胞肺癌也具有明显的抑制作用[44]。
石碌含笑叶片和花苞的挥发油对α-葡萄糖苷酶的IC50值分别为(0.73±0.04)g·L-1和(1.77±0.07)g·L-1,叶片挥发油高于花苞挥发油可能是因为叶片挥发油中存在大量倍半萜醇类,这与祁悦等[45]对喙花姜的块茎和根中的挥发油的研究一致,块茎和根的挥发油主要为单萜和倍半萜,且表现出一定的α-葡萄糖苷酶抑制活性。同时,陆廷亚等[46]通过实验证明大高良姜地下根茎挥发油对α-葡萄糖苷酶表现出一定的抑制作用,其中的挥发油成分也主要为倍半萜等。植物精油中成分较为复杂,虽然许多木兰科植物的精油能够表现出α-葡萄糖苷酶抑制作用,但具体与α-葡萄糖苷酶发生结合的组分以及结合的强度尚不清晰。后续试验可以通过体内实验验证这些具有α-葡萄糖苷酶抑制活性的组分在体内的降血糖作用及机制。由于精油对酶抑制作用的强弱以及体外实验的局限性,精油是否能够降低人体血糖或者作为降血糖药物的辅助剂,这仍需要通过进一步的实验验证。
-
从水蒸气蒸馏法提取的石碌含笑花苞和叶片的精油中分别检测到39个和31个化学成分,都以倍半萜醇类和倍半萜烯烃类为主。两种精油共有的成分18个,其中愈创木醇、异愈创木醇、α-姜黄烯等主要成分含量有明显差异,且醇类和烯烃类相对含量的差异是区别两种精油的重要指标。研究结果表明,石碌含笑叶片挥发油对α-葡萄糖苷酶的抑制率达到87.13%,IC50为(0.73±0.04)g·L-1;花苞挥发油对α-葡萄糖苷酶的抑制率为72.27%,其IC50为(1.77±0.07)g·L-1。石碌含笑的花苞和叶片的精油具有良好的α-葡萄糖苷酶抑制活性,为石碌含笑资源的综合利用及高值化加工提供新思路,也为其在降血糖等方面的研究提供理论依据。









 DownLoad:
DownLoad: