-
吗啡在临床上是效果良好的止痛药,用于治疗慢性疼痛,但是反复使用会导致成瘾,这极大地限制了它在临床上的应用[1]。此外,全球因使用吗啡导致成瘾的人越来越多,严重危害了个人身体健康和公共卫生安全[2]。目前,国内外研究吗啡成瘾的方法有很多,包括条件性位置偏爱模型[3-4]、自身给药模型[5-6]及行为敏化模型等[7-9]。条件性位置偏爱(CPP)模型具有周期短、操作简单的特点,被广泛用于研究药物成瘾,是研究药物滥用的经典动物模型。运用巴普洛夫反射原理,通过小鼠在伴药箱停留的时间多少,来反映小鼠对滥用药物的渴求程度,也是一种经典的筛选抗成瘾药物的动物模型[10]。CPP模型的建立有很多影响因素,如给药方法(增量法、恒量法)、恒量法中的吗啡浓度(5、10、20 mg·kg−1)[11-13]、吗啡注射次数(3、4、6次)[14-15]、给药方式(皮下注射,腹腔注射)[16-17]、给药时程间(6、8、10 d)、训练时间(30、40、50 min)的选择;CPP箱体个数的差异(三箱式、两箱式);CPP箱体环境的差异(箱壁颜色、箱底纹理)及动物种类(小鼠种类、大鼠种类)[18]等都会影响CPP模型建立的效果。在恒量吗啡给药中,不同研究存在吗啡给药时间间隔上的差异[19-20],主要有24 、48 h,目前这两种方法间的差异尚未见报道。本研究针对不同给药时间间隔对吗啡诱导小鼠CPP模型的影响,比较建立模型前后小鼠在各箱的停留时间和CPP模型的消退情况,对吗啡诱导CPP模型进行综合评价,为后续抗吗啡成瘾药物的筛选提供整体动物模型。
HTML
-
本实验动物为C57BL/6J小鼠,6周龄,雄性,购于湖南斯莱克景达实验动物有限公司【许可证号:SCXK(湘)2016—0002】,在标准环境(8:00—20:00明暗交替)下分笼饲养,每笼6只,让小鼠任意取食水和食物。实验开始前,给小鼠1周的时间适应实验室环境。实验随机分为4组,分别为生理盐水组(24 h),生理盐水组(48 h),吗啡组(24 h)和吗啡组(48 h),每组12只小鼠。硫酸吗啡由中国药品生物制品检定所提供(批号171201-200521)。
-
条件性位置偏爱箱(Anilab浙江宁波):是三箱式条件性位置偏爱箱,分为两侧箱和中间箱。两侧箱大小相等(长 × 宽 × 高=17.38 cm × 13.5 cm × 15 cm),左侧箱为白箱,有白色的箱壁和圆孔地板,右侧箱为黑箱,有黑色的箱壁和栅栏样地板。中间箱为连通箱,大小为(9.8 cm × 13.5 cm × 15 cm)。透明有机玻璃挡板可以放在中间箱中,将小鼠限制在相应的箱体里面。箱体上方有摄像头,用于记录实验期间小鼠的运动情况。偏爱箱放置于小型的隔音室里,避免声音对实验的干扰。
-
基础值指造模前小鼠在伴药箱停留的时间。基础值期前一天捉拿小鼠,用针头轻轻摩擦小鼠颈背部皮肤,消除小鼠在皮下注射过程中产生的恐惧情绪。在基础值期,将小鼠头朝同一方向放入中间箱后,让小鼠自由地在3个箱体中穿梭,记录小鼠15 min内在每个箱体里停留的时间,每天1次,共3 d,取后2 d 的平均值作为基础值,选取左右2箱中时间较少的箱体为伴药箱,另一侧箱体为非伴药箱,筛除对于特定箱体存在自然偏爱的小鼠(在某一箱体的时间大于540)。
-
用透明玻璃挡板封闭中间箱,使小鼠只能在特定的箱体里活动。吗啡组(24 h)小鼠,上午8:30注射吗啡(5 mg·kg−1)后立刻放入伴药箱40 min,下午2:30注射生理盐水后放入非伴药箱40 min,每天2次注射,连续给药4 d。吗啡组(48 h)小鼠,上午8:30给药,1、3、5、7 d给吗啡(5 mg·kg−1),2、4、6、8 d给生理盐水,每天注射1次,连续给药8 d。生理盐水组(24 h)和生理盐水组(48 h)操作与时间间隔相同的吗啡组的操作相同,将吗啡换为生理盐水。
-
造模期结束后,第二天将小鼠放入中间箱,让其自由穿梭15 min,记录各组小鼠在伴药箱停留的时间,计算CPP得分,观察各组对伴药箱的偏好情况。CPP得分(CPP scores)= CPP造模后在伴药箱停留的时间−造模前在伴药箱停留的时间。
-
筛选造模成功的小鼠进行消退,观察CPP模型的持续时间,采用半训练消退的方式检测,让小鼠每天在条件性位置偏爱箱中自由穿梭15 min,观察小鼠在伴药箱停留的时间,直到小鼠在伴药箱的停留时间与基础值相差不超过20%。
-
使用GraphPad Prism 5软件进行数据的统计分析,数据结果通过平均值 ± 标准误(Mean ± SEM)表示,根据实际数据采用单因素方差分析(one-way ANOVA),Dunnett’s Multiple Comparison Test检验分析或双因素方差分析(two-way ANOVA),Bonferroni post tests检验分析,P<0.05为显著性差异。
1.1. 实验动物和药物
1.2. 实验仪器
1.3. CPP模型的建立
1.3.1. 基础值期
1.3.2. 造模期
1.3.3. 表达期
1.3.4. 消退期
1.4. 数据统计分析
-
CPP造模前后小鼠在伴药箱停留时间见表1,结果表明,造模前各组小鼠在伴药箱停留时间无明显差异,经多次皮下注射吗啡后,与生理盐水组(24 h)相比,吗啡组(24 h)小鼠在伴药箱的时间极显著增加(P < 0.001);与生理盐水组(48 h)相比,吗啡组(48 h)小鼠在伴药箱的时间也极显著增加(P < 0.001)。计算CPP得分,结果见图1。通过单因素方差分析,与生理盐水组相比,吗啡组24 h和48 h组的CPP得分均明显增加,但二者之间无显著性差异,两种方法都能成功建立CPP模型。
给药时间间隔
Interval of drug
administration/h组别
Treatment
group造模前伴药箱时间/s
Time spent in drug paired compartment
prior to model establishment造模后伴药箱时间/s
Time spent in drug paired compartment
after model establishmentCPP 得分
CPP score24 生理盐水组 274.26 ± 17.01 287.35 ± 25.03 13.09 ± 16.63 吗啡组 260.45 ± 14.05 452.29 ± 22.52*** 191.85 ± 23.08*** 48 生理盐水组 278.87 ± 20.70 292.48 ± 17.17 13.61 ± 8.18 吗啡组 285.49 ± 12.04 462.47 ± 21.51### 176.98 ± 17.89### 注:***代表与生理盐水组(24 h)相比较具有极显著差异,P< 0.001;###代表与生理盐水组(48 h)相比较具有极显著差异,P< 0.001。
Note:*** indicates highly significant difference at P < 0.001 as against the saline group (24 h); ### indicates highly significant difference at P < 0.001 as compared to the saline group (48 h).Table 1. The effect of intervals of drug administration on establishment of conditioned place preference (CPP) model
-
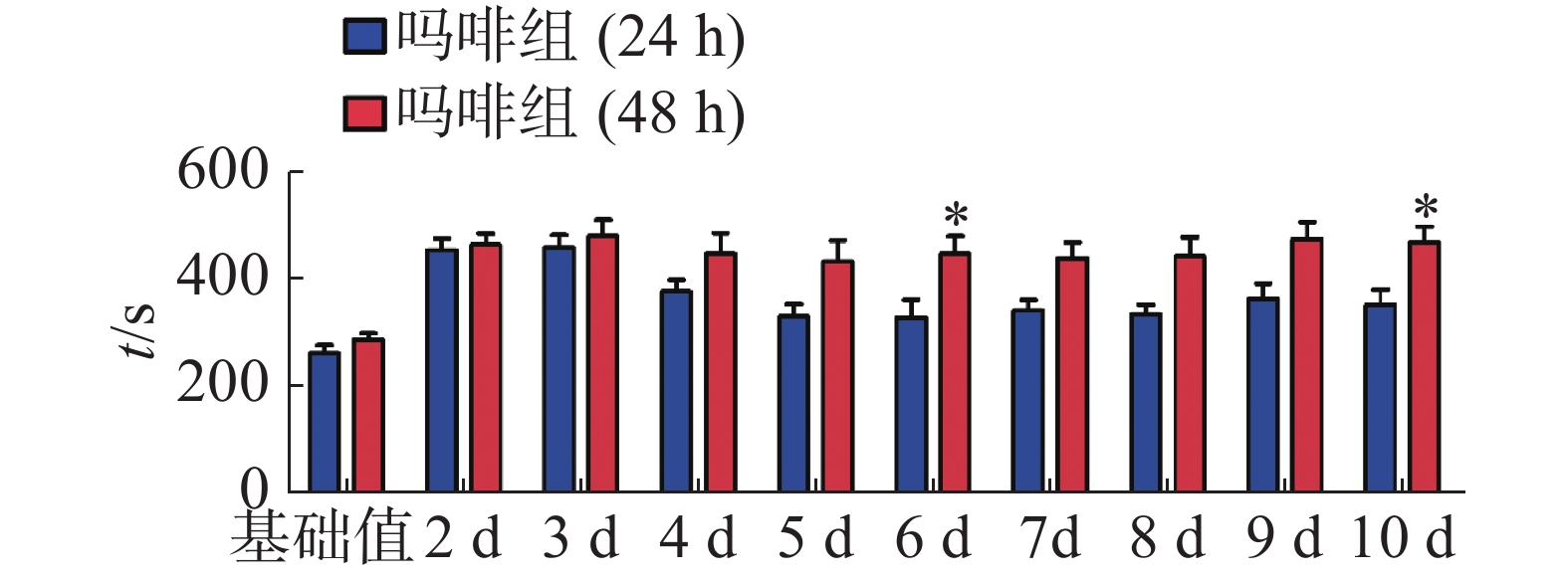
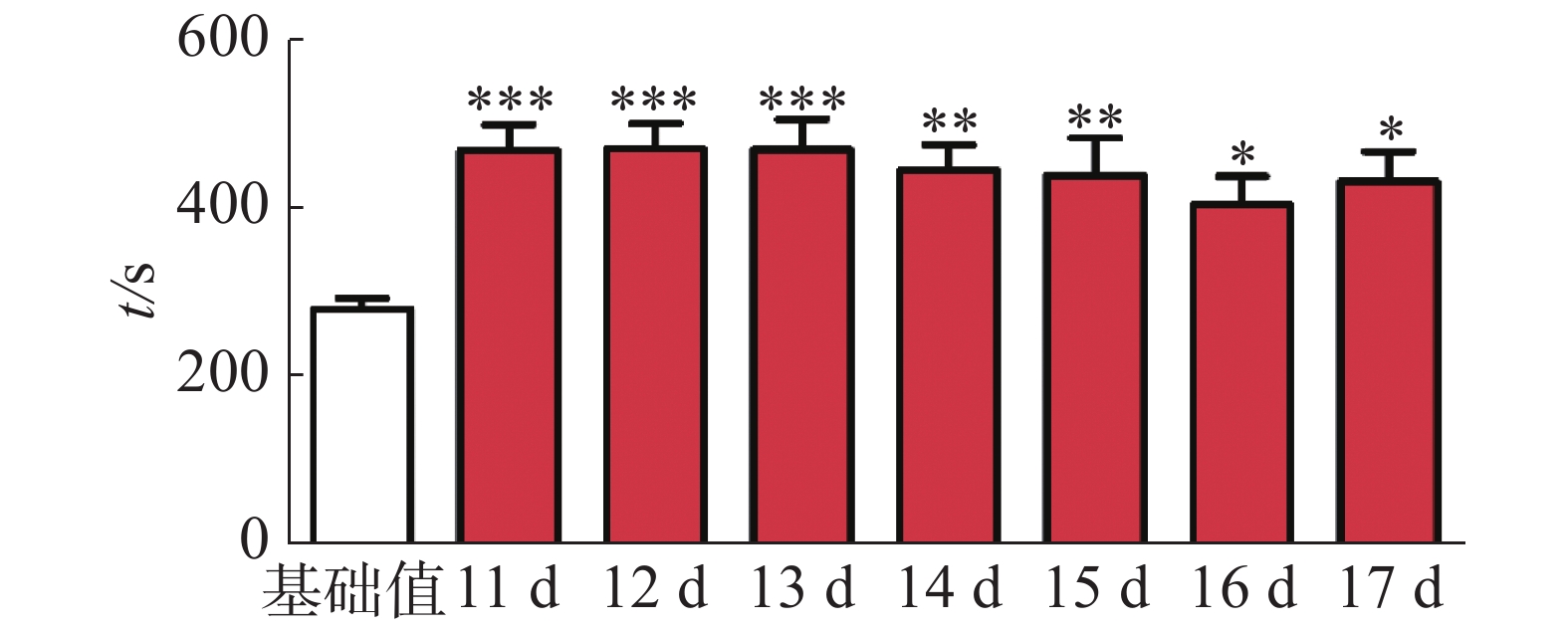
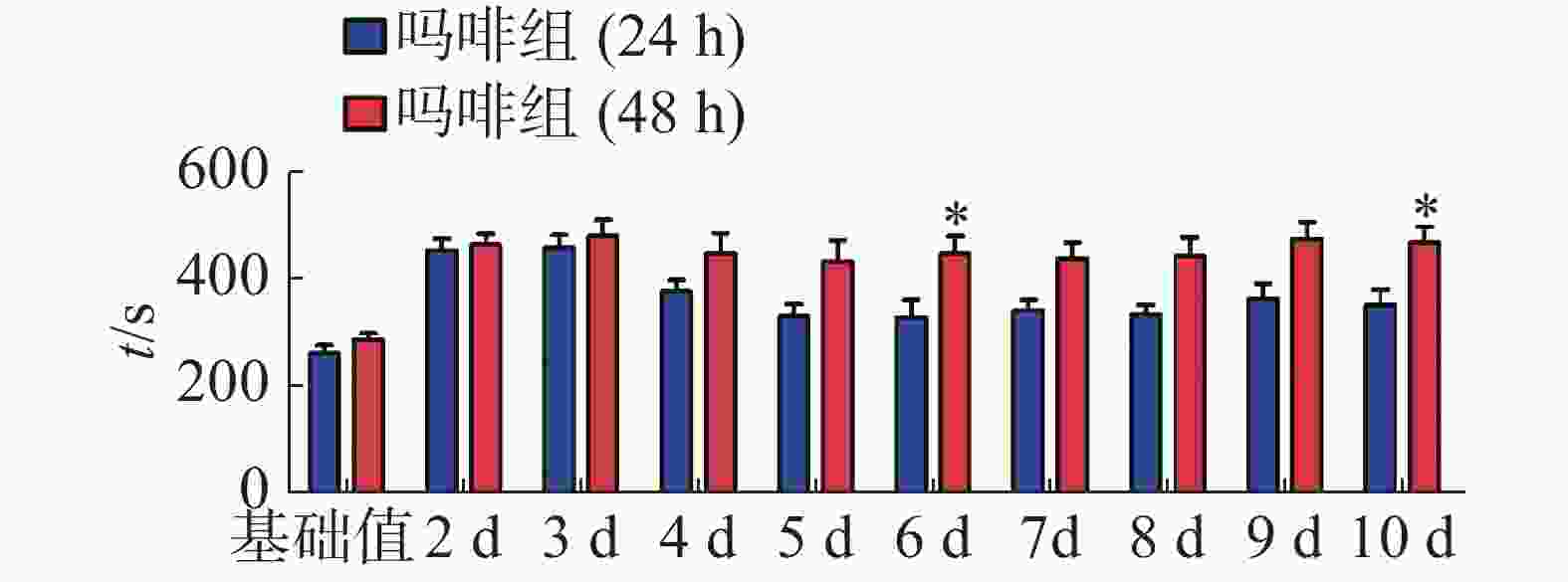
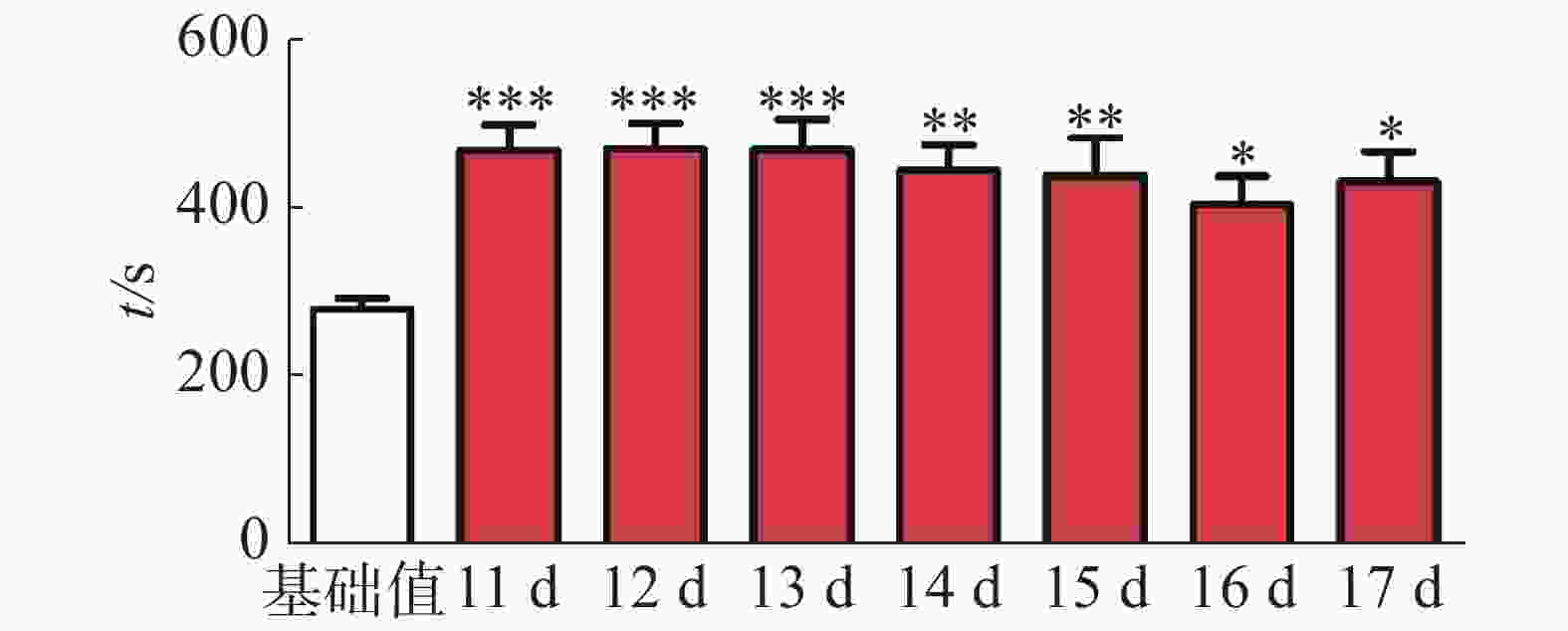
CPP表达期以后第2天进行消退训练,比较2种给药时间间隔建立CPP模型后的维持情况。每天进行1次15 min的测试。结果(图2)表明,前3 d内,两组小鼠在伴药箱的停留时间差别不大,说明短期(3 d)内时间间隔为24 h和48 h时建立的CPP模型无差别;从第4 天开始,吗啡组(24 h)小鼠在伴药箱停留的时间开始变小,出现消退情况(伴药箱时间与基础值的差值为基础值的20%以内),6~10 d时,消退情况较多,但消退情况不稳定(前天消退,后天又恢复)到10 d时大部分小鼠(8/12,约66%)消退且稳定,而吗啡组(48 h)小鼠在伴药箱的时间一直维持在440 s左右,10 d时,只有少部分(3/12,约25%)小鼠的CPP恢复至基础值左右,在6 d和10 d时,伴药箱的时间显著高于吗啡组(24 h)的时间(F(9,220) = 7.23;6 d,t=3.000,P<0.05;10 d,t=2.947,P< 0.05),这表明给药间隔时间为24 h建立的CPP模型维持时间短、易被消退;对吗啡组(48 h)小鼠继续测试7 d,结果见图3。从图3可见,吗啡组(48 h)在11~13 d时伴药箱内停留依然保持较高水平,与基础值相比具有极显著差异(P< 0.001),在第14天时出现消退情况,到第16天后部分(5/12,42%)小鼠被消退,所以吗啡给药间隔48 h建立的CPP模型可以维持15 d。
2.1. 不同给药时间间隔对CPP模型建立的影响
2.2. 不同给药时间间隔对CPP模型消退的影响
-
本研究探究了不同给药时间间隔(24、48 h)对5 mg·kg−1吗啡在C57BL/6J小鼠上建立CPP模型的影响。结果表明,5 mg·kg−1的吗啡能诱导CPP模型的建立,与文献[21]结果一致,吗啡给药间隔24 h建立的CPP模型与给药间隔48 h的相比,在3 d内伴药箱时间无明显变化,说明2种方法均能建立稳定的CPP模型。复吸是研究药物依赖的一个重要方面,而消退过程是复吸的前提,因此本研究也评估了CPP模型的消退,为后续吗啡成瘾中滥用药物的复吸(复发)研究奠定基础。CPP的消退是让小鼠反复的进入伴药箱(环境刺激)而不给予滥用药物(条件刺激),从而让小鼠对伴药箱的偏爱逐步消退的过程。本研究采用半训练消退的方法[22],每天将小鼠放入CPP箱中15 min,让其自由穿梭,连续9 d。结果表明,吗啡给药间隔24 h的CPP模型可以维持5 d,在10 d时大部分(8/12,约66%)小鼠已经稳定消退到基础值水平,而吗啡给药间隔48 h的CPP模型可以维持15 d,在第10 天时仅少量(3/12,约25%)小鼠消退,在第16 天时部分(5/12,42%)小鼠消退。所以给药间隔48 h的CPP模型维持时间更久,不容易被消退。这与前人研究报道的同剂量下增加给药时间间隔能有效提高吗啡诱导CPP模型稳定性的结果一致[23]。
吗啡诱导的条件性位置偏爱形成以后,动物会产生心理渴求和强制性觅药行为,这种觅药行为的形成与药物奖赏,突触信号转导,神经可塑性以及与药物依赖相关的畸形记忆息息相关。目前研究较多的是奖赏通路和神经突触的可塑性变化,奖赏通路涉及多个脑区(中脑腹侧被盖区、伏隔核、海马、前额叶皮质)的神经递质(谷氨酸、多巴胺、5-羟色胺、γ-氨基丁酸等)转运[24-26],其中主要是中脑腹侧被盖区到伏隔核的多巴胺投射通路。突触可塑性变化,表现为脑内突触树突分支树突棘密度的变化,突触间信息传输出现长时程增强(long term potential,LTP)和长时程抑制(long term depression,LTD)效应[27],前者涉及NMDA和mGluR受体激活,钙调蛋白激酶Ⅱ(CaMKⅡ)和一氧化氮合酶(nNOS)激活[28],引起细胞级联反应[29],调控基因表达[30],后者与炎症因子和神经元细胞的凋亡[31],两者一起参与吗啡成瘾的病理过程。给药时间间隔对可塑性的影响尚未见报道,给药时间间隔可能也会影响上述通路而参与CPP模型的建立。
综上所述,在吗啡诱导的条件性位置偏爱中,在短期实验内,两种方法CPP模型建立效果基本一致,给药的时间间隔越长,建立的CPP模型越稳定。在第4天吗啡给药,给药时间间隔为24 h时,经过10 d的半训练消退,CPP模型会大部分消退。如果为了研究药物对复吸情况的影响,建议选择时间间隔为24 h,因为该方法建立的CPP模型消退快,在第10天时部分小鼠已经完成消退。在第8天吗啡给药,给药时间间隔为48 h时,CPP可以维持15 d。如果实验为了研究药物对CPP模型消退的影响,则应选择间隔48 h,这样不容易出现因实验中测试次数过多而被消退的情况。








 DownLoad:
DownLoad: